Abstract
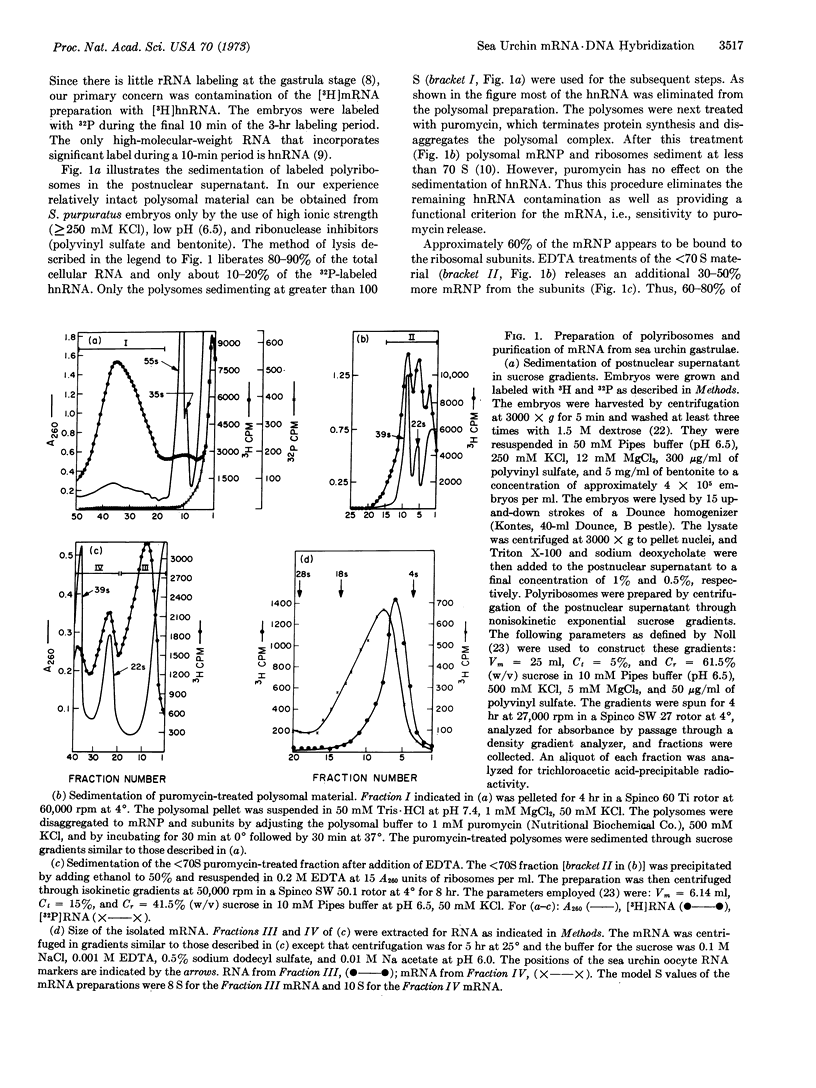
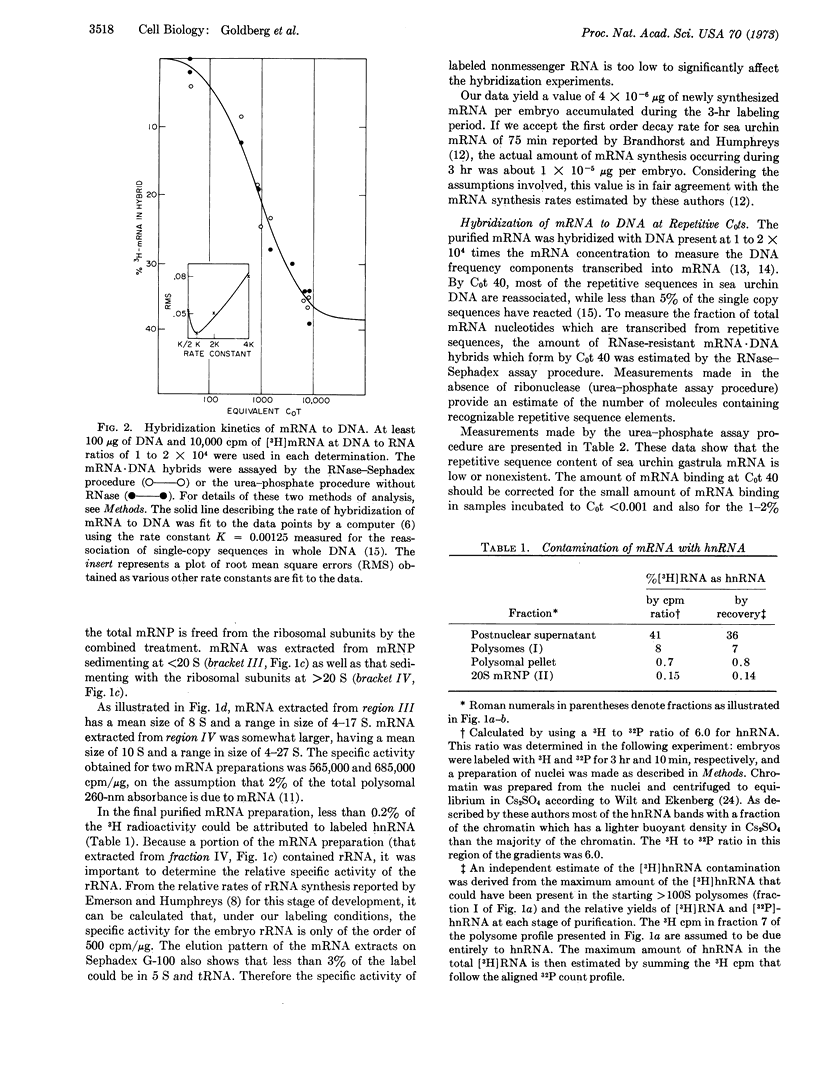
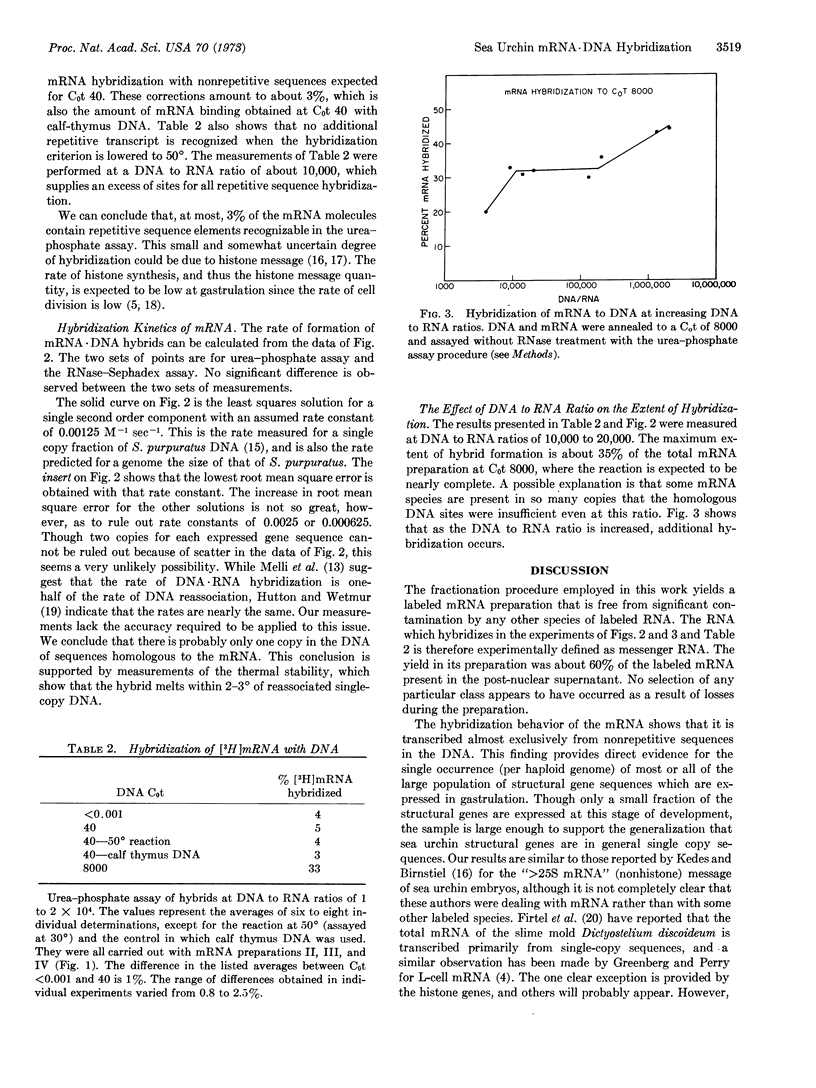
Messenger RNA was prepared from developing sea urchin gastrulae by puromycin release from polyribosomes. Approximately 60% of the total mRNA radioactivity of the postnuclear supernatant was recovered and shown to be free of any other labeled RNA species such as ribosomal and nuclear RNA. The mRNA was examined by hybridization to DNA present in great excess. The mRNA hybridizes almost exclusively with nonrepetitive DNA. Almost all of the messenger RNA molecules of sea urchin gastrulae therefore consist of transcripts from nonrepetitive sequences. It appears that the structural genes expressed at this stage are typically not repeated in the genome and the mRNA does not include recognizable repetitive sequence.
Keywords: 3H-labeled gastrula mRNA, hybridization, hydroxyapatite
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Release, identification, and isolation of messenger RNA from mammalian ribosomes. Proc Natl Acad Sci U S A. 1971 Apr;68(4):832–835. doi: 10.1073/pnas.68.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst B. P., Humphreys T. Stabilities of nuclear and messenger RNA molecules in sea urchin embryos. J Cell Biol. 1972 May;53(2):474–482. doi: 10.1083/jcb.53.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Dina D., Crippa M., Beccari E. Hybridization properties and sequence arrangement in a population of mRNAs. Nat New Biol. 1973 Mar 28;242(117):101–105. doi: 10.1038/newbio242101a0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Jacobson A., Lodish H. F. Isolation and hybridization kinetics of messenger RNA from Dictyostelium discoideum. Nat New Biol. 1972 Oct 25;239(95):225–228. doi: 10.1038/newbio239225a0. [DOI] [PubMed] [Google Scholar]
- Gelderman A. H., Rake A. V., Britten R. J. Transcription of nonrepeated DNA in neonatal and fetal mice. Proc Natl Acad Sci U S A. 1971 Jan;68(1):172–176. doi: 10.1073/pnas.68.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Hybridization properties of DNA sequences directing the synthesis of messenger RNA and heterogeneous nuclear RNA. J Cell Biol. 1971 Sep;50(3):774–786. doi: 10.1083/jcb.50.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINEGARDNER R. T. The isolation of nuclei from eggs and embryos of the sea urchin. J Cell Biol. 1962 Dec;15:503–508. doi: 10.1083/jcb.15.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys T. Measurements of messenger RNA entering polysomes upon fertilization of sea urchin eggs. Dev Biol. 1971 Oct;26(2):201–208. doi: 10.1016/0012-1606(71)90122-9. [DOI] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Renaturation of bacteriophage phiX174 DNA-RNA hybrid: RNA length effect and nucleation rate constant. J Mol Biol. 1973 Jul 15;77(4):495–500. doi: 10.1016/0022-2836(73)90218-0. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Birnstiel M. L. Reiteration and clustering of DNA sequences complementary to histone messenger RNA. Nat New Biol. 1971 Apr 7;230(14):165–169. doi: 10.1038/newbio230165a0. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Gross P. R., Cognetti G., Hunter A. L. Synthesis of nuclear and chromosomal proteins on light polyribosomes during cleavage in the sea urchin embryo. J Mol Biol. 1969 Oct 28;45(2):337–351. doi: 10.1016/0022-2836(69)90109-0. [DOI] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Birnstiel M. L., Purdom I. F., Williamson R. Genes coding for polysomal 9S RNA of sea urchins: conservation and divergence. Nature. 1972 Nov 24;240(5378):225–228. doi: 10.1038/240225a0. [DOI] [PubMed] [Google Scholar]
- Wilt F. H., Ekenberg E. Isolation of chromatin bearing nascent RNA from nuclei of sea urchin embryos. Biochem Biophys Res Commun. 1971 Aug 20;44(4):831–836. doi: 10.1016/0006-291x(71)90786-8. [DOI] [PubMed] [Google Scholar]


