Abstract
Photodynamic therapy (PDT) is a form of non-ionizing radiation therapy that uses a drug, called a photosensitizer, combined with light to produce singlet oxygen (1O2) that can exert anti-cancer activity through apoptotic, necrotic, or autophagic tumor cell death. PDT is increasingly being used to treat thoracic malignancies. For early-stage non-small cell lung cancer (NSCLC), PDT is primarily employed as an endobronchial therapy to definitively treat endobronchial or roentgenographically occult tumors. Similarly, patients with multiple primary lung cancers may be definitively treated with PDT. For advanced or metastatic NSCLC and small cell lung cancer (SCLC), PDT is primarily employed to palliate symptoms from obstructing endobronchial lesions causing airway compromise or hemoptysis. PDT can be used in advanced NSCLC to attempt to increase operability or to reduce the extent of operation required, and selectively to treat pleural dissemination intraoperatively following macroscopically complete surgical resection. Intraoperative PDT can be safely combined with macroscopically complete surgical resection and other treatment modalities for malignant pleural mesothelioma (MPM) to improve local control and prolong survival. This report reviews the mechanism of and rationale for using PDT to treat thoracic malignancies, details prospective and major retrospectives studies of PDT to treat NSCLC, SCLC, and MPM, and describes improvements in and future roles and directions of PDT.
Photodynamic therapy (PDT), either alone or in combination with other treatment modalities, is increasingly being used to treat thoracic malignancies. Unlike external-beam radiation therapy, which delivers ionizing irradiation, PDT delivered non-ionizing electromagnetic irradiation. PDT uses a photosensitizing agent, or photosensitizer, that can accumulate in tumor cells and is activated by light of a specific wavelength to produce reactive singlet oxygen, designated 1O2. This reactive oxygen species mediates cellular cytotoxicity. The PDT mechanism of action has previously been described.1 PDT exerts direct tumor cell killing through both apoptosis and necrosis and by damaging tumor vasculature.1,2 PDT also may induce an inflammatory reaction capable of stimulating a tumor-directed host immune response.3 As the target of PDT is not tumor deoxyribonucleic acid, in contrast to ionizing external beam radiation therapy, PDT-induced secondary cancers are highly unlikely to occur. Additionally, chemoresistance and radio-resistance do not influence the efficacy of PDT, PDT can be repeated to the same site without compromising its efficacy, and it does not compromise the ability to administer other treatment modalities in patients with recurrent or residual disease.1
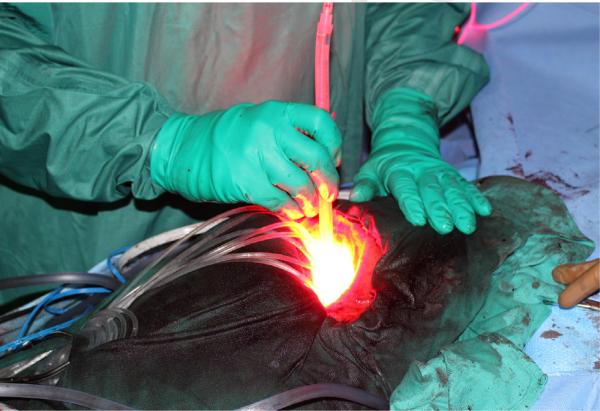
The optimal wavelength of light to activate the photosensitizer is dependent on the activation characteristics of the sensitizer, itself. After photosensitizing agent administration, tumor cells are irradiated with appropriate wavelength light from a laser or other source. Since the light required to activate common photosensitizing agents typically cannot pass beyond 5–10 mm of tissue, PDT is most commonly used to treat thoracic malignancies involving the lining of internal organs or cavities; it is less efficacious when being delivered for larger tumors without prior surgical debulking or use of interstitially placed light sources. For endobronchial PDT applications, a common light source is a laser-directed through fiberoptic cables and inserted through an endoscope into the lungs, whereas pleural PDT is more commonly delivered using microlenses and custom-designed applicators (Figure 1).4–6
Figure 1.
Intracavitary photodynamic therapy delivery. Following radical pleurectomy and gross macroscopic resection of malignant pleural mesothelioma, intraoperative PDT at 630 nm (red light) is delivered to the pleural surface using an optical fiber sheathed within a modified endotracheal tube. Seen existing the pleural cavity are the leads to isotropic light detectors that are externally attached to a real-time dosimetry system to monitor light fluence and dose.
NON-SMALL CELL LUNG CANCER
Approximately 224,210 patients will be diagnosed with lung and bronchus cancers in the United States in 2014, with the majority of these cases being non-small cell lung cancer (NSCLC). Despite standard treatments of surgery, chemotherapy, and radiation therapy, lung cancer remains the leading cause of death attributed to malignancies, and it is projected to account for nearly 160,000 deaths in the United States in 2014.7 PDT offers another treatment modality by which to combat this grave malignancy.
The photosensitizing agent that has been used most commonly to treat lung and other thoracic malignancies is porfimer sodium, which was approved by the US Food and Drug Administration (FDA) for cases of NSCLC where standard therapies are not appropriate and to palliate symptoms from airway obstruction. The FDA first approved PDT to treat microinvasive endobronchial NSCLC in early 1998, followed by approval to treat advanced partially obstructing endobronchial lung cancer in late 1998.8
Since then, there has been increasing interest in using PDT alone or in combination with standard modalities to definitely treat or palliate symptoms from NSCLC. PDT currently has roles in the treatment of roentgenographically occult, early-stage, and endobronchial NSCLC, synchronous primary bronchogenic carcinomas, and advanced NSCLC (Table 1).
Table 1.
Roles of PDT to Treat Patients With Thoracic Malignancies
| Non-small Cell Lung Cancer* |
| Definitive therapy for early-stage central endobronchial tumors |
| Definitive therapy for early-stage locally recurrent central tumors following Definitive surgery or radiation therapy |
| Definitive therapy for early-stage peripheral lung lesions |
| Definitive therapy for roentgenographically occult central tumors |
| Definitive therapy for synchronous primary carcinomas |
| Neoadjuvant therapy to convert originally inoperable patients to surgical candidates |
| Neoadjuvant therapy to reduce the extent of surgical resection (pneumonectomy → lobectomy) |
| Palliation to reduce endobronchial luminal obstruction and tumor stenosis, improve performance status and respiratory function, and resolve acute hemoptysis and poststenotic pneumonia |
| Treatment of disease with pleural spread as part of multi-modality therapy |
| Treatment of locally advanced disease as part of multi-modality therapy |
| Small-cell Lung Cancer |
| Palliation to reduce endobronchial luminal obstruction and tumor stenosis, improve performance status and respiratory function, and resolve acute hemoptysis and poststenotic pneumonia |
| Malignant Pleural Mesothelioma |
| Intraoperative adjuvant therapy following extrapleural pneumonectomy |
| Intraoperative adjuvant therapy following radical pleurectomy |
| Palliation to reduce endobronchial luminal obstruction and tumor stenosis, improve performance status and respiratory function, and resolve acute hemoptysis and poststenotic pneumonia |
Role of PDT for non-small cell lung cancer adapted, in part, from Simone CB 2nd, et al. J Thorac Dis. 2012;4(1):63-75.2
Advanced NSCLC
Only approximately 15% of patients with NSCLC present with localized disease confined to their primary tumor site at the time of diagnosis.9,10 More commonly, patients present with locally advanced and metastatic NSCLC, which is often associated with a high symptom burden and significant morbidity.11–13 While patients with malignancies commonly experience pain during the course of their disease,14,15 prior studies have reported higher rates of pain among patients with lung cancer compared with other cancer types.16,17 Advanced lung cancer patients are also likely to experience dyspnea, cough, and hemoptysis, all of which serve to limit quality of life.
PDT has been used to palliate symptoms from advanced NSCLC, and endobronchial PDT can effectively reduce airway obstruction and improve respiratory function and quality of life.2 Investigators in the United Kingdom prospectively evaluated PDT for palliation among 100 patients with stage IIIA–IV advanced bronchogenic carcinoma with endobronchial luminal obstruction, of which 82% had previously received radiation therapy or chemotherapy. PDT decreased endoluminal obstruction on average from 85.8% to 17.5%, with similar improvements in pulmonary function testing.18 PDT also was used to treat 133 symptomatic patients at the University of Alabama Hospital for endobronchial lung lesions, the majority of whom had NSCLC (n = 89). PDT achieved significant improvements in dyspnea in 74% of patients based on the Modified Medical Research Council Dyspnea Scale.19
Although Nd:YAG laser therapy has historically been the most used therapy for tumor ablation within the tracheobronchial tree, based on these studies and others, PDT has become a clearly established effective alternative to Nd:YAG. In fact, PDT may offer a safer and easier to perform alternative treatment to Nd:YAG.20,22 In a prospective randomized controlled trial comparing PDT (n = 14) to Nd:YAG laser therapy (n = 17) in 31 patients with partial or complete tracheobronchial obstruction from NSCLC, Spanish investigators found that symptomatic relief was more durable among patients treated with PDT, with a significantly longer time until treatment failure (P = .03) and longer median survival (P = .007).23
PDT also can serve as a management tool for hemoptysis by causing thrombosis and controlling small vessel bleeding.24 In fact, resolution of acute hemoptysis was achieved in 70% of patients treated at the University of California, Irvine with stage III–IV obstructive inoperable NSCLC with hemoptysis, dyspnea, and airway obstruction.25 Similar excellent control of hemoptysis was achieved with PDT in a prospective study in Austria.26
PDT also can be combined effectively with external-beam radiation therapy and brachytherapy. External-beam radiation therapy is perhaps the most established and effective palliative treatment modality for patients with central obstruction from NSCLC. Prospective studies have suggested a possible synergistic effect when combining PDT and ionizing radiation, particularly brachytherapy. A report from Beth Israel Deaconess Medical Center of 32 patients with bulky endobronchial NSCLC treated with PDT followed 6 weeks later by brachytherapy (192Ir, 4 Gy weekly × 5 weeks) documented 81% local control, 94% distant metastasis-free survival, and 100% overall survival at a mean follow-up of 24 months.27 In a small randomized trial by Vancouver investigators to determine if PDT improves outcomes when combined with external-beam radiation therapy for patients with inoperable NSCLC causing central airway obstructing, PDT before radiation therapy significantly improved responses and allowed for more durable local control.28
As part of multimodality therapy, PDT has been reported to be an effective component of definitive therapy for patients with locally advanced NSCLC.29 PDT has been used as a means to reduce the extent of surgical resection required by allowing patients with NSCLC who were initially planned to undergo pneumonectomy the ability to undergo lobectomy,30,31 and also to convert patients originally inoperable due to disease extent to become surgical candidates.31 PDT successfully reduced the extent of resection or converted inoperable disease to operable disease in 85% of the 26 patients with NSCLC treated at Tokyo Medical University Hospital, with four of five originally inoperable patients able to be converted to resectable and 18 of 21 patients originally candidates only for pneumonectomy able to undergo lobectomy.31
Additionally, among 41 patients with locally advanced NSCLC treated at Ohio State University Medical Center with induction PDT and chemo-therapy and/or radiation therapy, PDT-based induction converted 57% of patients initially deemed unresectable to be able to undergo definitive resection and allowed the 27% initially planned to need a pneumonectomy to be able to undergo lobectomy. Furthermore, PDT achieved pathological downstaging in 64% and a pathologic complete response in 18% undergoing surgery, with 46% of patients alive at 3 years following therapy.32
PDT also may have a role in the management of NSCLC patients with isolated pleural metastasis. In a phase II trial at the University of Pennsylvania, 22 patients with pleural metastases but no extrathoracic metastases were treated with surgery with complete (n = 17) or partial tumor debulking (n = 3), followed by intraoperative hemithoracic pleural PDT. A 73.3% local control rate was achieved at 6 months, and late pleural recurrences were rare in patients living 2 years. The cohort 3-year survival was 23% and median survival was 21.7 months from the time of surgery and PDT, compared with 6–9 months from the time of diagnosis for similar patients based on historical controls.33
Early-Stage NSCLC
Despite advances in imaging and increased use of positron emission tomography (PET) and PET/computed tomography (CT) scans that for NSCLC have been shown to have extremely high sensitivities in detecting disease and upstaging patients with otherwise more localized disease on CT scans,34,35 there remains a subset of patients with bronchoscopicallyconfirmed lung cancer who have disease that is otherwise undetectable on imaging. These lesions are termed roentgenographically occult. Roentgeno-graphically occult carcinomas are commonly detected through sputum cytology, and patients with these cancers often present with early-stage, centrally located squamous cell carcinomas.
Definite surgical resection has historically been the standard treatment approach for this patient population but can be associated with significant morbidity. Given that lymph node metastasis is exceedingly rare with roentgenographically occult bronchogenic carcinomas seen on endoscopy, focal therapy to the primary lesion alone with PDT can be curative.
National Kinki Central Hospital for Chest Diseases investigators reported an early experience treating 25 patients with 29 roentgenographically occult bronchogenic carcinomas with PDT from 1983–1990. They achieved a complete remission in 72% of lesions with PDT, including 89% (17/19) of lesions ≤ cm.36 Although the complete response rate was only 31% for the 36 patients with 39 roentgenographically occult lung cancer lesions of the trachea and bronchus treated with PDT at the National Cancer Center Hospital in Tokyo,37 a pooled analysis from five Japanese hospitals of 33 patients with 40 roentgenographically occult proximal bronchial carcinomas treated with PDT revealed a complete response in all lesions Figure 1 cm (n = 32) but in only three of eight carcinomas 41 cm.38
Investigators at Osaka Prefectural Habikino Hospital in Japan achieved a complete response in 64% for the 39 roentgenologically occult carcinomas in 29 patients treated with PDT, with complete responses more likely for lesions that were super-ficially infiltrating than lesions that had a nodular appearance (76% v 43%).39 From 1994–2006.
Tohoku University Hospital investigators treated 48 patients with roentgenographically occult bronchogenic squamous cell carcinomas with PDT. All patients had bronchoscopic longitudinal tumor lengths of ≤10 mm. PDT achieved a complete response in 94% of patients, and the 5-year and 10-year overall survival rates were 81% and 71%, respectively, for the cohort.40
Early-stage and endobronchial NSCLC, like roentgenologically occult bronchogenic carcinomas, can be effectively treated with PDT. University of Southern California Medical School investigators reported a 97% complete response rate in 35 patients with predominantly primary NSCLC (n = 29) within the tracheobronchial tree,41 and independent reports from Chinese investigators revealed 96% and 97% response rates in 21 patients with 24 bronchogenic carcinomas42 and 54 patients with 69 bronchogenic carcinomas,43 respectively.
Complete response rates to PDT were achieved in 73% of 50 patients with 59 early squamous cell carcinomas of the bronchus treated at National Kinki Central Hospital for Chest Diseases44 and 62% of 23 patients with 26 early central bronchogenic squamous cell carcinomas treated by Italian investigators, including a 100% overall response rate.45 A recent review of 15 trials and 626 patients with early central lung cancer treated with PDT to 715 lesions revealed PDT is well tolerated, with a PDT-related death in one patient (0.15%), photosensitivity skin reactions in 5%–28%, non-fatal hemoptysis in 0%–8%, and respiratory complications in 0%–18%. In this analysis, PDT achieved a complete response in 30%– 100% of patients, and the overall 5-year survival rate was approximately 61%.46
Lesion size has been shown to impact PDT success for treating early-stage and endobronchial NSCLC. In a phase II study by the Japan Lung Cancer Photodynamic Therapy Study Group from 1989–1992, 49 patients with 59 centrally located subsegmental or larger bronchi early-stage lung cancers were treated with PDT. A complete response was achieved in 85% of lesions, with the complete response median duration greater than 14 months. Complete responses were more likely for lesions with a longitudinal tumor extent of ≤ cm (n = 45) than >1 cm (n 14) (98% v 43%, P = .00001)47. Similarly, a complete response to PDT was achieved in 48% of lesions ≤ cm2 in surface area (n = 29) but not in any lesions >3 cm2 (n = 11) in a series of 38 patients with 40 lesions seen at the Mayo Clinic.48
In a large series of 240 patients with 283 central lung cancer lesions treated with PDT at Tokyo Medical University Hospital, a 99% overall response rate was achieved, with a 40% complete response rate. A complete response was achieved in 83% (79/95) of early-stage lesions, including 94% (65/69) of lesions <1.0 cm but in only 54% (14/26) of lesions ≤1.0 cm and 38% (6/16) of lesions ≤2.0 cm (P = .00001).49 A more recent report by these investigations included 93 patients with 114 central early-stage lung cancers with long-term follow-up after PDT. They again found the complete response rate to be higher for lesions <1.0 cm (77/83) than ≥1.0 cm (18/31) (93% v 58%, P <.001], whereas the 5-year survival rate was not influenced by tumor size (58% v 59%, P = .207). Of note, only 12% of lesions <1.0 cm that had an initial complete response to PDT recurred, and recurrences with low-to-moderate histologic atypia could successfully be salvaged with additional PDT.50
While PDT in early-stage and endobronchial lung cancer is most commonly used to treat patients unsuitable for surgical resection, it has become an established alternative treatment to surgery for patients with early-stage, small, centrally located NSCLC. Among 21patients who were surgical candidates with 23 early superficial squamous cell carcinomas who declined resection at Mayo Clinic, PDT achieved a complete response in 70% of lesions. However, due to persistent disease, disease recurrence, or a second primary lung malignancy, 48% of patients subsequently required surgery.51
Among patients who develop tumor recurrence following surgical resection of early-stage NSCLC, PDT may be a viable salvage option. Among 40 patients with early-stage medically inoperable carcinoma (n = 12) or recurrent carcinoma in situ following previous treatment for invasive lung cancer (n = 28) treated with PDT from 1989–2004, a 72% overall complete response rate was achieved. Although the complete response rates were similar for Tis and T1 lesions (73% v 69%, P 4.05), the median overall survival was longer for patients with Tis (120 months v 36 months, P = .03).52
PDT also has an established role in the management of patients with synchronous primary bronchogenic carcinomas. Such synchronous multiple primary lung cancers occur in up to 15% of lung cancer patients, with this percentage increasing with further improvements in imaging, adoption of surveillance guidelines, and increasing use of PET scanning.53 The treatment of synchronous intrathoracic primary tumors with PDT typically has been limited to patients who are medically inoperable due to medical comorbidities or who would be at significant risk of pulmonary morbidity from repeated surgeries or radiation therapy to definitely treat multiple tumors.54
Among 64 patients treated with PDT from 2004–2008 for early-stage lung cancer, 22 had multiple primary lung cancer lesions. In these patients, surgery was used commonly to manage the more peripheral, larger carcinomas, whereas PDT was the predominant modality for centrally located tumors. All 39 lesions treated with PDT exhibited a complete response to therapy. In a large series of 104 patients with synchronous lung primary malignancies of the trachea and lobar and segmental bronchi treated with PDT or endoluminal endoscopic surgery and PDT, a complete tumor regression was achieved with PDT in all tumors <1 cm in diameter.55
NSCLC Summary
For patients with advanced or metastatic NSCLC, PDT is an effective intervention to palliate symptoms by reducing endobronchial tumor obstruction, which can improve dyspnea and pulmonary function. PDT also can be used to manage acute hemoptysis and poststenotic pneumonia. PDT can be combined with other treatment modalities for advanced disease. In the neoadjuvant setting, PDT can increase the likelihood of patients being surgically operable and/or reduce the extent of operation required. PDT can be safely and effectively combined with external beam radiation therapy or endobronchial brachytherapy and may achieve further improvements in symptomatic relief with more durable responses and improved local control than can be achieved with conventional radiation therapy alone.
Among patients with roentgenologically occult bronchogenic carcinomas, PDT can achieve high complete response rates and is a well-established option for patients who are not medically operable due to comorbidities. PDT is associated with less morbidity than resection and may be considered as a treatment option for roentgenologically occult bronchogenic carcinomas even among select surgical candidates, particularly for those refusing resection. PDT is most effective for tumors with lengths ≤1 cm that have no extracartilaginous invasion and no radiologic findings on high-resolution CT imaging. Similarly, early-stage and endobronchial carcinomas ≤1 cm are treated effectively with PDT. PDT also has an established role in treating synchronous or metachronous primary endobronchial carcinomas, an increasingly common diagnosis. PDT should be considered for patients with synchronous endo-bronchial carcinomas who are medically inoperable or technically unresectable. Since repeated surgical resections or multiple courses of external beam radiation therapy to manage multiple carcinomas may result in significant patient morbidity, PDT also can be considered in properly selected patients who are surgical candidates, particularly for central tumors with diameters <1 cm. Patients treated with PDT for definitive therapy of early-stage NSCLC still need to be monitored closely after treatment to assess for local recurrences.
SMALL CELL LUNG CANCER
Small cell lung cancer (SCLC) has a high propensity for distant metastatic spread and as such, the use of PDT as a potentially curative modality for early-stage SCLC is more problematic. To date, literature on the clinical use of PDT to treat SCLC is extremely limited. Reports of SCLC being treated with PDT have been described in single-patient case studies56,57 or in larger series of bronchogenic carcinomas in which a minority of patients within the cohort had SCLC. Moghissi et al. reported on palliative PDT administration to 100 patients with stage IIIA–IV advanced inoperable bronchogenic carcinomas, 10 of whom had SCLC.18 Similarly, four of 133 patients reported on by Minnich et al19 and four of 81 patients reported on by Furukawa et al22 had SCLC. Only one of 258 lesions treated with PDT by Kato et al was SCLC.58 Results from these studies were not separately reported according to histology.
SCLC Summary
As SCLC has a high propensity for distant meta-stasis, PDT does not have a practical role as definitive therapy for this disease. Furthermore, SCLC is very responsive both to chemotherapy and radiation therapy, and palliation for patients with advanced or metastatic SCLC may best be achieved using those conventional treatment modalities. PDT can be considered, however, for palliation of symptomatic patients with SCLC who have progressive disease following standard treatment modalities or who are not candidates for standard therapies.
MALIGNANT PLEURAL MESOTHELIOMA
Malignant pleural mesothelioma (MPM) is a rare malignancy with a limited median survival on the order of 12 months.59 Most patients with MPM are not candidates for definitive surgery at diagnosis, either because they present with numerous comorbities and/or at an advanced age following a prolonged latency period after asbestos exposure or because they are diagnosed at an advanced stage with disease that is not amenable to gross total resection.
However, among patients with MPM who are candidates for definitive resection, surgery, when administered as a part of a multidisciplinary treatment regimen, can be associated with improved survival over nonsurgical modalities. An International Association for the Study of Lung Cancer trial that evaluated 3,101 patients across 15 centers from four continents showed that patients undergoing surgery with curative intent had improved survival compared with patients not treated with resection as part of their management plan.60 As such, the International Mesothelioma Interest Group Congress recently issued a consensus recommendation that surgery should be performed as part of a multi-modality treatment approach for MPM.61
Currently employed procedures for definitive surgery include an extrapleural pneumonectomy (EPP), which is a lung-sacrificing surgery that entails the en bloc resection of the parietal pleura, lung, pericardium, and diaphragm, and a radical or extended pleurectomy, which entails the resection of all pleural surfaces and gross disease while preserving the lung.62 The goal of both procedures, however, is to obtain a macroscopic complete resection,61,63 with the procedure type selected based on the disease distribution and surgeon preference and experience.61 EPP is the most oncologic surgery-like procedure, with less microscopic residual disease thought to be left behind compared with a lung-sparing surgery, and it better facilitates adjuvant hemithoracic radiotherapy that can improve local control with less concern of radiation-induced lung toxicity64. In contrast, a radical pleurectomy offers lower rates of perioperative morbidity and mortality65 and improved preservation of lung function.66
Unlike with lung cancer, in which PDT can be delivered as monotherapy or as part of multimodality therapy, PDT for mesothelioma only has a role in definitive therapy as part of a multimodality treatment regimen. PDT is able to be combined with EPP67 and radical pleurectomy,68 and it is generally well tolerated. PDT, however, does result in localized inflammation and fluid accumulation in the immediate postoperative setting and can modestly extend hospital stay beyond what is seen with the respective surgical procedures alone.
Among the earliest reports of using PDT to treat MPM was one by investigators at the National Cancer Institute.69 These investigators later performed the only randomized phase III trial that has assessed the benefit of PDT for MPM. From 1993–1996, patients were treated with maximum debulking surgery and postoperative cisplatin, interferon alpha-2b, and tamoxifen immunochemotherapy, and they were randomized to receive or not receive intraoperative intrapleural PDT. PDT employed porfimer sodium as the photosensitizer and novel intraoperative real-time light dosimetry. Sixty-three patients were randomized, including 48 patients who underwent optimal tumor debulking to ≤5 mm. PDT did not add to the number or severity of treatment-related complications from surgery and/or immunochemo-therapy. PDT, however, also did not influence the patterns of recurrence, median survival (14.1 months v 14.4 months), or median progression-free time (8.5 months v 7.7 months).70 It is possible that no clinical improvements were seen with PDT due to a high proportion of patients having macroscopic residual disease remaining following surgery, as opposed to a gross microscopic resection with only miscroscopic residual disease, for which PDT is more ideally suited to control given its limited depth of penetration.71
In another prospective trial, 40 patients with MPM who were enrolled in a phase II study underwent EPP or pleurectomy followed by intracavitary porfimer sodium-based PDT. The median survival was 36 months for stage I–II patients (n = 13) and 10 months for patients with more advanced disease (n = 24) (P <.0001). On multivariate analysis, PDT dose was determined to be an independent prognostic indicator for overall survival (P <.009).72
Twenty-eight patients with generally advanced MPM were treated on a phase I/II dose escalation study with increasing doses of meta-tetrahydroxyphenylchlorin-mediated intraoperative PDT. Although half of the study population had durable local tumor control following pleuropneumonectomy and PDT, PDT was associated with increased toxicity and accounted for one patient death in the immediate postoperative period.73 Polyhematoporphyrin-mediated PDT, however, was shown to be well tolerated without significant morbidity among 14 patients with advanced MPM treated from 1993–2002 with decortications and intraoperative PDT. Additionally, as compared with nine patients not treated with PDT in their prospective nonrandomized trial, patients treated with PDT had an improved median survival (P = .0179) and were less likely to have tumor regrowth 6 months after surgery (29% v 73%, P ≤.05).74
With a series of prospective and retrospective studies, University of Pennsylvania investigators have produced perhaps the most compelling data supporting the use of intraoperative PDT to treat MPM.75,76 They most recently reported on their prospective study in which 38 patients with MPM were treated with radical pleurectomy and intraoperative PDT from 2005–2010. In this patient population, all but one patient achieved a macroscopic complete resection despite this being a very advanced patient population with all but one patient having stage III/ IV MPM. A median survival of 31.7 months was achieved for the entire cohort, with a 41.2-month median survival for patients with epithelial histology (n = 31). Despite the prolonged overall survival, the progression-free survival was only 9.6 months for the cohort, potentially suggesting that there may be an autologous tumor vaccine-type response in microscopic residual disease induced by PDT.68
Malignant Pleural Mesothelioma Summary
Intraoperative PDT can be safely combined with surgery and other treatment modalities for malignant pleural mesothelioma. Prior to PDT delivery, a macroscopically complete surgical resection should be achieved, either by EPP or radical pleurectomy, due to the limited depth of penetration of PDT. PDT for malignant pleural mesothelioma appears promising and may improve local control and potentially prolong survival in properly selected patients who are able to undergo a macroscopically complete resection, with clinical outcomes appearing best when PDT is combined with a lung-sparing definitive surgery.
FUTURE DIRECTIONS FOR PDT
With novel treatment applications of PDT in the chest, the development and implementation of novel photosensitizers use, and the improvements in imaging and surveillance, the efficacy and use of PDT to treat thoracic malignancies, including NSCLC and MPM, is expected to increase in the future.
For NSCLC, investigators at Tokyo Medical University Hospital recently evaluated a novel PDT delivery. Among patients with peripheral lung cancers <1 cm in diameter who were unfit for surgery, under CT guidance they inserted needles percutaneously that contained an internal catheter positioned within the tumor. They then delivered light through the catheter to nine patients and achieved partial remission in seven patients. While this is an encouraging early result in a patient population that is not currently managed with PDT as a treatment option, the procedure was complicated by two cases of pneumothorax.77 Regardless, as this technique is refined and improved, the breadth of stage I NSCLC lesions able to be amenable to definitive PDT treatment is expected to increase. Improvements in interventional pulmonology navigation systems and an increasing ability to access a higher proportion of peripheral lesions endobronchially will further expand the use of PDT.
Novel photosensitizers also may make PDT more efficacious and widely accessible. Second-generation photosensitizers recently have been evaluated in clinical trials. Tokyo Medical University Hospital investigators conducted a phase II study of 41 patients assessing the use of mono-(L)-aspartylchlorin-e6 (NPe6) as photosensitizer for PDT to treat early-stage central, superficial lung squamous cell carcinoma lesions up to 2 cm in size. In comparison to an approximately six week photosensitivity with porfimer sodium, skin photosensitivity with NPe6 disappeared within two weeks in 85% of patients and within 18 days in all patients, with no serious adverse drug reactions observed and an 85% complete response rate.78 These investigators treated an additional 75 patients with 91 centrally located early-stage lung cancers using NPe6 as photosensitizer for PDT from 2004–2008. NPe6-based PDT achieved a complete response in 94% of lesions ≤1.0 cm (n = 70) and 90% for lesions >1.0 cm (n 21).79 Additional photosensitizers are under development to attempt to increase the depth of penetration, reduce the length of photosensitizity, and reduce PDT toxicity.
Improvements in imaging, endoscopy, and surveillance may increase the use and efficacy of PDT for thoracic malignancies. Although PDT can achieve complete response rates in the majority of early-stage centrally located lesions ≤1 cm, response rates generally decline significantly for larger lesions. Advances in imaging techniques, including the use of fluorescence endoscopy, will better visualize tumor extension along the bronchial wall. Use of these techniques in combination with PDT may help to prevent undertreatment of tumors at the time of PDT delivery or to improve surveillance of lesions following PDT. In fact, a 2011 report using a video endoscopy-based auto fluorescence bronchoscope system with conventional bronchoscopy served to increase the sensitivity of surveillance after PDT administration from 69% to 100%80. Similarly, adding endobronchial ultrasonography to endoscopy can better assess tumor thickness, which also may reduce the risk of undertreating lesions with definitive PDT. Increased adoption of CT surveillance for high-risk populations and continued improvements in imaging techniques should diagnose NSCLC lesions at an earlier stage that is more amenable to definitive therapy with PDT. Imaging improvements also may improve surveillance following PDT delivery and thus increase salvage rates for patients who recur after having an initial complete response to PDT.
For MPM, University of Pennsylvania and Roswell Park Cancer Institute investigators completed enrollment in early 2014 to a multi-centered phase I trial assessing 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) as a novel photosensitizer for PDT delivered intraoperatively. This drug has only a 2-week photosensitivity period. The drug was well-tolerated, with publication of trial results pending. In 2014, these investigators also began enrolling to a multi-centered University of Pennsylvania-led randomized trial of radical pleurectomy with or without porfimer sodium-based PDT using advanced real-time, isotropic light dosimetry for epithelial MPM to definitively determine if PDT improves overall survival following a macroscopic complete resection. Future work for MPM also is being aimed at determining the impact of PDT on the immune system. Additionally, intrapleural immunotherapy81 and intrapleural gene therapy82 are novel therapeutic approaches to treating MPM in patients who are not candidates for surgery with curative intent, and future studies are likely to combine surgery and PDT with these immune-based therapies.
Acknowledgments
Funding: This work was supported, in part, by National Institutes of Health, National Cancer Institute Grant No. P01 87971.
Footnotes
Financial disclosure and conflict of interest: none.
REFERENCES
- 1.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simone CB, 2nd, Friedberg JS, Glatstein E, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis. 2012;4:63–75. doi: 10.3978/j.issn.2072-1439.2011.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedberg JS. Photodynamic therapy for malignant pleural mesothelioma: the future of treatment? Expert Rev Respir Med. 2011;5:49–63. doi: 10.1586/ers.11.1. [DOI] [PubMed] [Google Scholar]
- 4.Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 6.Vrouenraets MB, Visser GW, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anti-cancer Res. 2003;23:505–22. [PubMed] [Google Scholar]
- 7.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 8.Federal Drug Administration [July 1, 2014];Medical Devices. 2014 Jun 30; Available at: http://www.fda.gov/Medical Devices/default.htm.
- 9.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 10.Simone CB, 2nd, Wildt B, Haas AR, Pope G, Rengan R, Hahn SM. Stereotactic body radiation therapy for lung cancer. Chest. 2013;143:1784–90. doi: 10.1378/chest.12-2580. [DOI] [PubMed] [Google Scholar]
- 11.Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: implications for the evaluation of palliative treatment. Br J Cancer. 1995;71:633–6. doi: 10.1038/bjc.1995.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simone CB, 2nd, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2:178–88. doi: 10.3978/j.issn.2224-5820.2013.08.02. [DOI] [PubMed] [Google Scholar]
- 13.Simone CB., 2nd The role of palliative care in patients with NSCLC. Curr Med Lit Lung Cancer. 2011;5:1–16. [Google Scholar]
- 14.Simone CB, 2nd, Vapiwala N, Hampshire MK, Metz JM. Cancer patient attitudes toward analgesic usage and pain intervention. Clin J Pain. 2012;28:157–62. doi: 10.1097/AJP.0b013e318223be30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simone CB, 2nd, Vapiwala N, Hampshire MK, Metz JM. Internet-based survey evaluating use of pain medications and attitudes of radiation oncology patients toward pain intervention. Int J Radiat Oncol Biol Phys. 2008;72:127–33. doi: 10.1016/j.ijrobp.2008.03.071. [DOI] [PubMed] [Google Scholar]
- 16.Greenwald HP, Bonica JJ, Bergner M. The prevalence of pain in four cancers. Cancer. 1987;60:2563–9. doi: 10.1002/1097-0142(19871115)60:10<2563::aid-cncr2820601036>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Simone CB, 2nd, Vapiwala N, Hampshire MK, Metz JM. Palliative care in the management of lung cancer: analgesic utilization and barriers to optimal pain management. J Opioid Manag. 2012;8:9–16. doi: 10.5055/jom.2012.0091. [DOI] [PubMed] [Google Scholar]
- 18.Moghissi K, Dixon K, Stringer M, Freeman T, Thorpe A, Brown S. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg. 1999;15:1–6. doi: 10.1016/s1010-7940(98)00295-4. [DOI] [PubMed] [Google Scholar]
- 19.Minnich DJ, Bryant AS, Dooley A, Cerfolio RJ. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg. 2010;89:1744–8. doi: 10.1016/j.athoracsur.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Taber SW, Buschemeyer WC, 3rd, Fingar VH, Wieman TJ. The treatment of malignant endobronchial obstruction with laser ablation. Surgery. 1999;126:730–3. [PubMed] [Google Scholar]
- 21.Karanov S, Kostadinov D, Shopova M, Kurtev P. Photodynamic therapy in lung and gastrointestinal cancers. J Photochem Photobiol B. 1990;6:175–81. doi: 10.1016/1011-1344(90)85087-d. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa K, Okunaka T, Yamamoto H, et al. Effectiveness of photodynamic therapy and Nd-YAG laser treatment for obstructed tracheobronchial malignancies. Diagn Ther Endosc. 1999;5:161–6. doi: 10.1155/DTE.5.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Jiménez JP, Martínez-Ballarín JE, Llunell A, Farrero E, Rodríguez A, Castro MJ. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur Respir J. 1999;14:800–5. doi: 10.1034/j.1399-3003.1999.14d13.x. [DOI] [PubMed] [Google Scholar]
- 24.McCaughan JS, Jr, Hawley PC, LaRosa JC, Thomas JH, Hicks WJ. Photodynamic therapy to control life-threatening hemorrhage from hereditary hemorrhagic telangiectasia. Lasers Surg Med. 1996;19:492–4. doi: 10.1002/(SICI)1096-9101(1996)19:4<492::AID-LSM17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Jones BU, Helmy M, Brenner M, et al. Photodynamic therapy for patients with advanced non-small-cell carcinoma of the lung. Clin Lung Cancer. 2001;3:37–41. doi: 10.3816/clc.2001.n.016. [DOI] [PubMed] [Google Scholar]
- 26.Tomaselli F, Maier A, Sankin O, et al. Acute effects of combined photodynamic therapy and hyperbaric oxygenation in lung cancer—a clinical pilot study. Lasers Surg Med. 2001;28:399–403. doi: 10.1002/lsm.1067. [DOI] [PubMed] [Google Scholar]
- 27.Freitag L, Ernst A, Thomas M, Prenzel R, Wahlers B, Macha HN. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax. 2004;59:790–3. doi: 10.1136/thx.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam S, Kostashuk EC, Coy EP, et al. A randomized comparative study of the safety and efficacy of photodynamic therapy using Photofrin II combined with palliative radiotherapy versus palliative radiotherapy alone in patients with inoperable obstructive non-small cell bronchogenic carcinoma. Photochem Photobiol. 1987;46:893–7. doi: 10.1111/j.1751-1097.1987.tb04865.x. [DOI] [PubMed] [Google Scholar]
- 29.Arsen'ev AI, Kanaev SV, Barchuk AS, et al. Use of endotracheobronchial surgery in conjunction with radiochemotherapy for advanced non-small lung cancer. Vopr Onkol. 2007;53:461–7. [PubMed] [Google Scholar]
- 30.Konaka C, Usuda J, Kato H. Preoperative photodynamic therapy for lung cancer. Nippon Geka Gakkai Zasshi. 2000;101:486–9. [PubMed] [Google Scholar]
- 31.Okunaka T, Hiyoshi T, Furukawa K, et al. Lung cancers treated with photodynamic therapy and surgery. Diagn Ther Endosc. 1999;5:155–60. doi: 10.1155/DTE.5.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross P, Jr, Grecula J, Bekaii-Saab T, Villalona-Calero M, Otterson G, Magro C. Incorporation of photodynamic therapy as an induction modality in non-small cell lung cancer. Lasers Surg Med. 2006;38:881–9. doi: 10.1002/lsm.20444. [DOI] [PubMed] [Google Scholar]
- 33.Friedberg JS, Mick R, Stevenson JP, et al. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J Clin Oncol. 2004;22:2192–201. doi: 10.1200/JCO.2004.07.097. [DOI] [PubMed] [Google Scholar]
- 34.Kligerman S, Digumarthy S. Staging of non-small cell lung cancer using integrated PET/CT. AJR Am J Roentgenol. 2009;193:1203–11. doi: 10.2214/AJR.09.3193. [DOI] [PubMed] [Google Scholar]
- 35.Geiger GA, Kim MB, Xanthopoulos EP, et al. Stage migration in planning PET/CT scans in patients due to receive radiotherapy for non-small-cell lung cancer. Clin Lung Cancer. 2014;15:79–85. doi: 10.1016/j.cllc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Kubota K, Furuse K, Kawahara M, et al. Photodynamic therapy of roentgenographically occult lung cancer. Kyobu Geka. 1992;45:80–3. [PubMed] [Google Scholar]
- 37.Ono R, Ikeda S, Suemasu K. Hematoporphyrin derivative photodynamic therapy in roentgenographically occult carcinoma of the tracheobronchial tree. Cancer. 1992;69:1696–701. doi: 10.1002/1097-0142(19920401)69:7<1696::aid-cncr2820690709>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Furuse K, Okunaka T, Sakai H, et al. Photodynamic therapy (PDT) in roentgenographically occult lung cancer by photofrin II and excimer dye laser. Gan To Kagaku Ryoho. 1993;20:1369–74. [PubMed] [Google Scholar]
- 39.Imamura S, Kusunoki Y, Takifuji N, et al. Photodynamic therapy and/or external beam radiation therapy for roentgenologically occult lung cancer. Cancer. 1994;73:1608–14. doi: 10.1002/1097-0142(19940315)73:6<1608::aid-cncr2820730611>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Endo C, Miyamoto A, Sakurada A, et al. Results of long-term follow-up of photodynamic therapy for roentgenographically occult bronchogenic squamous cell carcinoma. Chest. 2009;136:369–75. doi: 10.1378/chest.08-2237. [DOI] [PubMed] [Google Scholar]
- 41.Balchum OJ, Doiron DR, Huth GC. Photoradiation therapy of endobronchial lung cancers employing the photodynamic action of hematoporphyrin derivative. Lasers Surg Med. 1984;4:13–30. doi: 10.1002/lsm.1900040104. [DOI] [PubMed] [Google Scholar]
- 42.Li JH, Chen YP, Zhao SD, Zhang LT, Song SZ. Application of hematoporphyrin derivative and laser-induced photodynamical reaction in the treatment of lung cancer: a preliminary report on 21 cases. Lasers Surg Med. 1984;4:31–7. doi: 10.1002/lsm.1900040105. [DOI] [PubMed] [Google Scholar]
- 43.Liang YM, Tu QX, Liu GY, Zhang XS. Short term result of lung cancer treated by photodynamic therapy (PDT). Zhonghua Zhong Liu Za Zhi. 1987;9:50–2. [PubMed] [Google Scholar]
- 44.Kawaguchi T, Yamamoto S, Naka N, et al. Immunohistochemical analysis of Bcl-2 protein in early squamous cell carcinoma of the bronchus treated with photodynamic therapy. Br J Cancer. 2000;82:418–23. doi: 10.1054/bjoc.1999.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patelli M, Lazzari Agli L, Poletti V, Falcone F. Photodynamic laser therapy for the treatment of early-stage bronchogenic carcinoma. Monaldi Arch Chest Dis. 1999;54:315–8. [PubMed] [Google Scholar]
- 46.Moghissi K, Dixon K. Update on the current indications, practice and results of photodynamic therapy (PDT) in early central lung cancer (ECLC). Photodiagnosis Photodyn Ther. 2008;5:10–8. doi: 10.1016/j.pdpdt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Furuse K, Fukuoka M, Kato H, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol. 1993;11:1852–7. doi: 10.1200/JCO.1993.11.10.1852. [DOI] [PubMed] [Google Scholar]
- 48.Edell ES, Cortese DA. Bronchoscopic phototherapy with hematoporphyrin derivative for treatment of localized bronchogenic carcinoma: a 5-year experience. Mayo Clin Proc. 1987;62:8–14. doi: 10.1016/s0025-6196(12)61520-1. [DOI] [PubMed] [Google Scholar]
- 49.Kato H, Okunaka T, Shimatani H. Photodynamic therapy for early stage bronchogenic carcinoma. J Clin Laser Med Surg. 1996;14:235–8. doi: 10.1089/clm.1996.14.235. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa K, Kato H, Konaka C, Okunaka T, Usuda J, Ebihara Y. Locally recurrent central-type early stage lung cancer < 1.0 cm in diameter after complete remission by photodynamic therapy. Chest. 2005;128:3269–75. doi: 10.1378/chest.128.5.3269. [DOI] [PubMed] [Google Scholar]
- 51.Cortese DA, Edell ES, Kinsey JH. Photodynamic therapy for early stage squamous cell carcinoma of the lung. Mayo Clin Proc. 1997;72:595–602. doi: 10.1016/S0025-6196(11)63563-5. [DOI] [PubMed] [Google Scholar]
- 52.Corti L, Toniolo L, Boso C, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med. 2007;39:394–402. doi: 10.1002/lsm.20513. [DOI] [PubMed] [Google Scholar]
- 53.Chang YL, Wu CT, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg. 2007;134:630–7. doi: 10.1016/j.jtcvs.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer. 2011;73:237–42. doi: 10.1016/j.lungcan.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Sokolov VV, Telegina LV, Trakhtenberg AK, Kolbanov KI, Pikin OV, Frank GA. Endobronchial surgery and photodynamic therapy for the treatment of multiple primary lung cancer. Khirurgiia (Mosk) 2010;7:28–31. [PubMed] [Google Scholar]
- 56.Lee JE, Park HS, Jung SS, Kim SY, Kim JO. A case of small cell lung cancer treated with chemoradiotherapy followed by photodynamic therapy. Thorax. 2009;64:637–9. doi: 10.1136/thx.2008.112912. [DOI] [PubMed] [Google Scholar]
- 57.Kubota K, Furuse K, Kawaguchi T, Kawahara M, Ogawara M, Yamamoto S. A case of long-term survival with stage IV small cell lung cancer and early-stage central-type squamous cell lung cancer treated by photodynamic therapy. Jpn J Clin Oncol. 1999;29:45–8. doi: 10.1093/jjco/29.1.45. [DOI] [PubMed] [Google Scholar]
- 58.Kato H, Usuda J, Okunaka T, et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med. 2006;38:371–5. doi: 10.1002/lsm.20346. [DOI] [PubMed] [Google Scholar]
- 59.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 60.Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol. 2012;7:1631–9. doi: 10.1097/JTO.0b013e31826915f1. [DOI] [PubMed] [Google Scholar]
- 61.Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg. 2013;145:909–10. doi: 10.1016/j.jtcvs.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 62.Friedberg JS. Radical pleurectomy and photodynamic therapy for malignant pleural mesothelioma. Ann Cardiothorac Surg. 2012;1:472–80. doi: 10.3978/j.issn.2225-319X.2012.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol. 2011;6:1304–12. doi: 10.1097/JTO.0b013e3182208e3f. [DOI] [PubMed] [Google Scholar]
- 64.Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;122:788–95. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 65.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–6. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 66.Bölükbas S, Eberlein M, Schirren J. Prospective study on functional results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J Thorac Oncol. 2012;7:900–5. doi: 10.1097/JTO.0b013e31824de2dc. [DOI] [PubMed] [Google Scholar]
- 67.Du KL, Both S, Friedberg JS, Rengan R, Hahn SM, Cengel KA. Extrapleural pneumonectomy, photodynamic therapy and intensity modulated radiation therapy for the treatment of malignant pleural mesothelioma. Cancer Biol Ther. 2010;10:425–9. doi: 10.4161/cbt.10.5.12616. [DOI] [PubMed] [Google Scholar]
- 68.Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2012;93:1658–65. doi: 10.1016/j.athoracsur.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pass HI, Tochner Z, DeLaney T, et al. Intraoperative photodynamic therapy for malignant mesothelioma. Ann Thorac Surg. 1990;50:687–8. doi: 10.1016/0003-4975(90)90230-4. [DOI] [PubMed] [Google Scholar]
- 70.Pass HI, Temeck BK, Kranda K, et al. Phase III randomized trial of surgery with or without intra-operative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol. 1997;4:628–33. doi: 10.1007/BF02303746. [DOI] [PubMed] [Google Scholar]
- 71.Simone CB, 2nd, Friedberg JS. Intracavitary adjuvant treatments for malignant pleural mesothelioma: where are we heading? Lung Cancer Manag. In press. [Google Scholar]
- 72.Moskal TL, Dougherty TJ, Urschel JD, et al. Operation and photodynamic therapy for pleural mesothelioma: 6-year follow-up. Ann Thorac Surg. 1998;66:1128–33. doi: 10.1016/s0003-4975(98)00799-1. [DOI] [PubMed] [Google Scholar]
- 73.Schouwink H, Rutgers ET, van der Sijp J, et al. Intra-operative photodynamic therapy after pleuropneumonectomy in patients with malignant pleural mesothelioma: dose finding and toxicity results. Chest. 2001;120:1167–74. doi: 10.1378/chest.120.4.1167. [DOI] [PubMed] [Google Scholar]
- 74.Matzi V, Maier A, Woltsche M, Smolle-Jüttner FM. Polyhematoporphyrin-mediated photodynamic therapy and decortication in palliation of malignant pleural mesothelioma: a clinical pilot study. Interact Cardiovasc Thorac Surg. 2004;3:52–6. doi: 10.1016/S1569-9293(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 75.Friedberg JS, Mick R, Stevenson J, et al. A phase I study of Foscan-mediated photodynamic therapy and surgery in patients with mesothelioma. Ann Thorac Surg. 2003;75:952–9. doi: 10.1016/s0003-4975(02)04474-0. [DOI] [PubMed] [Google Scholar]
- 76.Friedberg JS, Mick R, Culligan M, et al. Photodynamic therapy and the evolution of a lung-sparing surgical treatment for mesothelioma. Ann Thorac Surg. 2011;91:1738–45. doi: 10.1016/j.athoracsur.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 77.Okunaka T, Kato H, Tsutsui H, Ishizumi T, Ichinose S, Kuroiwa Y. Photodynamic therapy for peripheral lung cancer. Lung Cancer. 2004;43:77–82. doi: 10.1016/j.lungcan.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Kato H, Furukawa K, Sato M, et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer. 2003;42:103–11. doi: 10.1016/s0169-5002(03)00242-3. [DOI] [PubMed] [Google Scholar]
- 79.Usuda J, Ichinose S, Ishizumi T, et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways 41.0 cm in diameter. Clin Cancer Res. 2010;16:2198–204. doi: 10.1158/1078-0432.CCR-09-2520. [DOI] [PubMed] [Google Scholar]
- 80.Ali AH, Takizawa H, Kondo K, et al. Follow-up using fluorescence bronchoscopy for the patients with photodynamic therapy treated early lung cancer. J Med Invest. 2011;58:46–55. doi: 10.2152/jmi.58.46. [DOI] [PubMed] [Google Scholar]
- 81.Lucchi M, Chella A, Melfi F, et al. A phase II study of intrapleural immuno-chemotherapy, pleurectomy/ decortication, radiotherapy, systemic chemotherapy and long-term sub-cutaneous IL-2 in stage II-III malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2007;31:529–33. doi: 10.1016/j.ejcts.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 82.Sterman DH, Haas A, Moon E, et al. A trial of intrapleural adenoviral-mediated Interferon-α2b gene transfer for malignant pleural mesothelioma. Am J Respir Crit Care Med. 2011;184:1395–9. doi: 10.1164/rccm.201103-0554CR. [DOI] [PMC free article] [PubMed] [Google Scholar]