Abstract
Saccharomyces cerevisiae normally cannot assimilate mannitol, a promising brown macroalgal carbon source for bioethanol production. The molecular basis of this inability remains unknown. We found that cells capable of assimilating mannitol arose spontaneously from wild-type S. cerevisiae during prolonged culture in mannitol-containing medium. Based on microarray data, complementation analysis, and cell growth data, we demonstrated that acquisition of mannitol-assimilating ability was due to spontaneous mutations in the genes encoding Tup1 or Cyc8, which constitute a general corepressor complex that regulates many kinds of genes. We also showed that an S. cerevisiae strain carrying a mutant allele of CYC8 exhibited superior salt tolerance relative to other ethanologenic microorganisms; this characteristic would be highly beneficial for the production of bioethanol from marine biomass. Thus, we succeeded in conferring the ability to assimilate mannitol on S. cerevisiae through dysfunction of Tup1-Cyc8, facilitating production of ethanol from mannitol.
INTRODUCTION
Macroalgae, consisting of green, red, and brown algae, are promising sources of biofuels for several reasons: (i) macroalgae are more productive than land crops; (ii) arable land is not required for algal cultivation, obviating the necessity for irrigation, fertilizer, etc.; and (iii) macroalgae contain no lignin (1–4). Both red and brown algae contain high levels of carbohydrates, and a method for producing biofuel from these carbohydrates would be of tremendous economic and environmental benefit.
Brown macroalgae contain up to 33% (wt/wt [dry weight]) mannitol, which is the sugar alcohol corresponding to mannose and a promising carbon source for bioethanol production (1, 5, 6). Although some bacteria, such as Escherichia coli and Zymobacter palmae, can assimilate mannitol, i.e., utilize mannitol and produce ethanol (6, 7), bacteria are generally sensitive to ethanol, as well as, several other growth-inhibitory compounds. Z. palmae and E. coli KO11 can produce ca. 1.3% (wt/vol) and 2.6% (wt/vol) ethanol from 3.8% (wt/vol) and 9.0% (wt/vol) mannitol, respectively; however, both strains are sensitive to 5% (wt/vol) ethanol (8, 9). Yeast is currently considered to have several advantages over ethanologenic bacteria, including high tolerance to ethanol and inhibitory compounds (10). Several yeast strains, such as Pichia angophorae and Saccharomyces paradoxus NBRC0259-3, can produce ethanol from mannitol (8, 11). However, compared to the well-characterized model organism Saccharomyces cerevisiae, the host-vector systems of these yeasts are not well equipped, and their genetics and physiologies are poorly defined.
Mannitol dehydrogenase is the key enzyme that catalyzes the pyridine nucleotide-dependent oxidation of d-mannitol to d-fructose (12). Despite the existence of genes encoding putative homologs of mannitol dehydrogenase (YEL070W and YNR073C), S. cerevisiae strains, including the S288C reference strain, are unable to assimilate mannitol for growth; a few exceptions exist, such as the polyploid strain BB1 (13). This inability of S. cerevisiae to assimilate mannitol has prevented construction of a system for production of ethanol from mannitol using yeast (i.e., a yeast-algal bioethanol production system), for which there is a great demand. A recent study described a genetically manipulated S. cerevisiae strain that acquired the ability to metabolize mannitol and alginate, another brown macroalgal carbon source, and further showed that expression of mannitol dehydrogenase and mannitol transporter was sufficient to allow growth on mannitol (14). However, the regulatory mechanisms of the genes involved in mannitol metabolism in S. cerevisiae remain poorly understood.
In this study, we found that S. cerevisiae can acquire the ability to assimilate mannitol for ethanol production by developing spontaneous mutations in TUP1 or CYC8. Tup1-Cyc8 (also known as Tup1-Ssn6) is a general transcriptional corepressor that is conserved in many eukaryotic organisms (15, 16). The complex is composed of four molecules of Tup1 and one molecule of Cyc8 (17). Tup1-Cyc8 is implicated in the repression of over 300 genes, including genes that are cell type-specific, glucose repressible, DNA damage inducible, or involved in flocculation or the hypoxic response (18, 19). Our results strongly suggest that the inability of wild-type S. cerevisiae to assimilate mannitol can be attributed to the repressive functions of the Tup1-Cyc8 corepressor. Thus, our findings shed light on previously unknown mechanisms of mannitol metabolism in S. cerevisiae.
MATERIALS AND METHODS
Microorganisms.
The S. cerevisiae strains used in the present study are listed in Table S1 in the supplemental material. E. coli strain KO11 (ATCC 55124) was purchased from the American Type Culture Collection. P. angophorae (CBS5830) (8) was purchased from CBS-KNAW Fungal Biodiversity Centre. S. paradoxus strain NBRC0259-3 was obtained previously (11).
Media and general techniques.
Standard yeast media were used (20). Yeast extract-peptone-dextrose (YPD), yeast extract-peptone-mannitol (YPM), and yeast extract-peptone-glycerol (YPG) media consisted of YP (2% yeast extract and 2% tryptone, pH 5.6) with 2% glucose, 2% mannitol, and 3% glycerol, respectively. SC and SM media consisted of 0.67% yeast nitrogen base without amino acids (BD) and complete amino acids/nucleosides (Clontech) with 2% glucose or 2% mannitol, respectively. In the case of cells carrying plasmid, dropout supplement −Ura (Clontech) was used instead of complete amino acids/nucleosides. Yeast strains were maintained on YPG plates to retain ρ+ cells, which have intact mitochondrial genomes (20, 21). Strains that exhibited growth defects on YPG plates (i.e., tup1Δ, cyc8Δ, and MK4437 strains) were maintained on YPD plates. All yeast cultures were grown at 30°C. For measurement of cell growth, cells were suspended in sterilized distilled water (SDW) and inoculated into 1 ml of SC or SM medium in a test tube at an optical density at 600 nm (OD600) of 0.1. Strains were grown for 3 days at 145 strokes per min (spm), and the OD600 values of the cultures were measured every day. In the case of flocculated cells, the OD600 was measured only on the third day after the culture was mixed with 0.1 volumes of 500 mM EDTA. For measurement of ethanol or sugar concentrations or mannitol dehydrogenase activity, cells on YPG plates were grown on YPM plates for approximately 3 days, suspended in SDW, and inoculated into 50 ml of YPM medium in a 100-ml Erlenmeyer flask at an OD600 of 0.1. Cells were precultured for 24 h at 95 spm, washed once with SDW, resuspended in SDW, and inoculated into 50 ml of YPD or YPM medium in a 100-ml Erlenmeyer flask at an OD600 of 0.1. To measure the salt tolerance, strains were grown in 1 ml of YPD medium (E. coli KO11 was grown in LBD medium [11] instead) in a test tube with or without 1 M NaCl for 1 day at 145 spm, and the OD600 of each culture was measured. In the case of flocculated cells, OD600 was measured after mixing the culture with 0.1 volumes of 500 mM EDTA.
Analytical methods.
Ethanol was assayed using an ethanol assay F-kit (Roche). Concentrations of glucose and mannitol were determined using a high-pressure liquid chromatography apparatus equipped with an Aminex HPX-87H (300 by 7.8 mm; Bio-Rad) column (65.5°C, elution with 5 mM H2SO4 at 0.65 ml/min) and a RID-10A detector (Shimadzu). Protein concentration was determined by using the Bradford reagent (Sigma) (22) with bovine serum albumin as a standard.
Microarray analysis.
Yeast cells that had been pregrown on YPG plates were suspended in SDW and inoculated into 50 ml of SC or SM medium in a 100-ml Erlenmeyer flask at an OD600 of 0.1 and then cultivated at 145 spm for 24 h (strain BY4742 in SC medium and strains MK3619 and MK3683 in SM medium) or 28 h (strain MK3683 in SC medium). RNA was extracted from the cultured cells using hot phenol (23), treated with DNase I, purified on an RNeasy column (Qiagen), and used for microarray analysis. RNA samples were analyzed using NimbleGen Systems according to the manufacturer's instructions. DNA microarrays from NimbleGen Systems include 5,747 target genes from S. cerevisiae S288C fixed on glass slides; each gene is represented by up to six unique probes consisting of 60-mer synthetic oligonucleotides. The arrays were scanned using a NimbleGen MS200 microarray scanner, and the raw data were analyzed using quantile normalization and the RMA algorithm (24). These normalized data were processed using ArrayStar (DNASTAR). Information regarding the function of each gene was obtained from the Saccharomyces Genome Database (http://www.yeastgenome.org/). This microarray analysis was conducted only once.
Gap-repair cloning.
Gap-repair cloning was performed as described previously (25). For the TUP1 gene, the open reading frame (ORF; 2,142-bp) and flanking 5′ (1,588-bp) and 3′ (1,228-bp) sequences were cloned into the SmaI site of YCplac33 (26) and digested with PmlI and MscI. For the CYC8 gene, the ORF (2,901-bp) and flanking 5′ (2,337-bp) and 3′ (1,389-bp) sequences were cloned into the SmaI site of YCplac33 and digested with MfeI and EagI. Digested fragments were used to transform yeast cells, and the gap-repaired plasmids were recovered from the transformed cells. Sequences of the cloned genes were compared to those deposited in the Saccharomyces Genome Database (http://www.yeastgenome.org/).
Assay of mannitol dehydrogenase activity.
Yeast cells cultured in 50 ml of YPD or YPM medium for 2 days at 95 spm were washed once with SDW, and half of the cells were resuspended in 750 μl of 50 mM sodium phosphate (pH 6.5). After acid-washed glass beads (Sigma) were added to the suspension, the cells were disrupted by using a FastPrep 24 (MP Biomedicals) at 6.5 m/s for two periods of 30 s separated by an interval of 30 s on ice. The cell lysates were spun at 20,000 × g for 10 min at 4°C, and the supernatant was used as cell extract. Mannitol dehydrogenase activity was continuously assayed at 30°C as described previously (27) in the following reaction mixture: 100 mM mannitol, 0.36 mM NAD+, 25 mM N-cyclohexyl-2-aminoethanesulfonic acid (CHES; pH 9.0), and cell extract (0.5 to 10 μg). One unit of enzyme activity was defined as 1.0 μmol of NADH produced in 1 min at 30°C.
Microarray data accession number.
The microarray data are available at the Gene Expression Omnibus (GEO) under accession number GSE55755.
RESULTS
Acquisition of the ability to assimilate mannitol by S. cerevisiae.
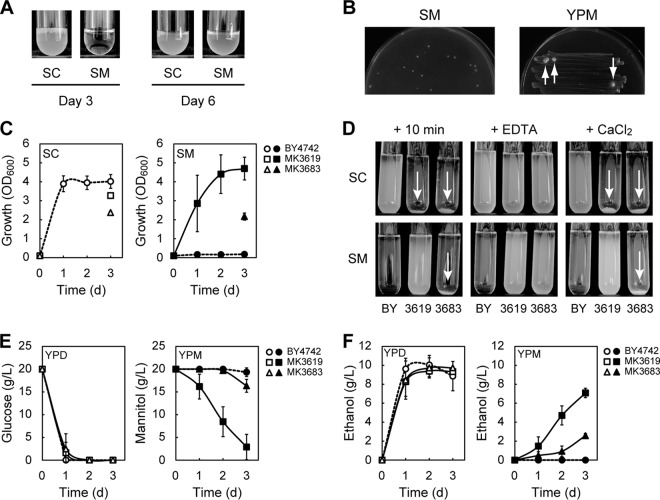
S. cerevisiae strain BY4742 is a derivative of the reference strain S288C. BY4742 cells that were maintained on YPG plates exhibited no growth in liquid synthetic mannitol medium (SM medium: synthetic medium containing mannitol as a sole carbon source) even after 3 days of cultivation, an observation consistent with the previously reported inability of S288C to grow on mannitol (13). After 6 days, however, the cultures reached saturation (Fig. 1A). This growth phenotype was confirmed three times. When the saturated cultures were inoculated into fresh SM medium at an OD600 of 0.1, the ensuing cultures reached saturation after only 2 days. These observations suggest that some of the BY4742 cells had spontaneously acquired the ability to utilize mannitol; these cells somehow adapted to the mannitol-containing medium, assimilated mannitol, and grew more rapidly than the unadapted cells.
FIG 1.
S. cerevisiae can acquire the ability to assimilate mannitol. (A) BY4742 cells were cultured for 3 or 6 days in SC or SM media, respectively. BY4742 cells that were maintained on YPG plates were inoculated into the medium at an OD600 of 0.10. (B) BY4742 cells that were maintained on YPG plates were grown to log phase in liquid YPD medium and collected. The collected cells (approximately 5 × 106 cells) were spread on SM plates. BY4742 cells grown on YPG plates were also streaked onto YPM plates. SM and YPM plates were incubated for 7 and 16 days, respectively, at 30°C. Visible colonies formed on the YPM plate are indicated by white arrows. (C) Growth of the indicated strains cultured in SC (open symbols) or SM (closed symbols) medium. In the case of flocculated cells, growth was measured only on the third day. (D) Ca2+-dependent flocculation. BY4742 (BY), MK3619, and MK3683 were cultured for 1 day in 5 ml of SC or SM medium, transferred to test tubes, and held for 10 min (+ 10 min). The cultures were mixed with 0.1 volumes of 500 mM EDTA and held for 10 min (+ EDTA). Cells were washed once with water, resuspended in 5 ml of 10 mM CaCl2, and held for 10 min (+ CaCl2). Flocculation is indicated by white arrows. (E and F) Sugar consumption (E) and ethanol production (F) of the indicated cells cultured in YPD (open symbols) or YPM (closed symbols) medium. (C to E) Results are the means of at least three independent experiments, and error bars represent the standard deviations (SDs).
To confirm this phenomenon, fresh BY4742 cells (approximately 5 × 106 cells) that had been grown to log phrase in liquid YPD medium were spread onto SM plates. After 7 days of incubation, several visible colonies appeared on the plates (Fig. 1B). This phenomenon was not specific to BY4742; we confirmed that other S. cerevisiae strains (AH109, BY4741, DBY877, EBY100, SEY6210, T8-1D, and YPH500) could also form visible colonies on SM plates after long incubations. Moreover, when fresh BY4742 cells were streaked on YPM plates and grown for 16 days, large visible colonies again formed (Fig. 1B). Next, we spread BY4742 cells (∼107) harvested from YPG plates independently on SM and YPM plates. After incubation for more than 5 days, 5 to 200 (SM plates, n = 4) or 6 to 16 (YPM plates, n = 2) large colonies formed per plate. These data indicated that only some of the approximately 5 × 106 to 1 × 107 cells that were spread or streaked on SM or YPM plates spontaneously had acquired the ability to utilize mannitol, ruling out the possibility that the BY4742 cells simply exhibited a prolonged lag phase in SM or YPM medium. Several colonies derived from BY4742 cells on SM or YPM plates were isolated as strains that had acquired the ability to utilize mannitol (Mtl+ strains) (Table 1), as described in the supplemental material. Two of these strains (MK3619 and MK3683) were initially chosen for further analysis.
TABLE 1.
Phenotypes of Mtl+ strains
| Strain | Flocculationa in: |
Mutationb in: |
||
|---|---|---|---|---|
| SM medium | SC medium | TUP1 | CYC8 | |
| MK3619 | – | + | G1382A (G461D) | ND |
| MK3683 | + | + | C325T (Q109X) | ND |
| MK4010 | – | – | WT | WT |
| MK4410 | – | + | T1805C (L602P) | ND |
| MK4412 | – | + | WTc | Δ1129–1138 (T376NYLTPLMRISKLQDWTX) |
| MK4416 | – | – | WT | Δ1139–1164 (Q380ASCKTGRKX) |
| MK4421 | + | + | Δ824–839 (P276RITTX) | ND |
| MK4437 | – | + | WT | WT |
| MK4443 | + | + | C1322A (S441X) | ND |
| MK4446 | + | + | Δ1122–1132 (S376PSIX) | ND |
| MK4447 | + | + | A58T (R20X) | ND |
| MK4449 | + | + | Δ1765 (Q589KALYPAHX) | ND |
| MK4450 | – | + | WT | G1066C (A356P) |
| MK4456 | – | – | WT | G1752A, C1753T (Q585X) |
Flocculation and nonflocculation are denoted by plus (+) and minus (–), respectively.
Changes in nucleotides are shown; changes in amino acid residues are indicated in parentheses. Deletion is indicated by a “Δ” symbol. A stop codon is indicated by an “X.” ND, not determined. WT, wild type (no mutation). If mutations were not found by gap-repair cloning and subsequent sequencing, the genomic sequence of TUP1 or CYC8 was checked for confirmation.
Mutation in TUP1 was verified only by gap-repair cloning and subsequent sequencing.
BY4742, MK3619, and MK3683 grew well in synthetic complete medium (SC medium: synthetic medium containing glucose as a sole carbon source) (Fig. 1C). As expected, MK3619 and MK3683 were able to grow in SM medium, although the parental strain BY4742 was not (Fig. 1C). Moreover, MK3619 and MK3683 exhibited Ca2+-dependent flocculation (MK3619 in SC medium and MK3683 in both SC and SM media), whereas BY4742 did not (Fig. 1D). Flocculation is a nonsexual, reversible cell aggregation (28). In contrast to BY4742, when MK3619 and MK3683 were cultured in YPM medium, both strains took up mannitol and produced ethanol (Fig. 1E and F). Compared to MK3683, MK3619 consumed much larger amounts of mannitol and produced ethanol much more efficiently. These results indicate that MK3619 and MK3683 had acquired the ability to assimilate mannitol. The growth of MK3619 and MK3683 on SM plates was dependent on functional mitochondria and oxygen (see Fig. S1 in the supplemental material), confirming that assimilation of mannitol requires respiration, as reported previously (11, 13).
Microarray analysis of transcripts in MK3619 and MK3683.
To figure out how MK3619 and MK3683 acquired the ability to assimilate mannitol, we conducted a microarray analysis. The whole transcriptomes of MK3619 in SM medium and MK3683 in both SC and SM media were compared to that of the parental strain BY4742 in SC medium (control). We initially focused on 26 genes that were upregulated more than 4-fold in all three samples relative to the control (see Table S2 in the supplemental material). These genes included maltase (MAL32), isomaltase (IMA1, IMA2), α-glucosidase (IMA3), anaerobic cell wall mannoprotein (DAN1, TIR1), putative sorbitol dehydrogenase (SOR2), putative mannitol dehydrogenase (YEL070W), putative hexose transporter (HXT10), and DNA damage-inducible (HUG1) genes, genes involved in thiamine biosynthesis (THI5, THI11, THI12, and THI13), and genes involved in mating (Fig. 1, MFA1, MFA2, MF(ALPHA)2, BAR1). It should be noted that genes encoding a putative mannitol dehydrogenase and hexose transporter were upregulated in both MK3619 and MK3683. These findings suggest that MK3619 and MK3683 had acquired the ability to assimilate mannitol by activating transcription of a set of genes required for mannitol utilization. We next focused on the 20 putative hexose transporter genes identified in S. cerevisiae to date (29). Among these genes, the expression of HXT10 in SM medium was remarkably induced in both MK3619 and MK3683 relative to the control (16.64-fold in MK3619 and 21.95-fold in MK3683), as mentioned above. HXT13, HXT15, and HXT17 were also upregulated by more than 2-fold relative to the control, and they were expressed at higher levels in SM medium than in SC medium (see Table S3 in the supplemental material). These results imply that Hxt13, Hxt15, Hxt17, and especially Hxt10 are involved in the uptake of mannitol.
Mechanisms underlying the acquisition of the ability to assimilate mannitol.
We hypothesized that during cultivation in SM medium, MK3619 and MK3683 had acquired spontaneous mutations affecting the transcription of a set of genes required for mannitol utilization. The microarray data and flocculation phenotype of the MK3619 and MK3683 strains were reminiscent of the tup1 mutant, which exhibits flocculation (30). Tup1 forms a corepressor complex with Cyc8 (also known as Ssn6) and regulates a large number of diverse genes, including genes that are cell type specific, glucose repressible, DNA damage inducible, or involved in flocculation or the hypoxic response (18, 19). MFA1, MFA2, DAN1, TIR1, and HUG1 are upregulated in tup1 mutants (15, 31–33). Because our microarray analysis had revealed the upregulation of these genes (MFA1, MFA2, DAN1, TIR1, and HUG1) in MK3619 and MK3683, as described above, we examined the growth of tup1Δ cells in SC and SM media. We found that the tup1Δ mutant was able to grow and flocculate in both SC and SM media (Fig. 2A and see Fig. S2A in the supplemental material). To determine whether MK3619 and MK3683 had mutations in the TUP1 gene locus, we cloned TUP1 from MK3619 and MK3683 using the gap-repair cloning method. Subsequent sequencing revealed that MK3619 contained the G1382A mutation, causing a G461D substitution in Tup1, and MK3683 contained the C325T amber mutation, causing truncation of most of the C-terminal portion of Tup1 (residues 109 to 713) (Fig. 2B and Table 1). The nucleotide sequence of TUP1 cloned from BY4742 was the same as that deposited in Saccharomyces genome database. To verify that the mannitol-assimilating abilities of MK3619 and MK3683 could be attributed to these mutations, we introduced the TUP1 alleles from each strain (MK3619, MK3683, and BY4742) into tup1Δ mutant using the low-copy-number plasmid YCplac33. Transformed cells exhibited the same flocculation pattern and mannitol-assimilating phenotype as the strain from which the TUP1 allele was derived, demonstrating that the acquired phenotypes could be ascribed to these mutations in TUP1 (Fig. 1C, 1D, and 2C; see also Fig. S2B in the supplemental material). Moreover, MK3619, MK3683, and the tup1Δ mutant grown in YPD or YPM medium exhibited similar mannitol dehydrogenase activities, whereas BY4742 had no such activity (Table 2). To determine whether other Mtl+ strains also had mutations in TUP1, we examined the TUP1 gene of the Mtl+ strains other than MK3619 and MK3683 (Table 1). One strain (MK4410) had a mutation causing a single amino acid substitution in Tup1, as in MK3619. Two strains (MK4443 and MK4447) had single-nucleotide substitutions in TUP1 leading to stop codons, as in MK3683. Three strains (MK4421, MK4446, and MK4449) had deletions in TUP1 resulting in frameshifts that created premature stop codons.
FIG 2.
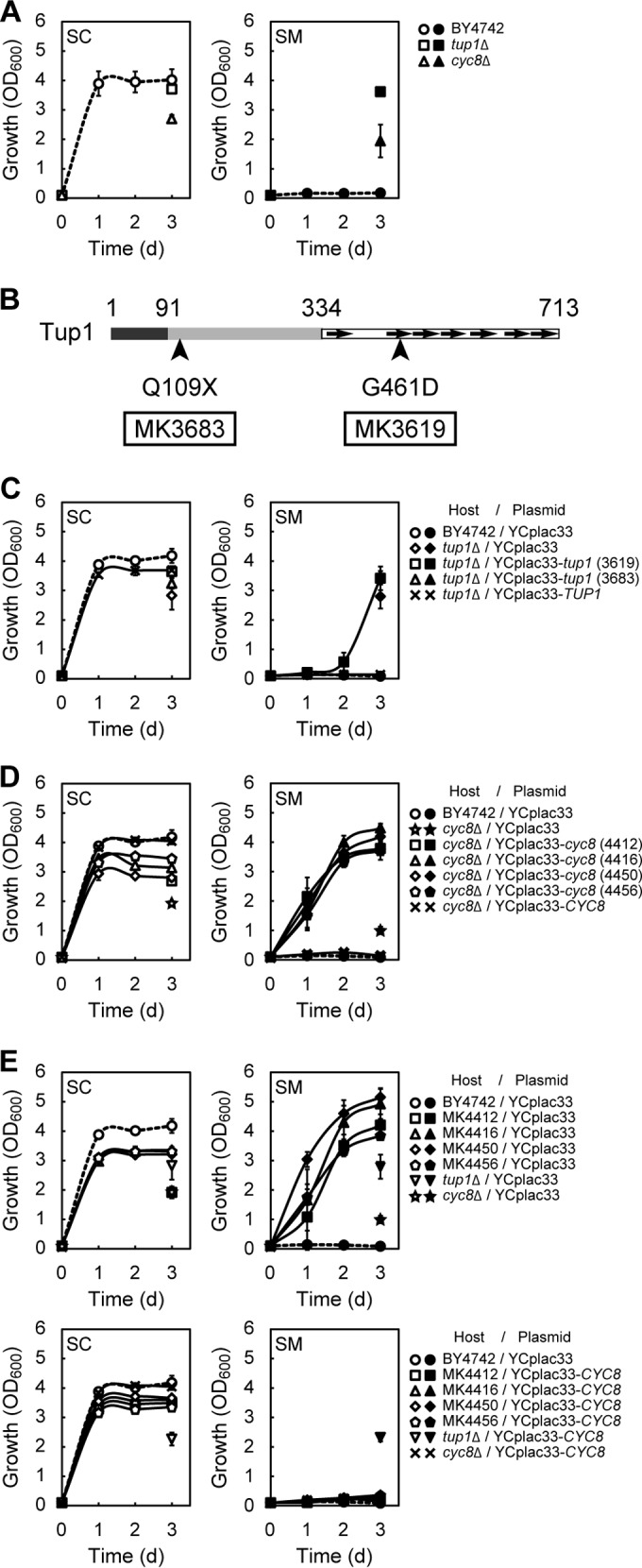
Mutations in TUP1 or CYC8 are responsible for acquisition of the ability to assimilate mannitol. (A, C, D, and E) Growth of the indicated strains cultured in SC (open symbols) or SM (closed symbols) medium. In the case of flocculated cells, growth was measured only on the third day. The results are the means of at least three independent experiments, and error bars represent the SDs. (C and D) Complementation analysis. TUP1 alleles from MK3619, MK3683, and BY4742 (C) or CYC8 alleles from MK4412, MK4416, MK4450, MK4456, and BY4742 (D) were cloned into the low-copy-number plasmid YCplac33 by the gap-repair cloning method. Constructed plasmids were introduced into tup1Δ (C) or cyc8Δ (D) strains. BY4742, the tup1Δ mutant, and the cyc8Δ mutant carrying empty YCplac33 were used as controls. Numbers in parentheses represent the strain from which each allele was obtained. (E) YCplac33 containing wild-type CYC8 (from BY4742) and YCplac33 alone were introduced into MK4412, MK4416, MK4450, MK4456, the tup1Δ mutant, and the cyc8Δ mutant. BY4742 carrying YCplac33 was used as a control. (B) Schematic structure of Tup1 (713 amino acid residues). N-terminal (dark gray), central (light gray), and C-terminal (white) domains are shown. WD-repeat motifs are represented as arrows (39). The mutated sites in MK3619 and MK3683 are indicated by arrowheads.
TABLE 2.
Mannitol dehydrogenase activities of yeast strains
| Strain | Mean mannitol dehydrogenase activity (U/mg) ± SDa in: |
|
|---|---|---|
| YPD medium | YPM medium | |
| BY4742 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| MK3619 | 0.80 ± 0.44 | 1.70 ± 0.56 |
| MK3683 | 1.03 ± 0.76 | 0.94 ± 0.46 |
| tup1Δ mutant | 1.50 ± 0.42 | 1.50 ± 0.15 |
| MK4416 | 4.93 ± 0.47 | 3.46 ± 0.51 |
Values represent the means of three independent experiments.
The remaining six Mtl+ strains (MK4010, MK4412, MK4416, MK4437, MK4450, and MK4456) had no mutations in TUP1 (Table 1). Because Tup1 forms a complex with Cyc8 (16, 34), we next focused on the CYC8 gene. As expected, similar to the tup1Δ mutant, the cyc8Δ mutant was able to grow and flocculate in both SC and SM media, although the growth of the cyc8Δ mutant was slightly slower than that of the tup1Δ mutant (Fig. 2A and see Fig. S2A in the supplemental material). We found that several of the Mtl+ strains (MK4412, MK4416, MK4450, and MK4456) had mutations in CYC8 (see Fig. S3A in the supplemental material and Table 1). The nucleotide sequence of CYC8 cloned from BY4742 was the same as that deposited in the Saccharomyces Genome Database. All of these strains were able to grow, but not flocculate, in SM medium (see Fig. S3B and C in the supplemental material). When we introduced each of these CYC8 alleles, as well as wild-type CYC8, into the cyc8Δ mutant, the transformed cells exhibited the same flocculation pattern and mannitol-assimilating phenotype as the strain from which the CYC8 allele was derived (Fig. 2D; see also Fig. S2C, S3B, and S3C in the supplemental material). These results confirmed that the acquired phenotypes could be ascribed to the mutations in CYC8. Furthermore, we introduced wild-type CYC8 into the tup1Δ mutant, the cyc8Δ mutant, and each Mtl+ strain that carried a mutation in CYC8. All of the transformed strains, except the tup1Δ mutant, lost the ability to assimilate mannitol (Fig. 2E and see Fig. S2D in the supplemental material). These results confirmed that the mannitol-assimilating phenotype displayed by Mtl+ strains MK4412, MK4416, MK4450, and MK4456 could be attributed to mutations on CYC8.
Based on these findings, we concluded that the majority of Mtl+ strains had mutations in TUP1 or CYC8 and that these mutations were responsible for the acquisition of mannitol-assimilating ability. These results suggest a central role of the Tup1-Cyc8 corepressor in regulation of mannitol utilization. In other words, the inability of wild-type S. cerevisiae to assimilate mannitol can be attributed to repression by the Tup1-Cyc8 corepressor.
Ethanol production from mannitol using S. cerevisiae.
Although all Mtl+ strains could assimilate mannitol, their flocculation patterns differed, i.e., there was no correlation between flocculation phenotype and the ability to assimilate mannitol (Table 1). Therefore, to minimize the effect of Tup1-Cyc8 dysfunction on pleiotropic phenotypes other than mannitol assimilation, we chose strains that did not have flocculation phenotypes in SM or SC media (i.e., MK4416 and MK4456) from among the Mtl+ strains with mutations in TUP1 or CYC8.
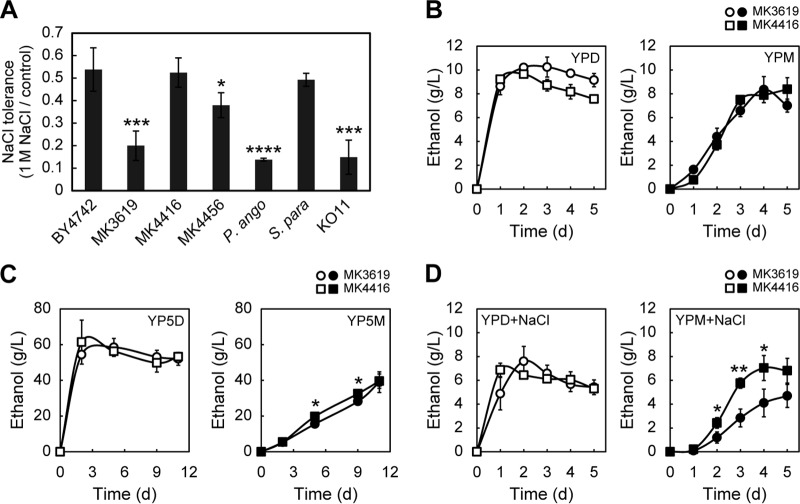
Tolerance to salt is advantageous for bioethanol production from marine biomass, because it leads to increased productivity. We recently reported that S. paradoxus NBRC0259-3 is the most suitable yeast strain for the production of ethanol from mannitol and is superior in this regard to two other microbes, P. angophorae and E. coli KO11 (11). Therefore, we compared the salt tolerances of MK4416 and MK4456 strains with that of S. paradoxus NBRC0259-3, P. angophorae, and E. coli KO11. Because we had confirmed that MK3619 produced ethanol from mannitol much more efficiently than MK3683 (Fig. 1E and F), we also tested the salt tolerance of this strain. MK3619, P. angophorae, and E. coli KO11 exhibited much stronger sensitivity to salt than did S. cerevisiae BY4742 and S. paradoxus NBRC0259-3 (Fig. 3A). On the other hand, MK4416 was more salt tolerant than MK4456 and was comparable in this regard to BY4742 and NBRC0259-3 (Fig. 3A). Therefore, we next examined the ethanol productivity of MK4416 relative to that of MK3619. Although MK3619 produced larger amounts of ethanol from glucose than MK4416, both MK3619 and MK4416 produced similar amounts of ethanol from mannitol (Fig. 3B). In the presence of a high concentration of mannitol (100 g/liter), MK3619 and MK4416 were able to produce ∼40 g/liter ethanol, a yield comparable to the yield from S. paradoxus NBRC0259-3 (Fig. 3C) (11). In the presence of salt (1 M NaCl), MK4416 was able to produce much more ethanol from mannitol than MK3619 (Fig. 3D). This result is consistent with the high salt tolerance of MK4416 (Fig. 3A). It is worth noting that MK4416 grown in YPD or YPM medium displayed much higher levels of mannitol dehydrogenase activity than MK3619 (Table 2). Thus, S. cerevisiae MK4416, which carries a mutation in CYC8, is the most useful and suitable strain for the production of ethanol from mannitol.
FIG 3.
Ethanol production from mannitol using S. cerevisiae. (A) Salt tolerances of the indicated strains. The values shown are the ratios of the OD600s of salt-treated cells to those of nontreated control cells. P. ango, S. para, and KO11 represent P. angophorae, S. paradoxus NBRC0259-3, and E. coli KO11, respectively. (B to D) Ethanol production of the indicated cells. The results are means of three independent experiments, and error bars represent the SDs. (B) Strains were cultured in YPD (open symbols) or YPM (closed symbols) media. (C) Strains were cultured in YP medium containing 100 g/liter glucose (YP5D, open symbols) or 100 g/liter mannitol (YP5M, closed symbols). (D) Strains were cultured in YPD (open symbols) or YPM (closed symbols) medium containing 1 M NaCl. (A to D) Student t test determinations: *, P < 0.05; **, P < 0.01; ***, P < 0.005; and ****, P < 0.001.
DISCUSSION
Although a few S. cerevisiae strains, such as the polyploid strain BB1, are (for unknown reasons) capable of efficient growth on mannitol, the reference strain S288C is unable to assimilate mannitol (13, 35, 36). Moreover, to date, the molecular mechanism underlying the ability to assimilate mannitol had remained unclear.
In the present study, we showed that S. cerevisiae strains that acquired the ability to assimilate mannitol by developing spontaneous mutations could be easily selected during prolonged culture in mannitol medium. S. cerevisiae is a powerful industrial tool because of its high stress tolerance, high ethanol productivity, and well-established genetic methods (10). Therefore, mannitol-assimilating S. cerevisiae strains would be an ideal platform for development of a yeast-algal ethanol production system that would be superior to systems that use S. paradoxus or ethanologenic bacteria. A recent study described a genetically engineered S. cerevisiae strain with the ability to metabolize mannitol (14). That study showed that the minimal genes required for growth on mannitol were mannitol dehydrogenase and a mannitol transporter, although the regulatory mechanism remained unexplained (14). These results are consistent with our microarray data, which showed that genes encoding a putative mannitol dehydrogenase and hexose transporters were upregulated in Mtl+ strains (see Table S2 in the supplemental material). Furthermore, our study identified Tup1-Cyc8 as a key regulator of the genes required for mannitol utilization, providing greater insight into the molecular mechanisms of mannitol metabolism. In addition, HXT10 and other hexose transporter genes were prominently upregulated in tup1 mutant cells (see Table S3 in the supplemental material). Currently, little is known about the function of Hxt10, but it appears to be unable to transport a significant amount of glucose (29, 37). Thus, Hxt10 may function predominantly as a transporter of mannitol.
The Tup1-Cyc8 corepressor is implicated in the repression of wide variety of genes (18, 19). The specificity of this repression is determined by specific DNA-binding repressors (16). To identify the DNA-binding repressor that acts together with Tup1-Cyc8, we examined the growth in SM medium of 97 strains from the EUROSCARF MATα haploid deletion set, including disruptants of known repressors such as mig1, rfx1, rox1, nrg1, and sko1 (see Table S4 in the supplemental material). All 97 disruptants were unable to grow in SM medium after 3 days of cultivation. One possible explanation is that an unknown novel repressor functions together with Tup1-Cyc8 to control mannitol metabolism. However, we speculate that several repressive mechanisms work cooperatively to regulate genes involved in mannitol metabolism. Tup1-Cyc8 represses transcription via multiple mechanisms (16, 19, 38): modifying chromatin structure via recruitment of HDACs, directing the positioning of nucleosomes, inhibiting the general transcription machinery via direct interactions, and masking the activation domains of DNA-binding proteins to prevent the recruitment of coactivators. When the CYC8 allele from MK4416 was overexpressed in BY4742, the overexpressing cells did not grow in SM medium (see Fig. S4 in the supplemental material). This result suggests that only a small amount of wild-type Cyc8 is sufficient to repress the genes involved in mannitol metabolism. Thus, we speculate that only a few wild-type Tup1-Cyc8 molecules, acting via multiple mechanisms, can ensure the repression of genes involved in mannitol metabolism.
Among Mtl+ strains, eight strains had mutations in the TUP1 gene (Table 1). The mutated sites were not biased and were distributed all over the gene. There were two types of mutations: amino-acid substitutions and mutations that created a stop codon. Whereas six strains with stop codon mutations (MK3683, MK4421, MK4443, MK4446, MK4447, and MK4449) exhibited clear flocculation phenotypes similar to that of the tup1Δ mutant, two strains with substitution mutations (MK3619 and MK4410) did not flocculate in SM medium (Table 1). Given that Tup1-Cyc8 regulates many kinds of genes, including some involved in flocculation (18, 19), these substitution mutations may predominantly affect mannitol assimilation but have little effect on flocculation. Moreover, both substitution mutations occurred in WD repeat motifs of Tup1 (Fig. 2B and Table 1). The WD repeat motifs of Tup1 are required for interactions with DNA-binding proteins (39, 40). Therefore, the substituted amino acids (G461 and L602) may be important for interactions between Tup1 and a DNA-binding protein involved in regulation of mannitol metabolism.
Four Mtl+ strains had mutations in CYC8 (Table 1). In three of these strains (MK4412, MK4416, and MK4450), mutations occurred in the C-terminal tetratricopeptide repeat (TPR) motifs of Cyc8 (41) (see Fig. S3A in the supplemental material). The TPR is a conserved degenerate 34-amino-acid repeat motif that mediates protein-protein interactions (42, 43). Previous reports showed that the C-terminal TPR motifs of Cyc8 are required for glucose repression (44). Thus, factors involved in glucose repression may also be required for the regulation of mannitol metabolism by Tup1-Cyc8.
Two Mtl+ strains (MK4010 and MK4437) did not have mutations in either TUP1 or CYC8 (Table 1). We found that both of these strains gradually lost the ability to assimilate mannitol during serial passage on YPG (for MK4010) or YPD (for MK4437) plates. By the eighth passage, both strains had completely lost the ability to grow in SM medium (see Fig. S5 in the supplemental material). Furthermore, we examined the effects of precultivation of the representative Mtl+ strains (Table 1) in SC liquid medium on their growth in SM liquid medium (see Fig. S6 in the supplemental material). The results showed that all tested Mtl+ strains, including MK4010 and MK4437, maintained their ability to grow in SM liquid medium after at least two passages of precultivation in SC liquid medium.
Overall, the findings reported here indicate that Tup1-Cyc8 is a key regulator of mannitol metabolism that represents a good target for development of a yeast-algal bioethanol production system. Furthermore, we succeeded in conferring the ability to assimilate mannitol on S. cerevisiae through dysfunction of Tup1-Cyc8, thereby facilitating production of ethanol from mannitol, a promising brown macroalgal carbon source.
Supplementary Material
ACKNOWLEDGMENTS
The pGK426 plasmid was provided by the National Bio-Resource Project of MEXT, Japan.
This study was supported by the Funding Program for Next-Generation World-Leading Researchers GS012 (to S.K.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02906-14.
REFERENCES
- 1.Huesemann M, Roesjadi G, Benemann J, Metting FB. 2010. Biofuels from microalgae and seaweeds, p 165–184. In Vertès A, Qureshi N, Yukawa H, Blaschek HP (ed), Biomass to biofuels: strategies for global industries. Wiley, New York, NY. [Google Scholar]
- 2.Adams JM, Gallagher JA, Donnison IS. 2009. Fermentation study on Saccharina latissima for bioethanol production considering variable pretreatments. J Appl Phycol 21:569–574. doi: 10.1007/s10811-008-9384-7. [DOI] [Google Scholar]
- 3.Yoon JJ, Kim YJ, Kim SH, Ryu HJ, Choi JY, Kim GS, Shin MK. 2010. Production of polysaccharides and corresponding sugars from red seaweed. Adv Mater Res 93-94:463–466. doi: 10.4028/www.scientific.net/AMR.93-94.463. [DOI] [Google Scholar]
- 4.John RP, Anisha GS, Nampoothiri KM, Pandey A. 2011. Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193. doi: 10.1016/j.biortech.2010.06.139. [DOI] [PubMed] [Google Scholar]
- 5.Zubia M, Payri C, Deslandes E. 2008. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J Appl Phycol 20:1033–1043. doi: 10.1007/s10811-007-9303-3. [DOI] [Google Scholar]
- 6.Horn SJ, Aasen IM, Østgaard K. 2000. Production of ethanol from mannitol by Zymobacter palmae. J Ind Microbiol Biotechnol 24:51–57. doi: 10.1038/sj.jim.2900771. [DOI] [Google Scholar]
- 7.Wargacki AJ, Leonard E, Win MN, Regitsky DD, Santos CN, Kim PB, Cooper SR, Raisner RM, Herman A, Sivitz AB, Lakshmanaswamy A, Kashiyama Y, Baker D, Yoshikuni Y. 2012. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335:308–313. doi: 10.1126/science.1214547. [DOI] [PubMed] [Google Scholar]
- 8.Horn SJ, Aasen IM, Ostgaard K. 2000. Ethanol production from seaweed extract. J Ind Microbiol Biotechnol 25:249–254. doi: 10.1038/sj.jim.7000065. [DOI] [Google Scholar]
- 9.Kim NJ, Li H, Jung K, Chang HN, Lee PC. 2011. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour Technol 102:7466–7469. doi: 10.1016/j.biortech.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 10.Hughes SR, Qureshi N. 2010. Biofuel demand realization, p 55–69. In Vertès A, Qureshi N, Yukawa H, Blaschek HP (ed), Biomass to biofuels: strategies for global industries. Wiley, New York, NY. [Google Scholar]
- 11.Ota A, Kawai S, Oda H, Iohara K, Murata K. 2013. Production of ethanol from mannitol by the yeast strain Saccharomyces paradoxus NBRC 0259. J Biosci Bioeng 116:327–332. doi: 10.1016/j.jbiosc.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Niehaus WG Jr, Dilts RP Jr. 1982. Purification and characterization of mannitol dehydrogenase from Aspergillus parasiticus. J Bacteriol 151:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quain DE, Boulton CA. 1987. Growth and metabolism of mannitol by strains of Saccharomyces cerevisiae. J Gen Microbiol 133:1675–1684. [DOI] [PubMed] [Google Scholar]
- 14.Enquist-Newman M, Faust AM, Bravo DD, Santos CN, Raisner RM, Hanel A, Sarvabhowman P, Le C, Regitsky DD, Cooper SR, Peereboom L, Clark A, Martinez Y, Goldsmith J, Cho MY, Donohoue PD, Luo L, Lamberson B, Tamrakar P, Kim EJ, Villari JL, Gill A, Tripathi SA, Karamchedu P, Paredes CJ, Rajgarhia V, Kotlar HK, Bailey RB, Miller DJ, Ohler NL, Swimmer C, Yoshikuni Y. 2014. Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 505:239–243. doi: 10.1038/nature12771. [DOI] [PubMed] [Google Scholar]
- 15.Keleher CA, Redd MJ, Schultz J, Carlson M, Johnson AD. 1992. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell 68:709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- 16.Smith RL, Johnson AD. 2000. Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem Sci 25:325–330. doi: 10.1016/S0968-0004(00)01592-9. [DOI] [PubMed] [Google Scholar]
- 17.Varanasi US, Klis M, Mikesell PB, Trumbly RJ. 1996. The Cyc8 (Ssn6)-Tup1 corepressor complex is composed of one Cyc8 and four Tup1 subunits. Mol Cell Biol 16:6707–6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrico PM, Zitomer RS. 1998. Mutational analysis of the Tup1 general repressor of yeast. Genetics 148:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malave TM, Dent SY. 2006. Transcriptional repression by Tup1-Ssn6. Biochem Cell Biol 84:437–443. doi: 10.1139/o06-073. [DOI] [PubMed] [Google Scholar]
- 20.Sherman F. 2002. Getting started with yeast. Methods Enzymol 350:3–41. doi: 10.1016/S0076-6879(02)50954-X. [DOI] [PubMed] [Google Scholar]
- 21.Fox TD, Folley LS, Mulero JJ, McMullin TW, Thorsness PE, Hedin LO, Costanzo MC. 1991. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol 194:149–165. doi: 10.1016/0076-6879(91)94013-3. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.McEntee CM, Hudson AP. 1989. Preparation of RNA from unspheroplasted yeast cells (Saccharomyces cerevisiae). Anal Biochem 176:303–306. doi: 10.1016/0003-2697(89)90313-8. [DOI] [PubMed] [Google Scholar]
- 24.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 25.Ma H, Kunes S, Schatz PJ, Botstein D. 1987. Plasmid construction by homologous recombination in yeast. Gene 58:201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- 26.Gietz RD, Sugino A. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 27.Perfect JR, Rude TH, Wong B, Flynn T, Chaturvedi V, Niehaus W. 1996. Identification of a Cryptococcus neoformans gene that directs expression of the cryptic Saccharomyces cerevisiae mannitol dehydrogenase gene. J Bacteriol 178:5257–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares EV. 2011. Flocculation in Saccharomyces cerevisiae: a review. J Appl Microbiol 110:1–18. doi: 10.1111/j.1365-2672.2010.04897.x. [DOI] [PubMed] [Google Scholar]
- 29.Boles E, Hollenberg CP. 1997. The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev 21:85–111. doi: 10.1111/j.1574-6976.1997.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 30.Lipke PN, Hull-Pillsbury C. 1984. Flocculation of Saccharomyces cerevisiae tup1 mutants. J Bacteriol 159:797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeRisi JL, Iyer VR, Brown PO. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 32.Abramova NE, Cohen BD, Sertil O, Kapoor R, Davies KJ, Lowry CV. 2001. Regulatory mechanisms controlling expression of the DAN/TIR mannoprotein genes during anaerobic remodeling of the cell wall in Saccharomyces cerevisiae. Genetics 157:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basrai MA, Velculescu VE, Kinzler KW, Hieter P. 1999. NORF5/HUG1 is a component of the MEC1-mediated checkpoint response to DNA damage and replication arrest in Saccharomyces cerevisiae. Mol Cell Biol 19:7041–7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams FE, Varanasi U, Trumbly RJ. 1991. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex. Mol Cell Biol 11:3307–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warringer J, Zorgo E, Cubillos FA, Zia A, Gjuvsland A, Simpson JT, Forsmark A, Durbin R, Omholt SW, Louis EJ, Liti G, Moses A, Blomberg A. 2011. Trait variation in yeast is defined by population history. PLoS Genet. 7:e1002111. doi: 10.1371/journal.pgen.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tornai-Lehoczki J, Peter G, Dlauchy D, Deak T. 1996. Some remarks on “a taxonomic key for the genus Saccharomyces” (Vaughan Martini and Martini 1993). Antonie Van Leeuwenhoek 69:229–233. doi: 10.1007/BF00399611. [DOI] [PubMed] [Google Scholar]
- 37.Ozcan S, Johnston M. 1999. Function and regulation of yeast hexose transporters. Microbiol Mol Biol. Rev 63:554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parnell EJ, Stillman DJ. 2011. Shields up: the Tup1-Cyc8 repressor complex blocks coactivator recruitment. Genes Dev 25:2429–2435. doi: 10.1101/gad.181768.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprague ER, Redd MJ, Johnson AD, Wolberger C. 2000. Structure of the C-terminal domain of Tup1, a corepressor of transcription in yeast. EMBO J 19:3016–3027. doi: 10.1093/emboj/19.12.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumura H, Kusaka N, Nakamura T, Tanaka N, Sagegami K, Uegaki K, Inoue T, Mukai Y. 2012. Crystal structure of the N-terminal domain of the yeast general corepressor Tup1p and its functional implications. J Biol Chem 287:26528–26538. doi: 10.1074/jbc.M112.369652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikorski RS, Boguski MS, Goebl M, Hieter P. 1990. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell 60:307–317. doi: 10.1016/0092-8674(90)90745-Z. [DOI] [PubMed] [Google Scholar]
- 42.Lamb JR, Tugendreich S, Hieter P. 1995. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci 20:257–259. doi: 10.1016/S0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- 43.Blatch GL, Lassle M. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21:932–939. [DOI] [PubMed] [Google Scholar]
- 44.Tzamarias D, Struhl K. 1995. Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8-Tup1 corepressor complex to differentially regulated promoters. Genes Dev 9:821–831. doi: 10.1101/gad.9.7.821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.