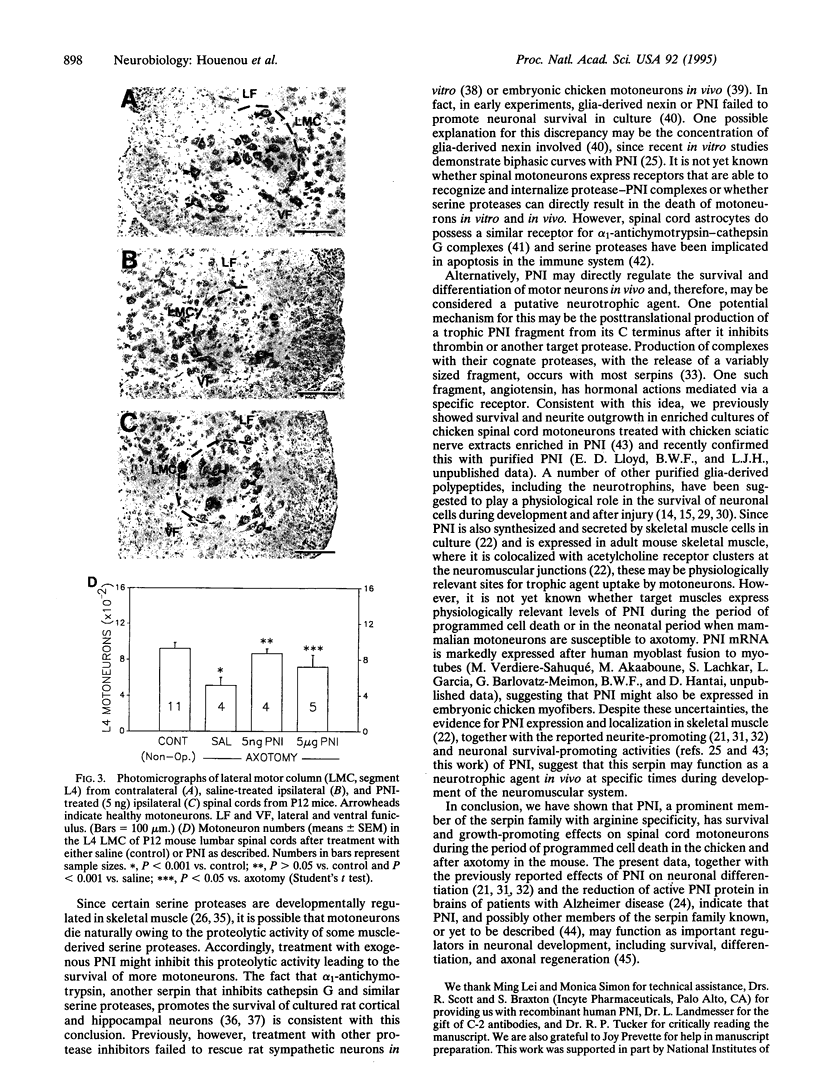
Abstract
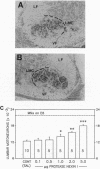
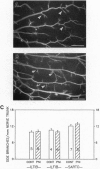
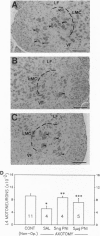
Protease nexin I (PNI) is a member of the family of serine protease inhibitors (serpins) that have been shown to promote neurite outgrowth in vitro from different neuronal cell types. These include neuroblastoma cells, hippocampal neurons, and sympathetic neurons. Free PNI protein is markedly decreased in various anatomical brain regions, including hippocampus, of patients with Alzheimer disease. Here, we report that PNI rescued spinal motoneurons during the period of naturally occurring (programmed) cell death in the chicken in a dose-dependent fashion. Furthermore, PNI prevented axotomy-induced spinal motoneuron death in the neonatal mouse. The survival effect of PNI on motoneurons during the period of programmed cell death was not associated with increased intramuscular nerve branching. PNI also significantly increased the nuclear size of motoneurons during the period of programmed cell death and prevented axotomy-induced atrophy of surviving motoneurons. These results are consistent with the possible role of PNI as a neurotrophic agent. They also support the idea that serine proteases or, more precisely, the balance of proteases and serpins may be involved in regulating the fate of neuronal cells during development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Chen M., Conn K. J., Festoff B. W. A receptor for cathepsin G:alpha 1-antichymotrypsin complexes on mouse spinal cord astrocytes. Neurology. 1993 Jun;43(6):1223–1227. doi: 10.1212/wnl.43.6.1223. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I. W., Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. I. A light and electron microscopic study of naturally occurring and induced cell loss during development. J Comp Neurol. 1978 Jan 1;177(1):33–57. doi: 10.1002/cne.901770105. [DOI] [PubMed] [Google Scholar]
- Crews L. L., Wigston D. J. The dependence of motoneurons on their target muscle during postnatal development of the mouse. J Neurosci. 1990 May;10(5):1643–1653. doi: 10.1523/JNEUROSCI.10-05-01643.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
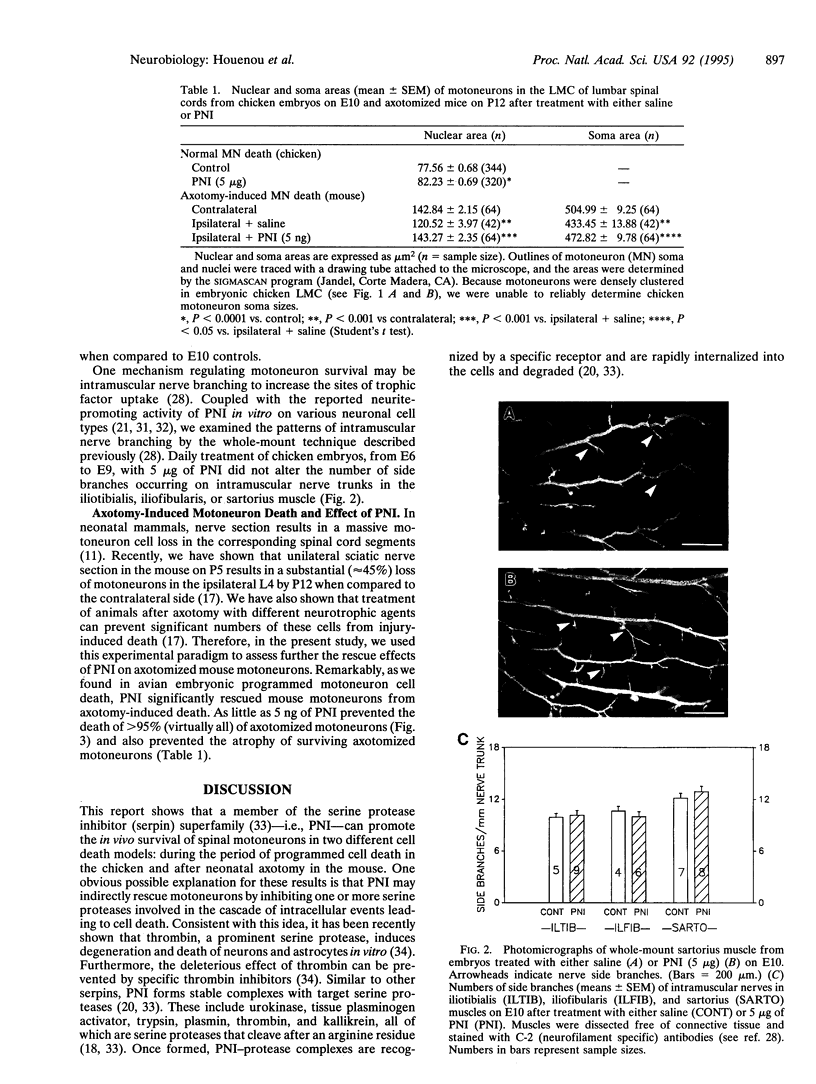
- Dahm L. M., Landmesser L. T. The regulation of intramuscular nerve branching during normal development and following activity blockade. Dev Biol. 1988 Dec;130(2):621–644. doi: 10.1016/0012-1606(88)90357-0. [DOI] [PubMed] [Google Scholar]
- Farmer L., Sommer J., Monard D. Glia-derived nexin potentiates neurite extension in hippocampal pyramidal cells in vitro. Dev Neurosci. 1990;12(2):73–80. doi: 10.1159/000111836. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Rao J. S., Hantaï D. Plasminogen activators and inhibitors in the neuromuscular system: III. The serpin protease nexin I is synthesized by muscle and localized at neuromuscular synapses. J Cell Physiol. 1991 Apr;147(1):76–86. doi: 10.1002/jcp.1041470111. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Rao J. S., Rayford A., Hantaï D. Plasminogen activators and their inhibitors in the neuromuscular system: II. Serpins and serpin: protease complex receptors increase during in vitro myogenesis. J Cell Physiol. 1990 Aug;144(2):272–279. doi: 10.1002/jcp.1041440213. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A. 1988 May;85(10):3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBURGER J., RICHET G. Enseignements tirés de la pratique du rein artificiel pour l'interprétation des désordres électrolytiques de l'urémie aiguë. Rev Fr Etud Clin Biol. 1956 Jan;1(1):39–55. [PubMed] [Google Scholar]
- Hantaï D., Rao J. S., Kahler C., Festoff B. W. Decrease in plasminogen activator correlates with synapse elimination during neonatal development of mouse skeletal muscle. Proc Natl Acad Sci U S A. 1989 Jan;86(1):362–366. doi: 10.1073/pnas.86.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., McCaig C. D. Motoneuron death and motor unit size during embryonic development of the rat. J Neurosci. 1984 Jan;4(1):13–24. doi: 10.1523/JNEUROSCI.04-01-00013.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houenou L. J., McManaman J. L., Prevette D., Oppenheim R. W. Regulation of putative muscle-derived neurotrophic factors by muscle activity and innervation: in vivo and in vitro studies. J Neurosci. 1991 Sep;11(9):2829–2837. doi: 10.1523/JNEUROSCI.11-09-02829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai H., Tanaka M., Hirai S. Alpha 1-antichymotrypsin has a trophic effect on hippocampal neurons in vitro. Neurosci Lett. 1991 Apr 29;125(2):163–165. doi: 10.1016/0304-3940(91)90017-n. [DOI] [PubMed] [Google Scholar]
- Kashihara Y., Kuno M., Miyata Y. Cell death of axotomized motoneurones in neonatal rats, and its prevention by peripheral reinnervation. J Physiol. 1987 May;386:135–148. doi: 10.1113/jphysiol.1987.sp016526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos V. E., Clatterbuck R. E., Winslow J. W., Cayouette M. H., Price D. L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993 Mar;10(3):359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C. Motoneuron cell death in the developing lumbar spinal cord of the mouse. Brain Res. 1982 Aug;256(4):473–479. doi: 10.1016/0165-3806(82)90192-4. [DOI] [PubMed] [Google Scholar]
- Li L., Oppenheim R. W., Lei M., Houenou L. J. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994 Jul;25(7):759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier R., Spreyer P., Ortmann R., Harel A., Monard D. Induction of glia-derived nexin after lesion of a peripheral nerve. Nature. 1989 Nov 30;342(6249):548–550. doi: 10.1038/342548a0. [DOI] [PubMed] [Google Scholar]
- Mizuguchi M., Kim S. U. Alpha 1-antichymotrypsin supports short-term survival of cerebral neurons in culture. Neurosci Lett. 1991 Apr 1;124(2):166–168. doi: 10.1016/0304-3940(91)90085-8. [DOI] [PubMed] [Google Scholar]
- Monard D., Niday E., Limat A., Solomon F. Inhibition of protease activity can lead to neurite extension in neuroblastoma cells. Prog Brain Res. 1983;58:359–364. doi: 10.1016/S0079-6123(08)60037-0. [DOI] [PubMed] [Google Scholar]
- Monard D., Solomon F., Rentsch M., Gysin R. Glia-induced morphological differentiation in neuroblastoma cells. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1894–1897. doi: 10.1073/pnas.70.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. V. Evidence on the role of cell death and neuromuscular function in the formation of specific peripheral connections. J Neurosci. 1981 Feb;1(2):141–151. doi: 10.1523/JNEUROSCI.01-02-00141.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W., Cole T., Prevette D. Early regional variations in motoneuron numbers arise by differential proliferation in the chick embryo spinal cord. Dev Biol. 1989 Jun;133(2):468–474. doi: 10.1016/0012-1606(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Houenou L., Pincon-Raymond M., Powell J. A., Rieger F., Standish L. J. The development of motoneurons in the embryonic spinal cord of the mouse mutant, muscular dysgenesis (mdg/mdg): survival, morphology, and biochemical differentiation. Dev Biol. 1986 Apr;114(2):426–436. doi: 10.1016/0012-1606(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Haverkamp L. J., Houenou L., Yin Q. W., McManaman J. Biological studies of a putative avian muscle-derived neurotrophic factor that prevents naturally occurring motoneuron death in vivo. J Neurobiol. 1993 Aug;24(8):1065–1079. doi: 10.1002/neu.480240806. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Tytell M., Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev Biol. 1990 Mar;138(1):104–113. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Yin Q. W., Collins F., MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991 Mar 29;251(5001):1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Yin Q. W., Prevette D., Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992 Dec 24;360(6406):755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- Pollin M. M., McHanwell S., Slater C. R. The effect of age on motor neurone death following axotomy in the mouse. Development. 1991 May;112(1):83–89. doi: 10.1242/dev.112.1.83. [DOI] [PubMed] [Google Scholar]
- Popiela H., Porter T., Beach R. L., Festoff B. W. Peripheral nerve extract promotes long-term survival and neurite outgrowth in cultured spinal cord neurons. Cell Mol Neurobiol. 1984 Mar;4(1):67–77. doi: 10.1007/BF00710943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J., Korzus E., Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994 Jun 10;269(23):15957–15960. [PubMed] [Google Scholar]
- Sarin A., Adams D. H., Henkart P. A. Protease inhibitors selectively block T cell receptor-triggered programmed cell death in a murine T cell hybridoma and activated peripheral T cells. J Exp Med. 1993 Nov 1;178(5):1693–1700. doi: 10.1084/jem.178.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y. A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992 Dec 24;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Elliott J. L., Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992 Nov;23(9):1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- Sommer J., Gloor S. M., Rovelli G. F., Hofsteenge J., Nick H., Meier R., Monard D. cDNA sequence coding for a rat glia-derived nexin and its homology to members of the serpin superfamily. Biochemistry. 1987 Oct 6;26(20):6407–6410. doi: 10.1021/bi00394a016. [DOI] [PubMed] [Google Scholar]
- Wagner S. L., Geddes J. W., Cotman C. W., Lau A. L., Gurwitz D., Isackson P. J., Cunningham D. D. Protease nexin-1, an antithrombin with neurite outgrowth activity, is reduced in Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8284–8288. doi: 10.1073/pnas.86.21.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Elliott J., Snider W. D. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992 Dec 24;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]