Abstract
Background:
We aimed to determine whether patients with arthroscopically repaired rotator cuff (RC) tears would have reduced pain and improved function after ultrasound-guided platelet-rich plasma (PRP) injections compared with placebo injection.
Hypothesis:
PRP compared with placebo (saline) was more effective in reducing pain at the site of an RC injury that has undergone arthroscopic repair.
Study Design:
Randomized controlled trial.
Level of Evidence:
Level 2.
Methods:
We conducted a 2-centered, blinded, randomized controlled trial comparing the level of pain in patients undergoing arthroscopic repair. Patients were randomized to either PRP or saline (placebo). They received 2 ultrasound-guided injections of the randomized product: 1 intraoperatively and 1 at 4 weeks postoperatively. The primary outcome measure was shoulder pain demonstrated using a visual analog scale (VAS) at 6 weeks postoperatively. Secondary outcomes included the EuroQol-5 Dimensions (EQ-5D); the Western Ontario Rotator Cuff Index (WORC); and the Disabilities of the Arm, Shoulder, and Hand Score (DASH), as well as adverse events and revision surgeries. Patients were assessed clinically preoperatively and at 2, 4, and 6 weeks postsurgery. A prespecified interim analysis was conducted after 50% of patients were recruited and followed.
Results:
We recruited 25 patients when interim power analysis led to an early trial termination. Follow-up was 96%. The mean difference between groups was not statistically significant (–1.81; 95% CI, –4.3 to 1.2; P = 0.16). The EQ-5D, WORC, and DASH scores also did not show significant differences between groups at week 6 (P = 0.5, 0.99, and 0.9, respectively). There were no revision surgeries, and 4 adverse events (3 PRP, 1 saline).
Conclusion:
There was no statistical difference in outcome measures when augmenting arthroscopically repaired RC tears with PRP.
Clinical Relevance:
Identifying therapies that improve outcomes in patients with RC tears remains a challenge and deserves ongoing investigation.
Keywords: platelet-rich plasma, arthroscopy, rotator cuff, pain
Rotator cuff (RC) tears are a common cause of shoulder pain.4,18 With a rapidly aging population, degenerative RC tears will become an increasingly prevalent clinical problem.15 Surgery can improve patient outcome, but impaired healing, surgical site infection, shoulder stiffness, and iatrogenic tendon injury have a relatively high prevalence at approximately 6% to 11%.20 Postoperative RC re-tears have been shown to occur in 11% to 94% of RC repairs, depending on the size of the tear and the level of tendon degeneration.9,21 There is a recent trend in using whole blood and its derivatives to promote the healing process and decreased pain associated with tendon tears.2,8
Platelet-rich plasma (PRP) contains high concentrations of platelets that, once activated, undergo degranulation to release growth factors with healing properties.1 These growth factors include, but are not limited to, platelet-derived growth factor, which stimulates cell mitosis; transforming growth factor β, which is implicated in collagen synthesis and morphogenesis; and vascular endothelial growth factor, which helps induce endothelial cell proliferation and migration, thus initiating the angiogenic response.11,19,22 Furthermore, platelets have been identified to have analgesic properties by releasing protease-activated receptor 4 peptides.2 In 2010, at the beginning of the current study, no randomized controlled trial had been completed examining the effect of PRP on arthroscopic RC repair surgery.
The primary objective of the current study was to determine whether patients treated with arthroscopic RC repair receiving either PRP or normal saline (placebo) intraoperatively and postoperatively at 4 weeks experienced less perioperative pain, as measured by the visual analog scale (VAS). Secondary objectives aimed to evaluate the effect of PRP versus normal saline on function and health-related quality of life metrics in the perioperative period and compare the rates of adverse events and revision surgeries. The primary hypothesis for this study is that PRP compared with placebo was more effective in reducing pain at the site of a RC injury that has undergone arthroscopic repair.
Methods
Study Overview
We conducted a prospective, randomized, controlled, double-blind trial comparing PRP and normal saline (placebo) in patients undergoing arthroscopic RC repair. In this study, “double-blind” includes study patients and data analyzers, including the Central Adjudication Committee (CAC). Prior to the initiation of this study, approval (REB #10-403) was obtained from the McMaster University / Hamilton Health Sciences Research Ethics Board. Two surgeons (K.R. and O.A.) at 2 clinical centres within Hamilton Health Sciences (McMaster University Medical Center and Hamilton General Site) participated in this study. Briefly, patients with RC tears who provided informed consent were randomized to receive either PRP or normal saline during their arthroscopic RC repair surgery and at 4 weeks after their arthroscopy procedure. Patients completed demographic and baseline questionnaires prior to their surgery. Patients were assessed at 2, 4, and 6 weeks postoperatively (Figure 1). The study aimed to include 50 patients based on an initial power calculation; however, a prespecified interim analysis was conducted after 50% recruitment to evaluate efficacy and statistical power. At the time of the interim analysis, it was determined that for a statistically significant difference in VAS pain score between the treatment and control groups, based on the current scores, a much larger sample would be required. The study stopped because of futility.
Figure 1.
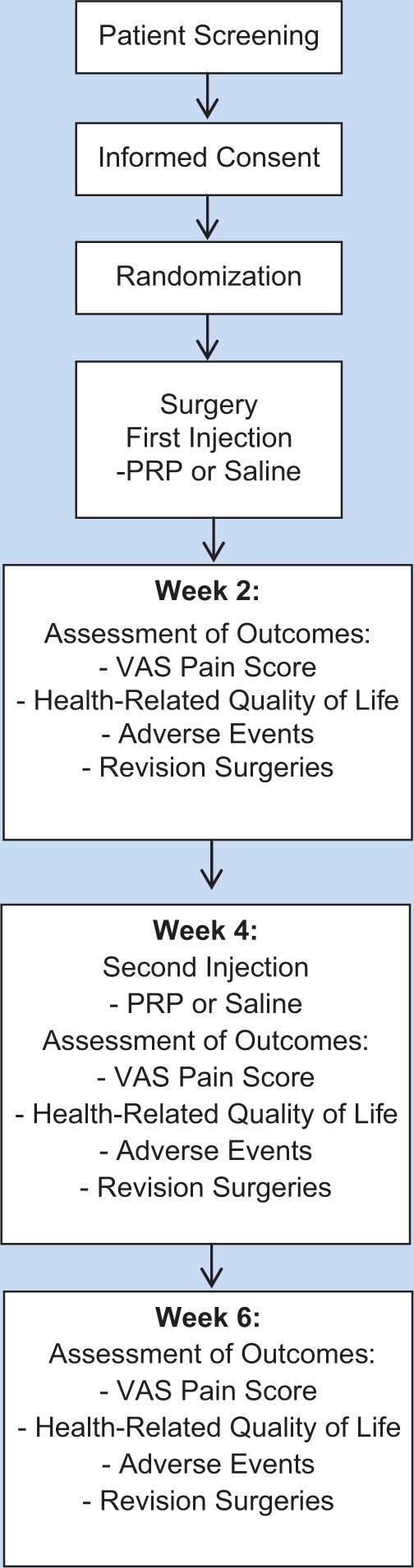
Flow diagram of study methodology. PRP, platelet-rich plasma; VAS, visual analog scale.
Patient Recruitment and Eligibility Criteria
All patients scheduled for arthroscopic RC repair surgery with the participating surgeons during the recruitment period, from October 2010 to July 2012, were screened for eligibility. Reasons for ineligibility were documented. Patients were enrolled in the study if they met the criteria outlined in Table 1. A blinded CAC, composed of 3 orthopaedic surgeons (M.B., J.M., and D.P.), independently assessed patients whose eligibility was in doubt to confirm their eligibility.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria Men or women between 18 and 70 years of age Primary, traumatic, or degenerative rotator cuff tears measuring 3 cm or less Rotator cuff tears requiring arthroscopic repair within 18 months of initial diagnosis Provision of informed consent |
| Exclusion criteria Rotator cuff tears secondary to fracture Patients with an associated dislocation at the time of randomization Rotator cuff tears that underwent prior surgical repair or revision arthroscopy Nonsurgical rotator cuff–associated treatment in the 1 month prior to randomization including corticosteroid injection and anti-inflammatory treatment Prior platelet-rich plasma injection Preexisting conditions associated with upper extremity pain, including arthritis, ongoing infection, carpal tunnel syndrome, cervical neuropathy or other nerve pathology, local malignancy, and systemic disorders (eg, uncontrolled diabetes, hypothyroidism) Gross shoulder instability Active infection Pregnant or plan to become pregnant in the next 12 months Preoperative platelet count less than 125,000 and preoperative hemoglobin of 7.5 g/dL or less Likely problems with follow-up (eg, patients with no fixed address, report a plan to move out of town, or intellectually challenged patients without adequate family support) Do not read and speak English In another ongoing trial that would interfere with the assessment of the primary or secondary outcomes Any other reason (in the judgment of the surgeon) |
Randomization
Eligible patients providing informed consent were randomized prior to surgery using an Internet-based randomization system to ensure concealment of patient allocation. Random variable block sizes were used to avoid an imbalance in the number of patients assigned to each treatment group. Patients were randomized to have their RC tear repairs augmented with either PRP or normal saline (placebo), receiving injections intraoperatively and again at 4 weeks postoperatively.
Operative Technique
All operations were performed under general anesthesia, with patients positioned in the beach chair position. Surgery commenced with a diagnostic arthroscopy of the glenohumeral joint. The cuff tear size was evaluated using a calibrated probe. A full subacromial bursectomy was performed. An acromioplasty was performed with release of the coracoacromial ligament and bony overhang of the acromion. The greater tuberosity was then further prepared. Next, a small awl was hand-tapped to make the pilot hole in the greater tuberosity for an Arthrex 5.5-mm BioCorkscrew suture anchor insertion. The anchor was then inserted in the mid-greater tuberosity footprint area. Each anchor was double loaded with number 2 Fibrewire sutures. One anchor was inserted for each centimeter of the supraspinatus and/or infraspinatus tear that required repair. The repair incorporated any delaminated areas. A single row repair was performed.
Platelet-Rich Plasma Preparation and Trial Interventions
PRP was prepared using the Arthrex Autologous Conditioned Plasma (ACP) Double Syringe System (Arthrex, Inc). This system allows for rapid and efficient concentration of platelets and growth factors from autologous blood for use at the treatment site.
The surgeon performed the intraoperative injection of either the prepared PRP or normal saline under direct visualization. Patients randomized to the normal saline group received a normal saline injection (6-9 mL). For patients randomized to the PRP group, half of the PRP volume (6-9 mL total volume) was injected directly into the RC tendon under direct visualization in several positions along the repair site (articular side). The injecting needle was then placed in the subacromial space, and all instrumentation was removed before performing the skin closure. After the skin closure, the subacromial space was suctioned of residual fluid, and the remaining PRP (3-4.5 mL) was injected into the subacromial space.
At the 4-week visit, under ultrasound guidance, the second PRP or normal saline injection (depending on randomization) was administered by the attending surgeon, trained to use a SonoSite Ultrasound (Bothwell). Blood draw was performed using an 18- to 20-gauge needle for all patients to maintain blinding. A 3-mL lidocaine skin injection was first administered to raise a skin wheal. Using a 22- to 25-gauge needle, 1 to 2 mL of the randomized product was injected at the supraspinatus tendon–bone interface, and the rest was injected on top of the supraspinatus tissue in the subacromial space (2-3 mL).
Postoperative Care
Patients were all discharged home on the day of surgery. The use of an ice pack was recommended, and Percocet was permitted for the first 48 hours postoperatively. All other postoperative pain management methods were avoided, including regional nerve blocks, pain pumps, and injections of any pain medication. Standard physiotherapy guidelines were followed.
Patient Follow-up
Patients were assessed in the perioperative period by their attending surgeon at 2, 4, and 6 weeks postsurgery. The patients completed the VAS for pain and quality of life and functional questionnaires at each of these clinic visits. They were also asked about adverse events and reoperations, and this information was verified by their surgeon and by a review of their medical records.
Outcome Measures
The primary outcome was change in pain severity, measured using a VAS at 6 weeks postsurgery. Patients were asked to rate their worst pain in their shoulder for the previous 24 hours on a 10-cm vertical scale, with “0” indicating no pain at all and “10” indicating the worst pain the patient could imagine. The VAS is considered to be a sensitive measure that is able to detect small changes in an individual’s perception of pain severity.5 This instrument is widely used and considered a more valid determinant of increases or decreases in pain over time than a questionnaire with a select number of responses, with good construct validity and high internal consistency.5
Secondary outcome measures include the EuroQol-5 Dimensions (EQ-5D), the Western Ontario Rotator Cuff Index (WORC), and the Disabilities of the Arm, Shoulder, and Hand Score (DASH) administered prior to the surgical intervention and at 2, 4, and 6 weeks postsurgery, as well as incidence of revision surgeries and adverse events.
Briefly, the EQ-5D is a comprehensive, compact health status classification and health state preference system.16 This questionnaire is widely used and has demonstrated validity and sensitivity in many populations. It is scored from −0.11 to 1.0, wherein 1.0 is perfect health and 0 is death.6
The WORC is a self-administered, 21-item questionnaire that assesses 5 domains, including pain and physical symptoms, sports and recreation, work function, social function, and emotional function. This instrument is a valid, reliable, and responsive measure of outcome specific to RC injuries.12,16,17
The DASH is a 30-item, self-reported questionnaire designed to measure physical function and symptoms in patients with musculoskeletal disorders of the upper limb. In addition to its wide use in evaluating function in orthopaedic patients, the DASH has been extensively validated and is a reliable and responsive measure.3,10,13
Any adverse events and complications deemed related to the affected shoulder by the reporting surgeon were documented. Revision surgeries were defined as any secondary operative procedures at the affected shoulder site. Planned secondary interventions from initial surgical procedures were not considered outcome events.
Adjudication of Adverse Events
The blinded CAC reviewed all reoperations and adverse events to see if they were related to the injected product and to be considered an outcome event. After independent assessment, consensus was reached for each decision.
Data Analysis
Statistical analyses were completed using SPSS for Windows 20.0.14 Descriptive statistics (frequencies for dichotomous data and means and standard deviations for continuous data) were calculated to describe the demographic characteristics of the patients. A t test was completed at 6 weeks for each of the 4 outcome measures to determine whether there was a significant difference in the mean scores between the saline group and the PRP group. A Levene test was completed first to determine whether to assume variance between the groups. The results of the Levene test for equality of variances are also provided. Analysis included t values, significance (P) values, mean differences, and 95% confidence intervals. A t test was also completed for mean difference comparing scores at 2 weeks postoperatively to those at 6 weeks postoperatively to determine whether there was a significant increase or decrease in scores when comparing the 2 groups.
Results
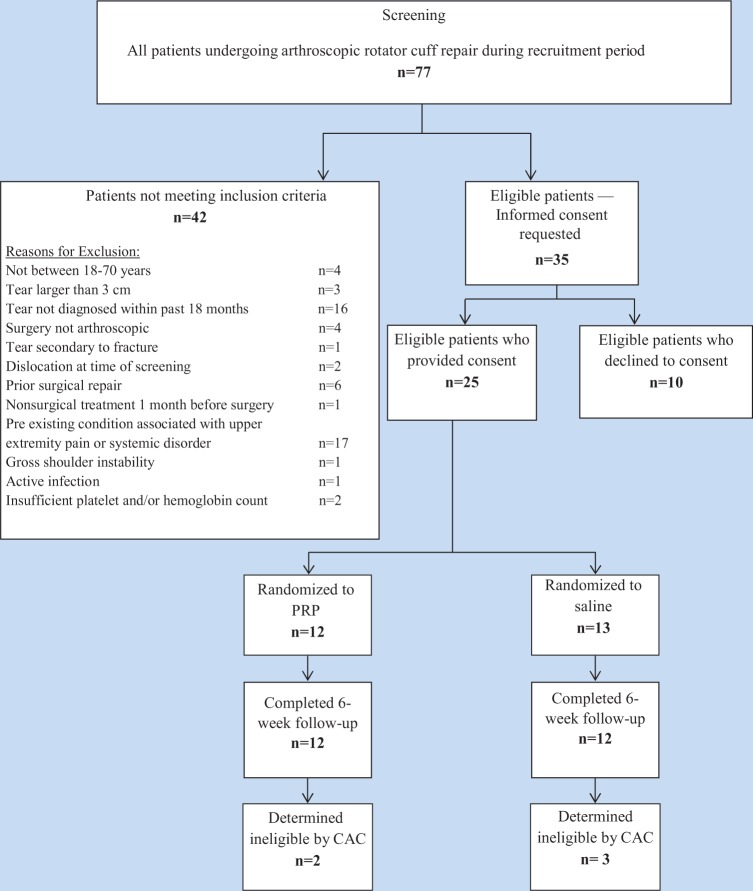
Seventy-seven patients were screened from October 2010 to July 2012. Of these, 52 were excluded and 25 patients met the study inclusion criteria and agreed to participate. The 25 patients were randomized to PRP (12 patients) and normal saline (13 patients) groups. All patients received the treatment they were randomized to. At the time of the interim analysis, 24 patients were followed until 6 weeks (12 PRP, 12 saline); 1 patient was lost to follow-up after the 4-week visit (Figure 2). The CAC assessed eligibility in a blinded manner and determined that 5 patients were treated for tears larger than 3 cm (2 PRP, 3 saline). One of these 5 patients also had prior surgical repair. These patients were included in the primary analysis according to the intention-to-treat principle. An exploratory analysis was conducted with these 5 patients excluded.
Figure 2.
Flow diagram of study participants. CAC, Central Adjudication Committee; PRP, platelet-rich plasma.
Demographics
The mean age of the study participants was 55 ± 6.4 years, and that patient age range was similar across the treatment groups (Table 2). There were 19 men and 6 women, and sex was also distributed similarly across the treatment groups. Fifteen patients had an RC tear in their dominant arm. All patients had either a degenerative RC tear (48%) or a traumatic tear (52%), and the type of tear was distributed evenly across the treatment arms. In 7 cases in the PRP group and 6 in the saline group, the tears affected the supraspinatus exclusively; the other cases involved both supraspinatus and infraspinatus tears.
Table 2.
Demographic data for the platelet-rich plasma (PRP) versus saline groups
| PRP (N = 12), n (%) | Saline (N = 13), n (%) | |
|---|---|---|
| Age, y (mean ± SD) | 55 ± 6.3 | 55 ± 6.4 |
| Male | 9 (75) | 10 (77) |
| Dominanta side affected | 9 (75) | 6 (46) |
| Degenerative cuff tear | 5 (42) | 7 (54) |
| Traumatic cuff tear | 7 (58) | 6 (46) |
| Supraspinatus tear | 7 (58) | 6 (46) |
| Supraspinatus and infraspinatus tears | 5 (42) | 7 (54) |
Dominance is the number of patients whose rotator cuff tear was in their dominant arm.
Primary Outcome: VAS Pain Scores
The mean difference between the groups was not statistically significant (P = 0.16). However, there was a trend toward greater numerical improvement in the difference of VAS scores between baseline and at 6 weeks for PRP compared with saline (Table 3). At 6 weeks postoperatively, the mean ± SD VAS pain scores were 3.6 ± 2.2 and 3.9 ± 3.3, respectively, for the PRP and saline groups (P = 0.80) (Table 3, Figure 3). An exploratory analysis of VAS pain scores at week 6 including only eligible patients left data for 19 patients to be analyzed, as 1 patient was lost to follow-up (10 PRP, 9 saline). In this group, mean ± SD VAS pain scores were 4.0 ± 2.8 and 3.7 ± 2.8, respectively, for the PRP and saline groups (P = 0.4). Removing these patients did not change the primary outcome.
Table 3.
Patient pain, quality of life, and function
| Outcome Measure | Visit | PRP Group, Mean ± SD (SE) | Saline Group, Mean ± SD (SE) |
|---|---|---|---|
| VAS Pain scores | Baseline | 5.6 ± 3.3 (0.9) | 4.1 ± 2.9 (0.8) |
| Week 2 | 5.1 ± 3.0 (0.9) | 4.6 ± 3.1 (0.9) | |
| Week 4 | 4.4 ± 2.5 (0.7) | 4.8 ± 2.6 (0.7) | |
| Week 6 | 3.6 ± 2.2 (0.6) | 3.9 ± 3.3 (1.0) | |
| Standardized mean difference between baseline and week 6 | −1.81 (95% CI = −4.3 to 1.2), P = 0.16 | ||
| Mean difference at week 6 | −0.32 (95% CI = −2.7 to 2.1), P = 0.8 | ||
| EQ-5D scores | Baseline | 0.614 ± 0.226 (0.065) | 0.674 ± 0.213 (0.059) |
| Week 2 | 0.596 ± 0.209 (0.060) | 0.596 ± 0.274 (0.079) | |
| Week 4 | 0.703 ± 0.103 (0.031) | 0.647 ± 0.244 (0.068) | |
| Week 6 | 0.660 ± 0.139 (0.042) | 0.609 ± 0.237 (0.069) | |
| Standardized mean difference between baseline and week 6 | 0.11 (95% CI = −0.05 to 0.28), P = 0.18 | ||
| Mean difference at week 6 | 0.051 (95% CI = −0.118 to 0.220), P = 0.5 | ||
| WORC scores | Baseline | 26.1 ± 15.2 (4.4) | 37.3 ± 16.1 (4.5) |
| Week 2 | 28.2 ± 16.0 (4.6) | 33.1 ± 13.6 (3.9) | |
| Week 4 | 35.2 ± 18.5 (5.3) | 44.6 ± 16.1 (4.6) | |
| Week 6 | 41.2 ± 17.2 (5.0) | 41.2 ± 21.2 (6.1) | |
| Standardized mean difference between baseline and week 6 | 11.3 (95% CI = −4.4 to 27.4), P = 0.15 | ||
| Mean Difference at Week 6 | −0.005 (95% CI = −16.4 to 16.3), P > 0.999 | ||
| DASH scores | Baseline | 73.0 ± 16.7 (4.8) | 65.5 ± 17.5 (4.8) |
| Week 2 | 81.9 ± 22.9 (6.6) | 92.2 ± 19.1 (5.5) | |
| Week 4 | 77.6 ± 12.7 (3.7) | 76.3 ± 19.6 (5.4) | |
| Week 6 | 70.5 ± 16.1 (4.6) | 69.2 ± 24.0 (6.9) | |
| Standardized mean difference between baseline and week 6 | −6.1 (95% CI = −21.4 to 9.2), P = 0.48 | ||
| Mean difference at week 6 | 1.23 (95% CI = −16.1 to 18.5), P = 0.9 | ||
DASH, Disabilities of the Arm, Shoulder and Hand score; EQ-5D, EuroQol-5 Dimensions; SE, standard error; VAS, visual analog scale; WORC, Western Ontario Rotator Cuff Index.
Figure 3.
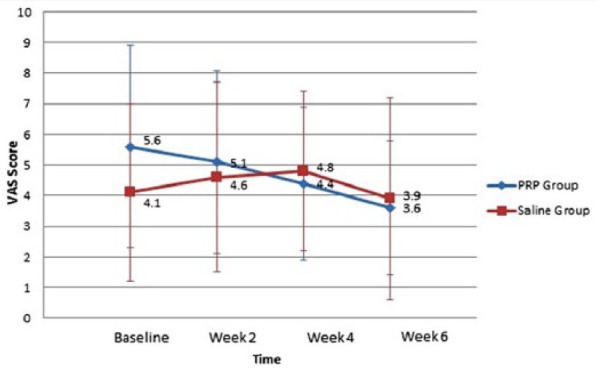
Mean VAS pain scores across follow-up time points for PRP and saline groups. PRP, platelet-rich plasma; VAS, visual analog scale.
Analysis of the treatment effect for standardized mean difference between baseline and week 6 between the 2 groups (1.81 points on the VAS scale) suggested that 150 patients would be required to achieve adequate study power. Analysis of the treatment effect for difference in mean at 6 weeks (0.3 points on the VAS scale) suggested that approximately 2400 more patients would be required to achieve adequate study power for this difference. Given the sample size requirements to find a difference in standardized mean difference between baseline and week 6 between the 2 groups, the trial was stopped early as initial enrollment plans would not approach adequate power.
Secondary Outcomes: HRQoL
The EQ-5D scores decreased in both groups from baseline to 2 weeks, showed improvement at week 4, and decreased again at week 6 (Table 3). No significant difference in scores was found between the PRP and saline groups at week 6 (P = 0.2). There was also no difference in the standardized mean difference for the groups between week 2 and week 6 (P = 0.2).
In the PRP group, the WORC scores increased consistently from baseline to week 6. In the saline group, scores decreased from baseline to week 2, increased at week 4, and decreased at week 6 (Table 3). Mean scores at week 6 were 41.2 in both groups, demonstrating no difference (P > 0.999). Standardized mean difference between week 2 and week 6 for the groups was also insignificant (P = 0.3).
In both treatment groups, the DASH scores were worse from baseline to week 2 and showed improvement at weeks 4 and 6 (Table 3). No significant differences were seen between the PRP and saline groups for the standardized mean difference at week 6 (P = 0.9) or between week 2 and week 6 (P = 0.2) for the DASH scores.
Adverse Events and Revision Surgeries
The CAC reviewed 4 adverse events: 1 case in the saline group of excessive pain, and 3 cases in the PRP group—infection at the site of injection, hand swelling from an intravenous line, and nausea and dizziness immediately postoperative. Of these, the infection in the PRP group was classified as a superficial surgical site infection and determined to be a study event. No revision surgeries occurred in either group during the 6-week follow-up period. Given the sample size, no conclusion can be made regarding differences in adverse event frequency between the 2 groups.
Discussion
At interim analysis, our study overall did not show a significant change in the standardized mean difference in VAS scores between the 2 groups at baseline and 6 weeks. In addition, our interim analysis demonstrated a lack of clinically important benefit to PRP after arthroscopic RC repair using quality of life measures at 6 weeks. Given the sample size requirements to find a difference in the standardized mean difference between the groups at the designated time points, the trial was stopped early as the initial enrollment plans would not approach adequate power. While the study could not demonstrate that the injected product influenced the pain score, the overall decrease in pain from baseline for both groups demonstrates that arthroscopic surgery to repair RC tears does provide pain relief. Five patients were confirmed by the blinded CAC to have RC tears larger than 3.0 cm, and 4 of them had pain scores documented at 6 weeks; their eventual removal from the dataset did not change the primary outcome.
Secondary objectives assessing function and quality of life exhibit varying patterns. The EQ-5D scores increased and decreased in both groups at each follow-up visit, and it is not clear whether there was overall improvement or not, failing to demonstrate any statistically significant findings. Of interest, the PRP group showed slightly improved scores from baseline to 6 weeks, whereas the saline group showed slightly worsened scores. The WORC scores improved in both groups at week 6 compared with baseline, demonstrating improvement in functioning after surgical repair without any specific benefit for augmentation with PRP. The DASH scores were also relatively similar between groups, worsening immediately after surgery and improving at weeks 4 and 6. The scores at 6 weeks were very similar between the 2 groups and better in the saline group; however, the PRP group showed improvement at week 6 compared with baseline, whereas the saline group’s mean score at week 6 was worsened from baseline.
No revision surgeries occurred over the 6-week follow-up period; however, there were 4 adverse events. Three of these occurred in the PRP group and 1 in the saline group. The CAC determined that the infection occurring at the site of surgery after the patient’s week 4 follow-up was to be considered a study event. No other adverse events were deemed to be study-related. Because of the small sample size, no conclusion can be made regarding differences in adverse event frequency between the 2 groups.
While the present study did demonstrate some slight trends in pain reduction in the PRP group, these overall findings are consistent with the mounting literature examining the use of PRP to augment arthroscopic RC repair surgeries. A randomized controlled trial by Castricini et al7 included 88 patients who underwent arthroscopic surgery to repair a torn RC with or without an intraoperative interposition of an autologous platelet-rich fibrin matrix between the RC and bone. This study reported no difference in shoulder function between the treatment and control group using the Constant score.7 A second study by Jo et al15 offered patients a choice of whether to receive an interpositional PRP gel or not (control) during surgical RC repair. Nineteen patients received PRP and 23 did not. Similar to the present study, this study reported that there was no difference in pain scores or functional scores at any point during the follow-up period.15 Both the previously mentioned studies did not use injections, but rather matrices placed between the cuff tissue and bone at the time of surgery, and this may limit comparisons with the present study. Randelli et al20 conducted a clinical trial involving 14 patients followed for 24 months. All patients were given a PRP injection along with an autologous thrombin component intraoperatively. This study demonstrated significant decreases in VAS pain scores at 6, 12, and 24 months in comparison with preoperative scores. However, no control was used for this trial, so the results cannot be attributed to the application of PRP.20 Randelli et al21 completed another study involving 53 patients, again where PRP injections were given intraoperatively in combination with an autologous thrombin component. Block randomization was implemented, and a control group was added. They reported that pain scores in the PRP group were significantly lower at 3, 7, 14, and 30 days postoperatively. Similar to our findings, however, they found no difference in outcomes at day 30. They also noted that there was no difference in the healing rate of the 2 groups when captured and analyzed via magnetic resonance imaging.21
In summary, 2 of the 3 previously conducted studies that involved a control did not report any benefit to the application of PRP intraoperatively; however, each study reported on different outcome measures, and at different time points, rendering a comparison difficult. Randelli et al21 captured pain scores immediately postoperatively at days 3, 7, 14, and 30, allowing for major differences between the treatment and control groups to be recognized. Jo et al15 and Castricini et al7 did not obtain frequent functional and pain measurements postoperatively and may have missed differences between the groups. Each trial involved an individual treatment at operation; the current trial exclusively included a second injection at 4 weeks postoperatively, allowing for the exploration of further effects that PRP may have had on pain outcomes.
There are several strengths in the present study. First, the patients were all randomized and blinded to their treatment groups. All study events were independently assessed by a blinded CAC. Quality of life and pain scores were collected at frequent time points postoperatively, allowing for an increased likelihood that subtle changes may be noticed; as pain is often at its highest immediately postoperatively, this is beneficial. Furthermore, the follow-up was 96% at 6 weeks.
An apparent limitation of this pilot study was the short follow-up period. Although data on pain, the primary outcome measure used for assessing the effect of PRP, were intended to be analyzed shortly after surgery, 6 weeks is an insufficient time period to capture all potential RC re-tears and revision surgeries. Another limitation of the study was the inability to screen for RC tear size before recruitment, which led to 5 patients being included with tear sizes above the 3-cm limit for inclusion. In addition, our results are primarily applicable to patients with traumatic and degenerative RC tears that are repaired initially within 18 months of diagnosis. Patients with preexisting conditions associated with upper extremity pain or systemic disorders may not have outcomes similar to those included in the present study.
Conclusion
The present study demonstrated no conclusive benefit for reducing postoperative pain after augmenting arthroscopically repaired RC tears with PRP compared with saline at 6 weeks; therefore, the primary hypothesis is rejected. It failed to demonstrate that patients receiving PRP injections intraoperatively and at 4 weeks postoperatively have decreased pain and superior functional outcomes than those receiving normal saline. Identifying therapies that improve outcomes in patients with RC tears remains a challenge and deserves ongoing investigation.
Acknowledgments
The authors thank Franca Mossuto and Lilly-Ann Reynolds for all of their assistance.
Footnotes
The following authors declared potential conflicts of interest: Alisha Hak, BSc, and Sheila Sprague, PhD, received payment for writing or reviewing the manuscript from GRS Inc; Mohit Bhandari, MD, PhD, FRCSC, is a paid consultant for Smith & Nephew, Stryker, Amgen, Zimmer, Moximed, and Bioventus.
References
- 1. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15. [DOI] [PubMed] [Google Scholar]
- 2. Asfaha S, Cenac N, Houle S, et al. Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol. 2007;150:176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128-146. [PubMed] [Google Scholar]
- 4. Breazeale NM, Craig EV. Partial-thickness rotator cuff tears. Pathogenesis and treatment. Orthop Clin North Am. 1997;28:145-155. [DOI] [PubMed] [Google Scholar]
- 5. Briggs M, Closs JS. A descriptive study of the use of visual analog scales and verbal rating scales for the assessment of postoperative pain in orthopedic patients. J Pain Symptom Manage. 1999;18:438-446. [DOI] [PubMed] [Google Scholar]
- 6. Brooks R, Rabin RE, de Charro F, eds. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective. Dordrecht, Netherlands: Kluwer Academic; 2003. [Google Scholar]
- 7. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair. Am J Sports Med. 2011;39:258-265. [DOI] [PubMed] [Google Scholar]
- 8. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28:1718-1727. [DOI] [PubMed] [Google Scholar]
- 9. Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair. Clin Orthop Relat Res. 2010;468:1476-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-related health change after surgery. BMC Musculoskelet Disord. 2003;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hollinger JO, Hart C, Gruber R, Doll B. Protein therapeutics and bone healing. In: Lynch SE, Wisner-Lynch LA, Nevins M, Marx RE, eds. Tissue Engineering: Applications in Oral and Maxillofacial Surgery and Periodontics. 2nd ed. Chicago, IL: Quintessence; 2008:3-25. [Google Scholar]
- 12. Holtby R, Razmjou H. Measurement properties of the Western Ontario rotator cuff outcome measure: a preliminary report. J Shoulder Elbow Surg. 2005;14:506-510. [DOI] [PubMed] [Google Scholar]
- 13. Hudak PL, Amadio PC, Bombardier C; The Upper Extremity Collaborative Group (UECG). Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand). Am J Ind Med. 1996;29:602-608. [DOI] [PubMed] [Google Scholar]
- 14. IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. [Google Scholar]
- 15. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? Am J Sports Med. 2011;39:2082-2090. [DOI] [PubMed] [Google Scholar]
- 16. Kirkley A, Alvarez C, Griffin S. The development and evaluation of a disease-specific quality-of-life questionnaire for disorders of the rotator cuff: The Western Ontario Rotator Cuff Index. Clin J Sport Med. 2003;13:84-92. [DOI] [PubMed] [Google Scholar]
- 17. Kirkley A, Griffin S, Dainty K. Scoring systems for the functional assessment of the shoulder. Arthroscopy. 2003;19:1109-1120. [DOI] [PubMed] [Google Scholar]
- 18. Matava MJ, Purcell DB, Rudzki JR. Partial-thickness rotator cuff tears. Am J Sports Med. 2005;33:1405-1417. [DOI] [PubMed] [Google Scholar]
- 19. Mishra A, Woodall J, Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113-125. [DOI] [PubMed] [Google Scholar]
- 20. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair: a pilot study. Disabil Rehabil. 2008;30:1584-1589. [DOI] [PubMed] [Google Scholar]
- 21. Randelli PS, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518-528. [DOI] [PubMed] [Google Scholar]
- 22. Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sports injuries. Sports Med. 2009;35:1-10. [DOI] [PubMed] [Google Scholar]