Abstract
Separated strands of simian virus 40 (SV40) DNA fragments were used in hybridization experiments to study the RNA transcribed by Escherichia coli RNA polymerase from the chromatin of cells transformed by SV40. The template activity of chromatin of the transformed cell line 11A8 (mouse-embryo cells) examined is about 17% that of purified DNA, suggesting that most of the chromatin DNA is repressed by chromosomal proteins. The SV40-specific RNA present in the RNA transcribed in vitro from 11A8 chromatin hybridizes specifically with the minus strand of SV40 DNA. Little or no reaction occurs with the plus strand of viral DNA. The SV40-specific RNA transcribed in vitro from chromatin of transformed cells shares sequences with the RNA produced during the early phase of SV40 lytic infection, and is similar to that present in the 11A8 cell line in vivo. Although the influence of chromosomal proteins on this pattern of transcription was not definitely determined, preliminary evidence indicates that an asymmetric pattern of transcription may also occur when 11A8 DNA is transcribed by E. coli RNA polymerase.
Keywords: RNA polymerase, RNA·DNA hybridization
Full text
PDF

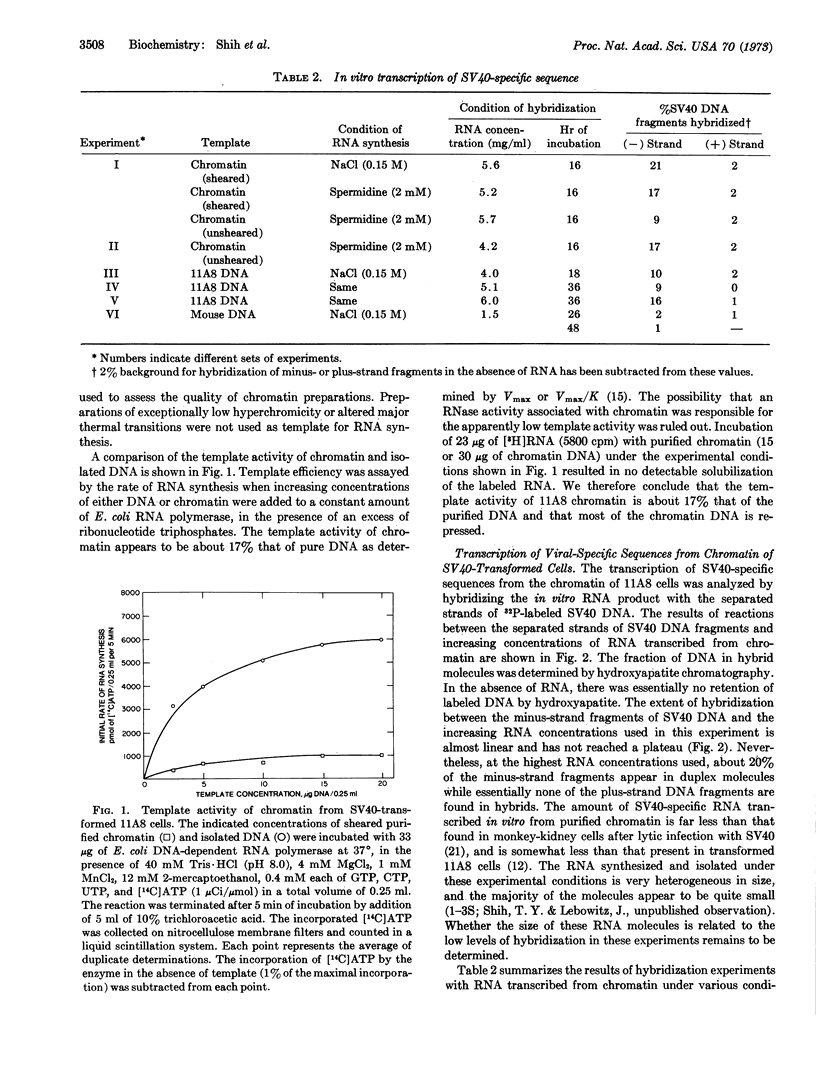
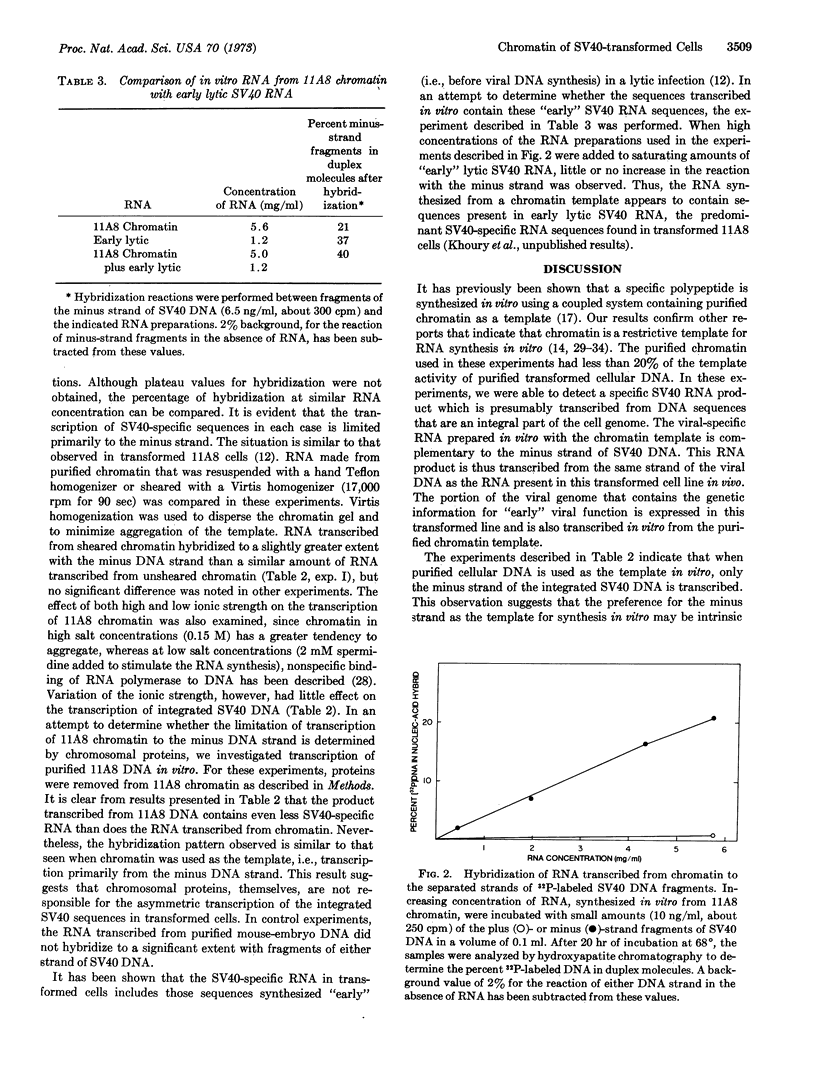

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Astrin S. M. In vitro transcription of simian virus 40 sequences in SV3T3 chromatin. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2304–2308. doi: 10.1073/pnas.70.8.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNER J., HUANG R. C., GILDEN R. V. CHROMOSOMALLY DIRECTED PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Nov;50:893–900. doi: 10.1073/pnas.50.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhor I., Kung G. M., Bonner J. Sequence-specific interaction of DNA and chromosomal protein. J Mol Biol. 1969 Jan;39(2):351–364. doi: 10.1016/0022-2836(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Virus-specific RNA in cells productively infected or transformed by polyoma virus. J Mol Biol. 1966 Apr;16(2):359–373. doi: 10.1016/s0022-2836(66)80179-1. [DOI] [PubMed] [Google Scholar]
- Bonner J., Dahmus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- Burns W. H., Black P. H. Analysis of simian virus 40-induced transformation of hamster kidney tissue in vitro. V. Variability of virus recovery from cell clones inducible with mitomycin C and cell fusion. J Virol. 1968 Jun;2(6):606–609. doi: 10.1128/jvi.2.6.606-609.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- Huang R. C., Huang P. C. Effect of protein-bound RNA associated with chick embryo chromatin on template specificity of the chromatin. J Mol Biol. 1969 Jan;39(2):365–378. doi: 10.1016/0022-2836(69)90323-4. [DOI] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Patterns of simian virus 40 deoxyribonucleic acid transcription. II. In transformed cells. J Virol. 1973 Jan;11(1):54–60. doi: 10.1128/jvi.11.1.54-60.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J. Transformation by polyoma virus and simian virus 40. Adv Cancer Res. 1972;16:141–180. [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Chromosomal RNA of calf thymus chromatin. Biochim Biophys Acta. 1969 May 20;182(1):30–35. doi: 10.1016/0005-2787(69)90517-6. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Template properties of DNA-polypeptide complexes. J Mol Biol. 1970 Jun 14;50(2):333–344. doi: 10.1016/0022-2836(70)90196-8. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Thermal denaturation and template properties of DNA complexes with purified histone fractions. J Mol Biol. 1970 Mar;48(3):469–487. doi: 10.1016/0022-2836(70)90059-8. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Lake R. S. Studies on the structure of metaphase and interphase chromatin of Chinese hamster cells by circular dichroism and thermal denaturation. Biochemistry. 1972 Dec 5;11(25):4811–4817. doi: 10.1021/bi00775a026. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Martin M. A. A general method of gene isolation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1697–1700. doi: 10.1073/pnas.70.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. E., Bonner J. Studies on the role of histones in relation to the template activity and precipitability of chromatin at physiological ionic strengths. J Mol Biol. 1971 Jun 28;58(3):675–684. doi: 10.1016/0022-2836(71)90032-5. [DOI] [PubMed] [Google Scholar]
- Smith K. D., Church R. B., McCarthy B. J. Template specificity of isolated chromatin. Biochemistry. 1969 Nov;8(11):4271–4277. doi: 10.1021/bi00839a007. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. II. Specificity of RNA synthesis. Biochim Biophys Acta. 1971 Jan 1;228(1):212–222. doi: 10.1016/0005-2787(71)90561-2. [DOI] [PubMed] [Google Scholar]
- Tan C. H., Miyagi M. Specificity of transcription of chromatin in vitro. J Mol Biol. 1970 Jun 28;50(3):641–653. doi: 10.1016/0022-2836(70)90090-2. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]


