Abstract
Vibrio parahaemolyticus is a halophile that is the predominant cause of bacterial seafood-related gastroenteritis worldwide. To survive in the marine environment, V. parahaemolyticus must have adaptive strategies to cope with salinity changes. Six putative compatible solute (CS) transport systems were previously predicted from the genome sequence of V. parahaemolyticus RIMD2210633. In this study, we determined the role of the four putative betaine-carnitine-choline transporter (BCCT) homologues VP1456, VP1723, VP1905, and VPA0356 in the NaCl stress response. Expression analysis of the four BCCTs subjected to NaCl upshock showed that VP1456, VP1905, and VPA0356, but not VP1723, were induced. We constructed in-frame single-deletion mutant strains for all four BCCTs, all of which behaved similarly to the wild-type strain, demonstrating a redundancy of the systems. Growth analysis of a quadruple mutant and four BCCT triple mutants demonstrated the requirement for at least one BCCT for efficient CS uptake. We complemented Escherichia coli MHK13, a CS synthesis- and transporter-negative strain, with each BCCT and examined CS uptake by growth analysis and 1H nuclear magnetic resonance (NMR) spectroscopy analyses. These data demonstrated that VP1456 had the most diverse substrate transport ability, taking up glycine betaine (GB), proline, choline, and ectoine. VP1456 was the sole ectoine transporter. In addition, the data demonstrated that VP1723 can transport GB, proline, and choline, whereas VP1905 and VPA0356 transported only GB. Overall, the data showed that the BCCTs are functional and that there is redundancy among them.
INTRODUCTION
Vibrio parahaemolyticus is a Gram-negative halophile inhabiting a wide range of aquatic ecosystems and causes bacterium-related seafood gastroenteritis in humans. Recent years have seen an increase in the incidence of infections worldwide, mostly during the warmer summer months (1–3). Vibrio parahaemolyticus is found in estuarine and marine environments as free-living organisms or in associations with fish and other marine species (4–7). Human infections occur usually through consumption of contaminated shellfish such as oysters. Vibrio parahaemolyticus is generally faced with salt concentrations of 3.5% salinity (35 ppt), but in estuarine systems and in shallow oyster beds during the summer months, this concentration can be much lower or higher. Salinity shifts in the environment pose tremendous osmotic challenges to bacteria that must respond swiftly by equating their intracellular osmotic potential with that of the external environment in order to maintain the positive turgor pressure required for normal growth (8–10). This is typically achieved via two strategies: the accumulation of inorganic ions, such as potassium ions (K+), and the accumulation of low-molecular-weight organic compounds, termed compatible solutes (CSs), which can be amassed in high concentrations without disturbing vital cellular function (8–10). The accumulation of CSs such as trehalose; free amino acids such as glutamate, glutamine, and proline; and their derivatives glycine betaine (GB) and ectoine is achieved either by de novo synthesis or via uptake from the surrounding environment by specific transporters (8–13). The transport of CSs is a strategy that is widespread among distinct evolutionary taxa of bacteria and has a lower energetic cost to the cell than de novo synthesis (8–13).
We previously described the presence of six putative CS transporter systems and two CS biosynthesis systems within the genome of Vibrio parahaemolyticus (14–17). The biosynthesis cluster ectABC-aspK, required for ectoine synthesis from an aspartic acid precursor, is present on chromosome I, and the betIBA cluster, required for GB synthesis from choline, is present on chromosome II (16, 17). A previous study by Naughton and colleagues using one-dimension proton nuclear magnetic resonance (1H NMR) demonstrated that at high salinity, V. parahaemolyticus was capable of de novo synthesis of ectoine, whereas an ΔectB knockout strain was not (16). In V. parahaemolyticus, both ectoine and GB are bona fide CSs (that is, they cannot be used as carbon sources), and the most effective CSs or precursors for this bacterium are betaine > choline > proline ≥ glutamate > ectoine (17). Expression analysis showed that the ectA and betA biosynthesis genes were more highly induced in log-phase cells and were also induced by NaCl upshock (17).
Four of the six CS transport systems identified in V. parahaemolyticus belong to the betaine-carnitine-choline transporter (BCCT) family, and two are members of the ATP-binding cassette (ABC) family, also known as ProU (ProU1, contained in chromosome I, and ProU2, contained in chromosome II) (16). Three out of four BCCTs (VP1456, VP1723, and VP1905) are located in chromosome I, while VPA0356 is found in chromosome II (16). Members of the BCCT family use proton/sodium-motive force to translocate substrates across the membrane and display certain distinguishing features: they transport quaternary ammonium compounds and encode a protein of 500 amino acid residues on average, organized into 12 putative transmembrane domains (13). Furthermore, they possess a conserved stretch of tryptophan residues in their eighth transmembrane domain (TM8), which is believed to be involved in the binding of substrates (18). Studies of the roles of individual BCCTs in osmotolerance in a number of bacteria have been performed (13, 19–23). For example, in Corynebacterium glutamicum and Bacillus subtilis, the BCCTs BetP and OpuD were shown to be osmotically induced and had a high affinity for GB (19, 20). Previous studies with Escherichia coli showed that BetT mediated high-affinity uptake of choline (24). In Vibrio cholerae, OpuD (VC1279), the only BCCT present in this species, was found to play a role in the uptake of GB under conditions of high osmolarity (21). In Pseudomonas aeruginosa, among the three BCCTs present in this organism, BetT3 was shown to function as the major choline transporter under hyperosmolar conditions (22). This species can also utilize choline and GB as carbon sources, which has been proposed to be an important phenotype for murine lung infection (25–27).
The function of each of the four BCCTs in V. parahaemolyticus is unknown. To address this, we first investigated the role of VP1456, VP1723, VP1905, and VPA0356 in the osmotic stress response by examination of the expression patterns of each of the transporters after high-salt upshock. Next, using deletion mutant analyses of each of the systems, we determined whether all or any of these transporters are essential for the osmotic shock response. Finally, by heterologous expression analysis of E. coli MKH13 and 1H NMR spectroscopy, we examined individual BCCTs for the uptake of GB, proline, choline, and ectoine.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. A streptomycin-resistant (Smr) clinical isolate of V. parahaemolyticus, RIMD2210633, was used as the wild type (WT) (28, 29). This strain was used to construct several mutant strains harboring either a single or multiple in-frame deletions in their BCCT genes. Unless otherwise stated, all strains were routinely cultured aerobically (250 rpm) at 37°C in either lysogeny broth (LB) medium (Fisher Scientific, Fair Lawn, NJ) or defined M9 salts base minimal medium (47.8 mM Na2HPO4, 22 mM KH2PO4, 18.7 mM NH4Cl, 8.6 mM NaCl) (Sigma) supplemented with 2 mM MgSO4, 0.1 mM CaCl2, and 0.4% (wt/vol) glucose (final concentration) as the sole carbon source (designated M9G medium). Genetic manipulations were done by using E. coli strains MKH13 and DH5α-λpir and the diaminopimelic acid (DAP) auxotrophic strain β2155-λpir. The E. coli β2155 DAP auxotroph was grown on medium supplemented with 0.3 mM DAP (Sigma-Aldrich). The following antibiotics were used: ampicillin (100 μg/ml), chloramphenicol (Cm) (25 μg/ml), and streptomycin (Sm) (200 μg/ml). The CSs betaine, choline, ectoine, proline, and l-carnitine were sterilized by filtration with a 0.2-μm filter (Corning, NY) and added to the growth medium at a concentration of 500 μM.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype(s) and/or description | Reference |
|---|---|---|
| Strains | ||
| V. parahaemolyticus | ||
| RIMD 2210633 | WT O3:K6 clinical isolate; Smr | 29 |
| SOY1456 (ΔVP1456) | WT with a VP1456 deletion | This study |
| SOY1723 (ΔVP1723) | WT with a VP1723 deletion | This study |
| SOY1905 (ΔVP1905) | WT with a VP1905 deletion | This study |
| SOYA0356 (ΔVPA0356) | WT with a VPA0356 deletion | This study |
| SOYBCCT12 | WT with ΔVP1456 ΔVP1723 | This study |
| SOYBCCT124 | WT with ΔVP1456 ΔVP1723 ΔVPA0356 | This study |
| SOYBCCT123 | WT with ΔVP1456 ΔVP1723 ΔVP1905 | This study |
| SOYBCCT13 | WT with ΔVP1456 ΔVP1905 | This study |
| SOYBCCT34 | WT with ΔVP1905 ΔVPA0356 | This study |
| SOYBCCT134 | WT with ΔVP1456 ΔVP1905 ΔVPA0356 | This study |
| SOYBCCT234 | WT with ΔVP1723 ΔVP1905 ΔVPA0356 | This study |
| SOYBCCT1342 | WT with ΔVP1456 ΔVP1723 ΔVP1905 ΔVPA0356 | This study |
| Escherichia coli | ||
| DH5α-λpir | λpir ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 hsdR17 deoR thi-1 supE44 gyrA96 relA1 | |
| DH5α-λpir(pJETΔVP1456) | pJET-ΔVP1456 transformed into E. coli DH5α-λpir | This study |
| DH5α-λpir(pJETΔVP1723) | pJET-ΔVP1723 transformed into E. coli DH5α-λpir | This study |
| DH5α-λpir(pJETΔVP1905) | pJET-ΔVP1905 transformed into E. coli DH5α-λpir | This study |
| DH5α-λpir(pJETΔVPA0356) | pJET-ΔVPA0356 transformed into E. coli DH5α-λpir | This study |
| β2155 DAP auxotroph | Donor for bacterial conjugation; thr1004 pro thi strA hsdS lacZΔM15 (F′ lacZΔM15 lacTRQJΔ36 proA+ proB+) ΔdapA Ermr pirRP4 (Kmr from SM10) | |
| β2155(pDSΔVP1456) | pDSΔVP1456 transformed into E. coli β2155 | This study |
| β2155(pDSΔVP1723) | pDSΔVP1723 transformed into E. coli β2155 | This study |
| β2155(pDSΔVP1905) | pDSΔVP1905 transformed into E. coli β2155 | This study |
| β2155(pDSΔVPA0356) | pDSΔVPA0356 transformed into E. coli β2155 | This study |
| MKH13 | MC4100 (ΔbetTIBA) Δ(putPA)101 Δ(proP)2 Δ(proU); Spr | 31 |
| MKH13(pBBVP1456) | pBBVP1456 transformed into MKH13 | This study |
| MKH13(pBAVP1723) | pBAVP1723 transformed into MKH13 | This study |
| MKH13(pBAVP1905) | pBAVP1905 transformed into MKH13 | This study |
| MKH13(pBAVPA0356) | pBAVPA0356 transformed into MKH13 | This study |
| Plasmids | ||
| pDS132 | R6K γori mobRP4 sacB Cmr; suicide vector | 38 |
| pDSΔVP1456 | ΔVP1456 cloned into pDS132 suicide vector | This study |
| pDSΔVP1723 | ΔVP1723 cloned into pDS132 suicide vector | This study |
| pDSΔV1905 | ΔV1905 cloned into pDS132 suicide vector | This study |
| pDSΔVPA0356 | ΔVPA0356 cloned into pDS132 suicide vector | This study |
| pJET1.2 | General cloning vector (Fermentas); Ampr | |
| pJETΔVP1456 | ΔVP1456 cloned into pJET1.2 vector | This study |
| pJETΔVP1723 | ΔVP1723 cloned into pJET1.2 vector | This study |
| pJETΔVP1905 | ΔVP1905 cloned into pJET1.2 vector | This study |
| pJETΔVPA0356 | ΔVPA0356 cloned into pJET1.2 vector | This study |
| pBBR1MCS | Broad-host-range cloning vector; Cmr | 39 |
| pBBVP1456 | VP1456 cloned into pBBR1MCS | This study |
| pBAD33 | Expression vector; AraC Cmr | |
| pBAVP1723 | VP1723 cloned into pBAD33 | This study |
| pBAVP1905 | VP1905 cloned into pBAD33 | This study |
| pBAVPA0356 | VPA0356 cloned into pBAD33 | This study |
Bacterial growth analysis.
A single bacterial colony was used to inoculate M9G medium containing 1% (wt/vol) NaCl (M9G–1% NaCl) and grown overnight at 37°C. A 2% inoculum of the culture grown overnight was subsequently used to inoculate M9G–1% NaCl medium and grown at 37°C to log phase for 5 h. A 0.1% aliquot of the 5-h culture was used to inoculate 200 μl of M9G–6% NaCl in the absence or presence of CSs. Growth analysis was carried out at 37°C with intermittent shaking for 24 h by using a Tecan Sunrise microplate reader (Tecan US Inc., Durham, NC). Samples were analyzed in triplicate, and at least two biological replicates were performed. The optical densities at 595 nm (OD595) of bacterial cultures were plotted by using Prism 5 (graphing and data analysis software) as averages of means of data from two biological replicates.
Mutant construction.
Vibrio parahaemolyticus RIMD2210633 strains harboring a single or multiple in-frame, nonpolar deletions in the BCCT genes were constructed by splicing by overlap extension (SOE) PCR and homologous recombination (30). SOE PCR primers were designed to create deletions in VP1456, VP1723, VP1905, and VPA0356 (Table 2) (16, 17). Briefly, by using V. parahaemolyticus RIMD2210633 genomic DNA as a template, two PCR products (AB and CD) of the BCCT gene of interest were generated by using primer pairs SOE A and SOE B and SOE C and SOE D in the first PCR. In the second PCR, using primer pair SOE A and SOE D, the fragments generated from the first round of PCR were spliced together to create a truncated version of the gene via their overlapping complementary tag sequences. The resultant truncated fragment was ligated into the suicide vector pDS132 and transformed into E. coli β2155, a DAP auxotroph strain. Recombinant plasmid pDS132 containing the truncated fragment was then introduced into V. parahaemolyticus RIMD2210633 by conjugation on an LB–1% NaCl–DAP agar plate. Transconjugant colonies were streaked onto LB–3% NaCl agar plates supplemented with Sm and Cm. Colony PCR was performed with SOE A and SOE D and flanking primer pairs (SOE FL-F and SOE FL-R). A single bacterial colony was subsequently selected and cultured aerobically at 37°C for 8 h in LB–3% NaCl containing Sm and Cm. A 0.1% aliquot of this culture was used to inoculate LB–3% NaCl broth lacking antibiotics and grown overnight. The culture grown overnight was serially diluted and plated onto LB–3% NaCl agar plates supplemented with Sm and sucrose. Colonies resulting from homologous recombination that replaced the wild-type gene with a truncated version were passaged onto selective sucrose agar plates. The recombinant clones that had undergone a double crossover were confirmed by colony PCR using primers SOE FL-F and SOE FL-R and DNA sequencing. The protocol described above was repeated to create all the BCCT mutants (Table 1). To create double and triple BCCT mutants, a single or double BCCT mutant was used as the background, and the process was repeated at the conjugation stage to introduce the desired deletion combinations. For the construction of the BCCT quadruple mutant, a triple mutant was used as the background, and the selection of the double-crossover colonies was carried out on agar plates in the presence of NaCl and sucrose concentration gradients.
TABLE 2.
Primers used in this studya
| Target | Primer | Sequence (5′→3′) |
|---|---|---|
| Cloning | ||
| VP1456 (BCCT1) | VP1456F (XbaI) | TCTAGATCTTGTGAGTTGAAGACACTTG |
| VP1456R (HindIII) | AAGCTTAAAAATGCCGAGCAATGAAT | |
| VP1723 (BCCT2) | VP1723F (XbaI) | TCTAGAAACTTGTGCTTGGTGATGTG |
| VP1723R (SacI) | GAGCTCACGGCACACTTTCGCATG | |
| VP1905 (BCCT3) | VP1905F (KpnI) | GAGAGGTACCGATCTTCCGCTTTCAC |
| VP1905R (PstI) | GAGACTGCAGAGCAGGGTGCTGGCTTC | |
| VPA0356 (BCCT4) | VPA0356F (XbaI) | TCTAGAAGCGGCTTTTTGAACATCCT |
| VPA0356R (HindIII) | AAGCTTCCAATTAAGGGCTCTTTGCAT | |
| SOE PCR | ||
| VP1456 | VP1456A (XbaI) | TCTAGAATCAATGGGGACAGCGATAA |
| VP1456B | cagctgagatctggtaccCGAAGCGAATTTTATCACCAA | |
| VP1456C | ggtaccagatctcagctgCGTACCGAACTTTCCGCTTA | |
| VP1456D (SacI) | GAGCTCCAACCATTTTCGCGTTTGTTC | |
| VP1456FL-F | GTCGATTACAATGGCGGATT | |
| VP1456FL-R | GTGGCACATTGTGAATGCTC | |
| VP1723 | VP1723A (XbaI) | TCTAGAACGATATGGTCTGCCAGCTT |
| VP1723B | cagctgagatctggtaccGGGACGTTTAATCCCACCAT | |
| VP1723C | ggtaccagatctcagctgGGTCTAATGGATGAACCTCGTC | |
| VP1723D (SacI) | GAGCTCCCAATTTCTGGATAAAGCACCC | |
| VP1273FL-F | TGCGCTTTTAAACACCATTG | |
| VP11723FL-R | ATGTCCAACGGAGGACAATC | |
| VP1905 | VP1905A (XbaI) | TCTAGAGAGGAACGATGACAAAGGGTA |
| VP1905B | cagctgagatctggtaccCGAGCCAAGACATGAATGAA | |
| VP1905C | ggtaccagatctcagctgATGTTTGATGTGCTGCCATTT | |
| VP1905D (SacI) | GAGCTCTTGATCGATTATTGACGCTCTG | |
| VP1905FL-F | AATAGCGCGGATGATCTGAC | |
| VP1905FL-R | TTGAATGCGCTTGCAATATC | |
| VPA0356 | VPA0356A (XbaI) | TCTAGACTTGATGTGAGGGGAAATGC |
| VPA0356B | cagctgagatctggtaccTGTGTCGATGTCTGGTTTCG | |
| VP0356C | ggtaccagatctcagctgATCATTTCGGTGCTGTTCTTG | |
| VP0356D (SacI) | GAGCTCTTTGCATTTTATGGGGTTGG | |
| VPA0356FL-F | GCCCACTTCAAACTGTCGTT | |
| VPA0356FL-R | CTCGATTCGATGTCATTCCA | |
| VP1456 | VP1456RT-F | GTTCGGTCTTGCGACTTCTC |
| VP1456RT-R | CCCATCGCAGTATCAAAGGT | |
| VP1723 | VP1723RT-F | AACAAAGGGTTGCCACTGAC |
| VP1723RT-R | TTCAAACCTGTTGCTGCTTG | |
| VP1905 | VP1905RT-F | TGGACGGTATTCTACTGGGC |
| VP1905RT-R | CGCCTAACTCGCCTACTTTG | |
| VPA0356 | VPA0356RT-F | CAAGGCGTAGGCCGCATGGT |
| VPA0356RT-R | ACCGCCCACGATGCTGAACC | |
| VPr001 (16S rRNA) | VPr001RT-F | ACCGCCTGGGGAGTACGGTC |
| VPr001RT-R | TTGCGCTCGTTGCGGGACTT |
Underlining indicates restriction enzyme sites; nucleotides in lowercase type indicate complementary overlaps. Abbreviations: F, forward; R, reverse; RT, reverse transcriptase; FL, flanking.
Functional complementation of E. coli strain MKH13 with VP1456, VP1723, VP1905, and VPA0356.
To clone each BCCT gene, V. parahaemolyticus RIMD2210633 genomic DNA was used as a template for PCR amplification of each BCCT gene with gene-specific primer pairs by using a Hot Start high-fidelity polymerase kit (Qiagen) (Table 2). The generated PCR product (∼1,500 bp in length, which included the endogenous promoters) was subcloned into pJET1.2 by using the CloneJET PCR cloning kit (Fermentas/Thermo Scientific), transformed into E. coli strain DH5α, and plated onto LB–1% NaCl–ampicillin agar. Colonies were screened by PCR, plasmid DNA was extracted and digested, and the purified fragment was ligated into pBBR1MCS in the case of VP1456 and pBAD33 in the cases of VP1723, VP1905, and VPA0356, each also digested with the same enzymes. The recombinant plasmids were transformed into E. coli MKH13 and grown on LB–1% NaCl supplemented with Cm. Colonies were screened by colony PCR and confirmed by DNA sequencing. The E. coli MKH13 strain is a derivative of MC4100 [F− araD139 Δ(argF-lac)U169 rpsL150 relA1 deoC1 ptsF25 rbsR flbB5301] (31). The large argF-lac deletion Δ(argF-lac)169 includes the betTIBA locus; thus, this strain cannot convert choline to GB or transport choline (31–33). The E. coli MKH13 genome contains additional deletions of genes encoding the PutP, ProP, and ProU transport systems; is unable to transport proline, GB, or choline; and cannot grow at 4% NaCl (31). Functional complementation experiments with E. coli MKH13 were first performed by growing cells overnight in M9G–1% NaCl supplemented with Cm. A 2.5% inoculum of this culture was used to inoculate 200 μl of M9G–4% NaCl–Cm containing 500 μM CS with and without arabinose. Bacterial cultivations were performed at 37°C, and the OD595 was measured hourly for a period of 24 h in a Tecan Sunrise microplate reader. For each condition tested, the sample was assayed in triplicate, with at least two biological replicates.
Determination of the affinity of the BCCTs for GB.
To determine the affinity of each BCCT for GB, growth analyses of E. coli MKH13 recombinant strains were performed in the presence of limiting GB concentrations of 0, 5, 25, 50, 75, 100, 125, and 150 μM. A 2.5% inoculum of cultures of E. coli MKH13 strains grown overnight in M9G–1% NaCl to an OD595 of ∼1.0 was used to inoculate M9G–4% NaCl medium containing 0.05% arabinose and GB. Cultures were incubated for 24 h, and the specific growth rates of the recombinant E. coli MKH13 strains for a given GB concentration were calculated.
Extraction and identification of intracellular organic solutes by 1H NMR.
1H NMR spectroscopy analysis was performed on cellular extracts of E. coli MKH13(pBCCT). For 1H NMR experiments examining ectoine and choline uptake in E. coli MKH13(pBCCT), a single colony was used to inoculate M9G–1% NaCl and grown aerobically at 37°C to an OD595 of ∼0.5. The cells were pelleted by centrifugation, washed with M9G–1% NaCl medium, and subjected to 1 h of osmotic upshock in M9G–4% NaCl supplemented with 500 μM ectoine or choline. Cells were centrifuged, and the pellet was washed once with M9G–4% NaCl medium. The pelleted cells were lysed by a series of freeze-thaw cycles (three times) in dry ice, and cellular extracts were solubilized in 750 μl of 99.99% ethanol (Sigma-Aldrich). After a 10-min centrifugation at 4,000 × g, ethanol fractions containing organic, soluble compounds free of cellular debris were transferred into clean tubes. Ethanol was removed via evaporation in a rotary evaporator. Organic materials were subsequently dissolved in 500 μl of deuterium oxide (D2O) solvent (Cambridge Isotope Laboratories Inc.), and insoluble materials were removed by centrifugation. Compatible solutes (soluble organic compounds) dissolved in D2O were transferred into a 5-mm NMR tube (Wilmad Lab Glass), and 1H NMR spectral data were recorded with a Bruker Avance AVIII 400-MHz NMR spectrometer equipped with a Bruker broadband fluorine observation (BBFO) probe. The spectrometer was run with Bruker Topspin software. NMR spectra were processed by using ACD/Lab processing software, academic edition (Advanced Chemistry Development Inc., Canada).
Isolation of RNA and gene expression analysis.
RNA isolation and VP1456, VP1723, VP1905, and VPA0356 gene expression analyses were performed on cells grown in LB–3% NaCl and under NaCl upshock growth conditions by using either log- or stationary-phase cells. Analyses and assays were carried out in accordance with Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (34). A single bacterial colony was used to inoculate LB–3% NaCl and grown aerobically overnight at 37°C. The resultant culture was diluted 1:50 in fresh LB–3% NaCl medium and grown at 37°C to log (4 h) or stationary (10 h) phase. Osmotic upshock experiments were performed on cultures harvested by centrifugation at 1,000 × g for 10 min and transferred into LB–6% NaCl or LB–9% NaCl medium for 30 min at 37°C with shaking. Total RNA was subsequently isolated by using the RNAprotect Bacteria reagent and RNeasy minikit (Qiagen) according to protocols recommended by the manufacturer. RNA was subjected to DNase treatment for 30 min at 37°C to remove DNA, followed by heat inactivation and subsequent DNase removal by centrifugation. Isolated RNA was quantified by using a Nanodrop spectrophotometer (Thermo Scientific) and examined by gel electrophoresis on 0.8% agarose to assess quality. In addition, a PCR assay was performed to check that no DNA was present. Next, first-strand cDNA synthesis was carried out by using 500 ng of total RNA as the template in a reaction primed with 200 ng of random hexamers (Invitrogen), using Superscript II reverse transcriptase (Invitrogen). Expression analysis of BCCT genes was performed with cDNA as the template by using fluorescence-based quantitative real-time PCR (qPCR), utilizing Hot Start-IT SYBR green qPCR master mix reagent (USB, Santa Clara, CA) and gene-specific primer pairs (Table 2). qPCR analysis was carried out with a 20-μl volume on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA) under the following cycling conditions: 95°C for 2 min for one cycle and 95°C for 10 s and 60°C for 30 s for 40 cycles. The quantitative cycle (Cq) values were determined, and the relative quantification of gene expression was calculated by the 2−ΔΔCq method with normalization across samples by using the 16S rRNA gene as a control (35). qPCR was performed with two technical replicates and at least two biological replicates.
Statistical analysis.
An unpaired Student t test was used to compare the means between two treatments (treated and untreated). One-way analysis of variance (ANOVA), followed by Bonferroni's multiple-comparison posttest, was used to analyze multiple groups. The significances of the data were computed as P values and are represented by asterisks in the figure legends. Graphing and statistical analyses of the data were performed by using Prism 5.
RESULTS
Four BCCT homologues present in V. parahaemolyticus.
Previously, we demonstrated by 1H NMR analysis that V. parahaemolyticus can accumulate GB, proline, choline, ectoine, and glutamate, but we did not examine the transporters involved (17). Bioinformatics analysis identified four single-component BCCTs in V. parahaemolyticus RIMD2210633, the open reading frames (ORFs) VP1456, VP1723, VP1905, and VPA0356 (16, 17). All four transporters are present in all sequenced V. parahaemolyticus genomes. Three of the four BCCTs, VP1456, VP1723, and VP1905, shared between 50 and 80% amino acid identities to each other. VP1456 and VP1905 shared the highest (80%) sequence identity, whereas VPA0356 shared the lowest sequence identity (∼30%) with the other three. VP1456 had the highest amino acid identity to the E. coli BCCT ProP protein, at 44%. VP1905 shared the highest amino acid identity with VC1279, the sole V. cholerae BCCT, at 82% identity, with VP1456 next at 68%, then VP1723 at 49%, and, finally, VPA0356 at 29%.
VP1456, VP1905, and VPA0356 are upregulated in LB medium upon NaCl upshock.
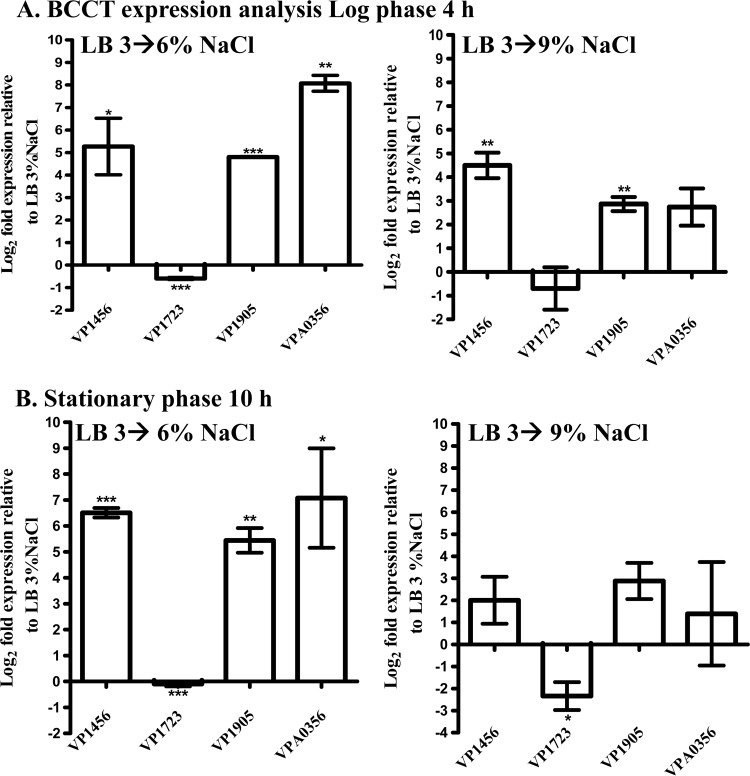
To address the question of whether the four putative BCCT genes in V. parahaemolyticus are induced by NaCl upshock, qPCR analysis was performed on cultures grown to log or stationary phase in LB–3% NaCl and subjected to 6% and 9% NaCl upshocks. It was found that under these conditions, VP1456, VP1905, and VPA0356 were induced upon NaCl upshock, while VP1723 was either unchanged or slightly downregulated (Fig. 1A and B). For instance, log-phase expression analyses upon upshock in 6% NaCl gave log2-fold induction increases of 5.2, 4.9, and 8.0 for VP1456, VP1905, and VPA0356, respectively (Fig. 1A). Log-phase expression analyses with a 9% NaCl upshock gave log2-fold change increases of 4.5 for VP1456 and 2.9 for VP1905 and VPA0356, and VP1723 was unchanged (Fig. 1A). Expression analysis of stationary-phase cells subjected to 6% and 9% NaCl upshocks yielded similar expression trends as those described above for VP1456, VP1905, and VPA0356, with 6.5-, 5.5-, and 7-log2-fold increases, respectively (Fig. 1B). The VP1723 gene was unchanged after 6% NaCl upshock but showed a 2.5-log2-fold decrease after a 9% NaCl upshock (Fig. 1B). Overall, these data demonstrated the induction of the VP1456, VP1905, and VPA0356 genes from log- and stationary-phase cells after NaCl upshock.
FIG 1.
Expression analysis of the BCCT genes in V. parahaemolyticus RIMD2210633 following NaCl upshock in LB medium. Cultures were grown in LB medium–3% NaCl to log phase (A) or stationary phase (B) and then subjected to 6% and 9% NaCl upshocks. These experiments were performed in duplicate, with two biological replicates. Changes in expression levels are relative to expression levels observed in either log-phase (A) or stationary-phase (B) cultures not subjected to osmotic upshock. The data were statistically analyzed by using an unpaired Student t test with a 95% confidence interval. The P values obtained are shown as asterisks, which denote the significant differences between upshocked samples and untreated controls. The error bars indicate means ± standard errors. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
BCCTs are redundant in V. parahaemolyticus.
To determine whether each the four BCCTs present in V. parahaemolyticus is essential, in-frame, nonpolar, single deletions of each of the genes were constructed. Analysis of the growth of each mutant in M9G–6% NaCl medium was examined in the absence or presence of CSs or their precursors. No difference in growth between the wild-type and mutant strains was observed under these conditions, indicating redundancy (data not shown). We next constructed double mutants to serve as backgrounds for the construction of BCCT triple and quadruple mutants. A double mutant was constructed in the ΔVP1456 (SOYBCCT1) single mutant background to create a ΔVP1456 ΔVP1723 strain (SOYBCCT12). V. parahaemolyticus triple mutant strains were constructed by using this double mutant to create the ΔVP1456 ΔVP1723 ΔVP1905 (BCCT123) and ΔVP1456 ΔVP1723 ΔVPA0356 (BCCT124) strains. A quadruple mutant could not be constructed by using either of these triple mutants. Therefore, we constructed two additional triple mutants, one in the BCCT13 and another in the BCCT23 double mutant backgrounds, creating the ΔVP1456 ΔVP1905 ΔVPA0356 (BCCT134) and ΔVP1723 ΔVP1905 ΔVPA0356 (BCCT234) strains, respectively. By using BCCT134 as a background strain, we were able to create a quadruple BCCT mutant, BCCT1342, by using a modified mutant construction protocol that included additional selection steps using NaCl and sucrose gradients. All mutants were tested with LB–3% NaCl and M9G–3% NaCl, and all mutants grew similarly to the wild type (data not shown).
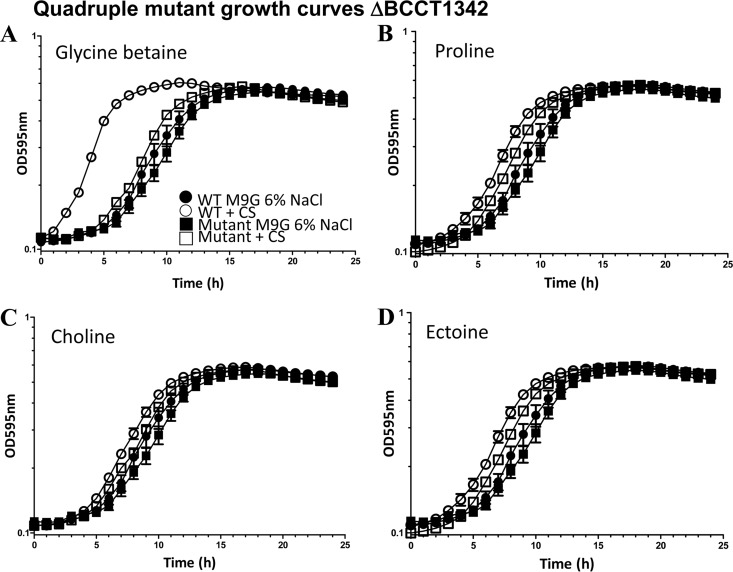
First, we performed growth curve analysis of the BCCT1342 quadruple mutant in M9G–6% NaCl in the absence or presence of GB, proline, choline, ectoine, or l-carnitine. In M9G–6% NaCl medium with no CS added, we obtained similar growth patterns for both the mutant and wild-type strains, with an extended lag phase of ∼6 to 7 h, indicating no overall defect in the mutant (Fig. 2A to D). In M9G–6% NaCl supplemented with GB, the lag time for the wild type was reduced from ∼6 h to ∼1 h, whereas for the quadruple mutant strain, the lag time was reduced marginally to ∼5 h. This result indicates that GB is not transported efficiently into the cell, suggesting that at least one of the BCCTs is required for its uptake (Fig. 2A). Growth curve analyses of the mutant in M9G–6% NaCl medium supplemented with choline, proline, or ectoine also yielded a slight reduction in the lag time but not to wild-type levels, indicating that these CSs are being transported but that a BCCT is required for efficient transport (Fig. 2B to D). Taken together, the growth curve analysis demonstrates that additional transporters are available for CS uptake but that a BCCT is required for the efficient transport of CS. We found that V. parahaemolyticus does not use l-carnitine as a CS (data not shown).
FIG 2.
Growth analyses of V. parahaemolyticus RIMD2210633 and quadruple mutant strain BCCT1342 (ΔVP1456 ΔVP1905 ΔVPA0356 ΔVP1723). Strains were grown in M9G–1% NaCl for 5 h and then inoculated into M9G–6% NaCl medium in the absence and presence of exogenously supplied CS. Data for growth analyses carried out for 24 h are shown in the graphs. Each assay was performed in triplicate, and data are shown as pooled data from two biological replicates. The error bars indicate means ± standard errors.
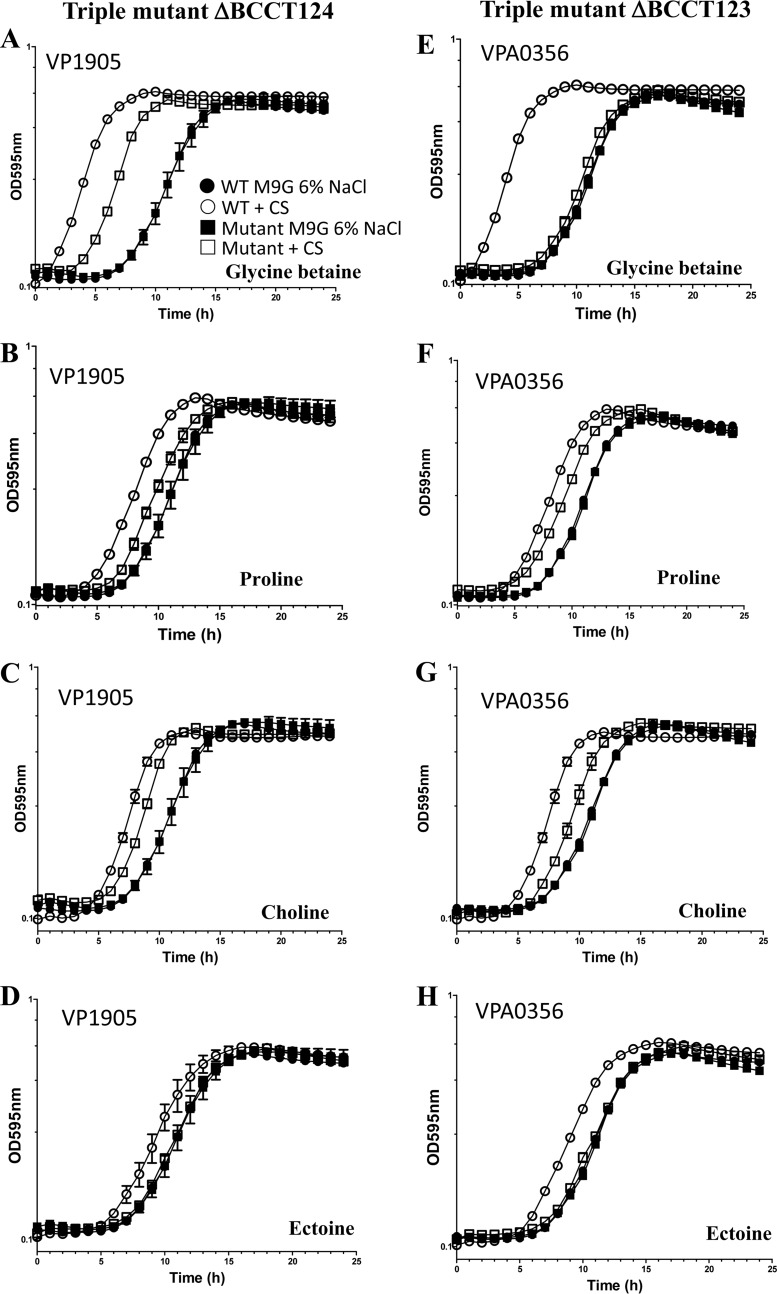
The construction of four different triple mutants allowed us to examine a single BCCT in the V. parahaemolyticus background. Growth curve analyses using M9G–6% NaCl medium with no CS yielded similar growth patterns, with an extended lag phase of 7 h for each strain, indicating no overall defect in the mutants compared to the wild type under these conditions (Fig. 3A to H and 4A to H). However, in M9G–6% NaCl supplemented with GB, the lag time for the wild type was reduced from 7 h to 1 h, whereas for BCCT124, which has only BCCT3 (VP1905) present, the lag time was reduced from 7 h to 4 h. This result indicates that GB is still being transported into the cell but not at the same level as in the wild type, suggesting that at least one additional transporter is required for maximum uptake (Fig. 3A). Since the reduction of the lag phase was greater than that of the quadruple mutant, this suggests that VP1905 is a transporter of GB. For triple mutant strain BCCT123, which has only BCCT4 (VPA0356) present, only a slight reduction in the lag time occurred with growth on GB, which indicates that VPA0356 transports this substrate with very low efficiency (Fig. 3E). In medium supplemented with proline or choline, a slight reduction in the lag time for both mutant strains was noted but again not to the same extent as for the wild type. However, the lag-phase shift in these mutants was similar to the shift in the quadruple mutant, suggesting that these BCCTs do not transport these CSs (Fig. 3B, C, F, and G). The addition of ectoine to the medium yielded no reduction of the lag time for either of the triple mutants, indicating that VP1905 and VPA0356 are not involved in ectoine uptake (Fig. 3D and H).
FIG 3.
Growth analyses of triple mutant strains BCCT124 and BCCT123. Strains were grown in M9G–1% NaCl for 5 h and then inoculated into M9G–6% NaCl medium in the absence and presence of exogenously supplied CS. Data for growth analyses carried out for 24 h are shown in the graphs. Each assay was performed in triplicate, and data are shown as the pooled data from two biological replicates. The error bars indicate means ± standard errors.
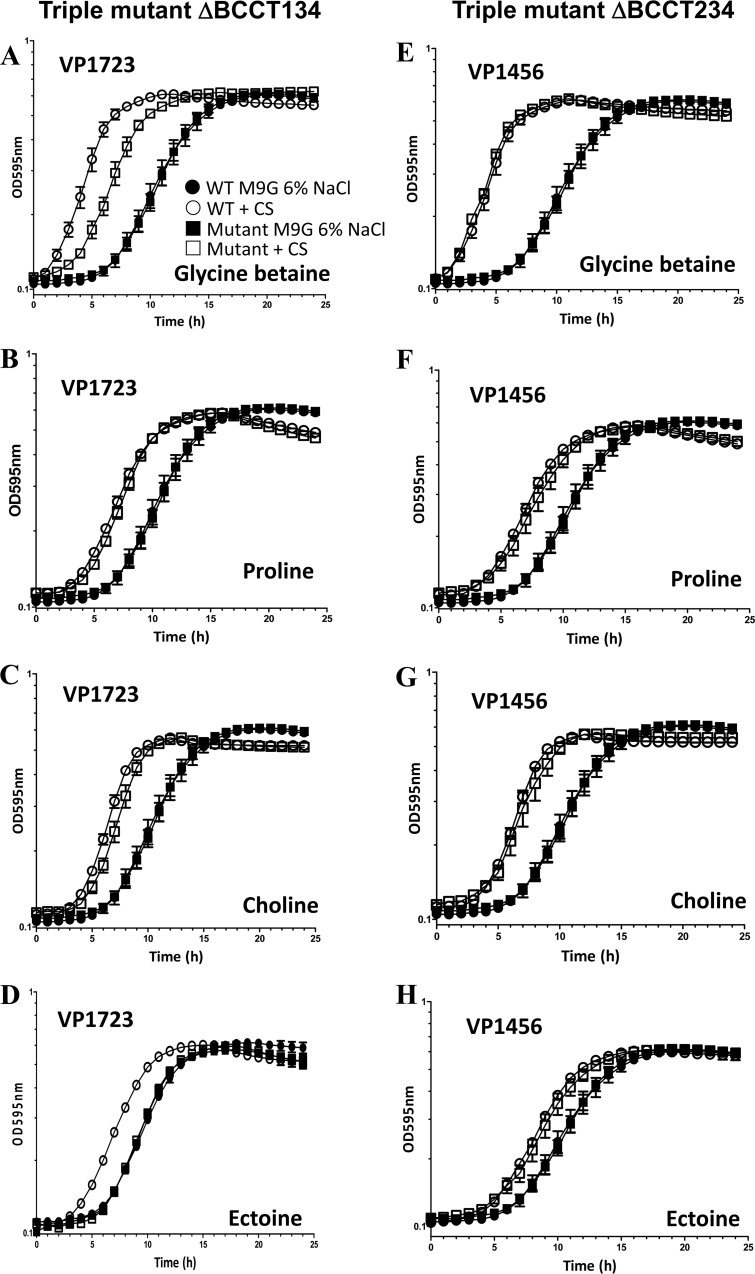
FIG 4.
Growth analyses of triple mutant strains BCCT134 and BCCT234. Strains were grown in M9G–1% NaCl for 5 h and then inoculated into M9G–6% NaCl medium in the absence and presence of exogenously supplied CS. Data for growth analyses carried out for 24 h are shown in the graphs. Each assay was performed in triplicate, and data are shown as the pooled data from two biological replicates. The error bars indicate means ± standard errors.
In the growth curve analysis of BCCT134, which contains BCCT2 (VP1723), in the presence of GB, the triple mutant showed a reduction in lag-phase growth. The shift in the lag phase was not to the wild-type level but was greater than that seen for the quadruple mutant, suggesting that VP1723 is a GB transporter and that an additional BCCT is required for efficient GB uptake (Fig. 4A). This mutant showed a nearly identical reduction in the lag phase in the presence of proline and choline, indicating that VP1723 is an important transporter of these compounds (Fig. 4B and C). No change in the lag phase was seen with the addition of ectoine (Fig. 4D). For triple mutant strain BCCT234, which contains an intact copy of VP1456, the addition of GB, proline, choline, or ectoine showed a reduction in the lag phase to the same extent as for the wild type (Fig. 4E to H), suggesting that VP1456 is also an important and efficient transporter of these CSs and has broad substrate specificity.
The BCCTs VP1456, VP1723, VP1905, and VPA0356 are CS transporters.
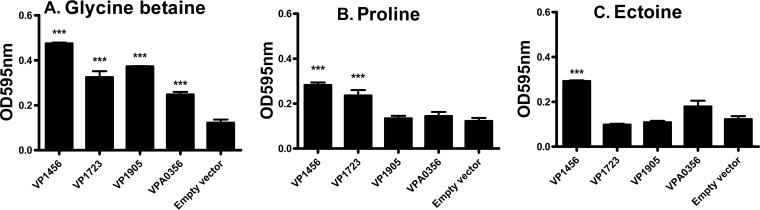
To further examine the transport abilities of each BCCT in relation to a given CS, functional complementation analyses of E. coli MKH13 were performed (Fig. 5). E. coli strain MKH13 is a ΔputPA ΔproP ΔproU mutant in an MC4100 background and is thus unable to transport proline, GB, and choline or convert choline to GB (31–33). Escherichia coli MKH13 grew in M9G–1% NaCl but not in 4% NaCl (data not shown). All complemented E. coli MKH13 strains, except for the negative empty-vector control, grew in the presence of GB (Fig. 5A). In the presence of proline, E. coli MKH13(pVP1456) or MKH13(pVP1723) grew significantly better than the control, indicating that these BCCTs can transport proline (Fig. 5B). Neither E. coli MKH13(pVP1905) nor MKH13(pVPA0356) grew in the presence of proline, suggesting that they cannot transport proline. Only E. coli MKH13(pVP1456) grew significantly better than the negative control in the presence of ectoine (Fig. 5C).
FIG 5.
Functional complementation of E. coli MKH13 harboring individual BCCT genes. E. coli MKH13 strains were grown in M9G–1% NaCl medium overnight and transferred into M9G–4% NaCl medium in the absence and presence of exogenously supplied CS. Data for growth analyses carried out for 24 h are shown in the graphs. For each condition tested, the sample was assayed in triplicate, with at least two biological replicates. (A) M9G–4% NaCl plus GB; (B) M9G–4% NaCl plus proline; (C) M9G–4% NaCl plus ectoine. The error bars represent means ± standard errors. ***, P ≤ 0.001.
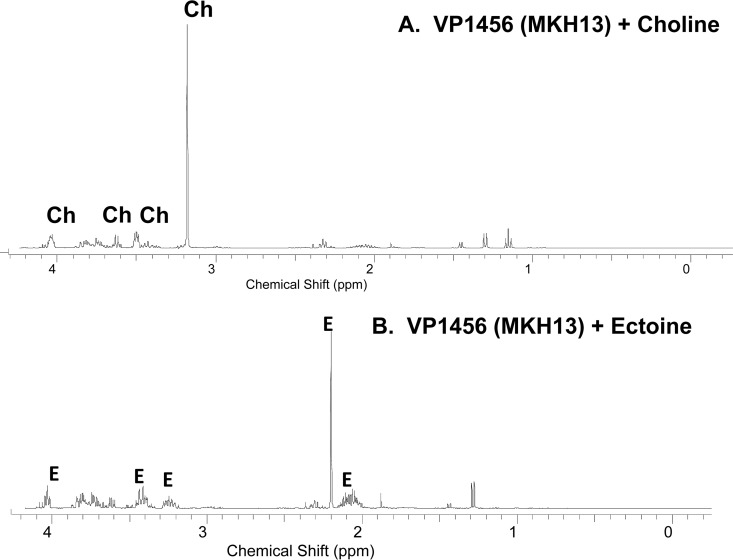
To determine whether any of the BCCTs take up choline, we performed 1H NMR analysis since choline accumulation is toxic to E. coli MKH13, as it cannot convert choline to GB. To perform these experiments, E. coli MKH13 cells transformed with individual BCCTs were grown in M9G–1% NaCl to early exponential phase and switched to M9G–4% NaCl supplemented with choline for 1 h, and 1H NMR analysis was then performed, which demonstrated that E. coli MKH13 cells transformed with VP1456 (but not VP1723, VP1905, or VPA0356) accumulated choline intracellularly (Fig. 6A). 1H NMR uptake analyses of recombinant E. coli MKH13 cells containing individual BCCTs in M9G–4% NaCl supplemented with ectoine also demonstrated accumulation of ectoine only in VP1456-transformed cells (Fig. 6B). These data confirm that VP1456 is the sole ectoine BCCT and that VP1456 has a broad substrate range in both V. parahaemolyticus and E. coli. The failure to show choline uptake for VP1723 in E. coli by 1H NMR analysis is likely the result of experimental differences in these assays. In the E. coli assay, only a 1-h time point was examined, which may be too short to show uptake for a low-affinity transporter, and functional differences between species may also play a part in the different outcomes.
FIG 6.
1H NMR spectroscopy of choline and ectoine transport in complemented E. coli MKH13 strains. 1H NMR spectra were acquired with a Bruker Avance III 400-MHz NMR spectrometer. The chemical shifts (δ) are expressed in ppm. The spectral peaks corresponding to the detected compounds of interest are labeled with the initials of the compound name on the top. 1H NMR experiments were performed at least twice, with two biological replicates. E, ectoine peaks; Ch, choline peaks.
VP1456, VP1723, and VP1905 transport GB with high efficiency.
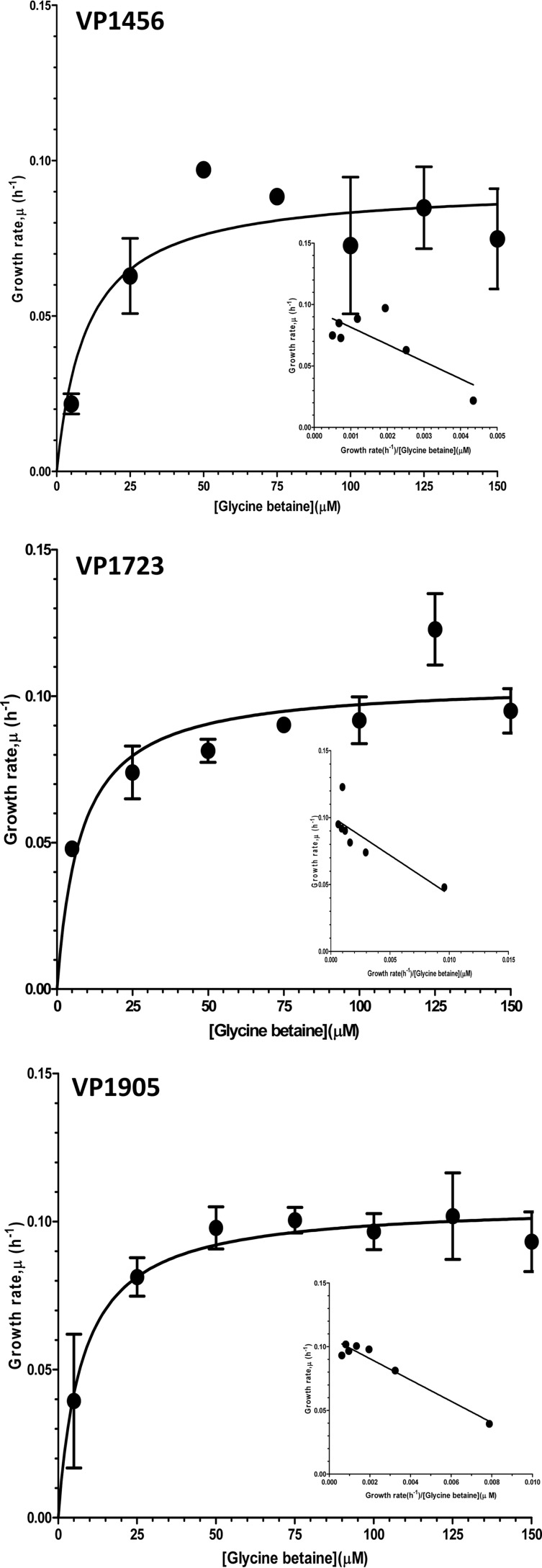
GB is one of the most commonly used CSs among bacteria. To examine whether the BCCTs take up GB with high or low efficiency, growth analyses of individual BCCTs in E. coli MKH13 in the presence of limiting concentrations of GB were performed. Using Monod's nonlinear regression and Eadie Hofstee linear plots, the parameters of bacterial growth kinetics at limiting GB concentrations were empirically determined, and the binding affinity of each BCCT was inferred (Fig. 7). It was found that VP1456 transported GB with high affinity, at a Ks of 5.9 ± 1.8 μM; VP1723 transported GB at a Ks of 14.0 ± 5.0 μM; and VP1905 transported GB at a Ks of 8.4 ± 0.9 μM (Fig. 7). Thus, the BCCT binding affinities for GB were in the descending order VP1456 > VP1905 > VP1723. VPA0356 showed very low affinity for GB relative to the three BCCTs mentioned above (data not shown).
FIG 7.
Determination of the affinity of BCCTs for GB. The affinity of each BCCT for GB was determined by growth analysis of recombinant E. coli MKH13 strains in the presence of limiting GB concentrations. Bacterial growth was monitored for 24 h, and the specific growth rates of the recombinant E. coli MKH13 strains for a given GB concentration were calculated.
DISCUSSION
The uptake and/or synthesis of CSs is paramount to the growth and survival of bacteria under high-salinity conditions (8, 9, 16, 17, 21, 36). Vibrio parahaemolyticus inhabits a wide range of ecosystems subjected to constant shifts in salinity. In this study, we investigated the specific role of four BCCTs in the osmotic stress tolerance response. We showed that three out of four BCCTs, VP1456, VP1905, and VPA0356, were induced by NaCl upshock but that VP1723 showed no change or reduced expression at high salinity. This suggests that salinity alone is not a trigger for the expression of VP1723 and that other factors are involved. For P. aeruginosa, it was shown that certain BCCTs are not induced at the level of transcription but at the level of transport activity, which may also be the case for VP1723 (22).
To investigate the function of the four BCCTs in the uptake of CS, single in-frame deletion mutants were created, but no differences in growth patterns were observed under the conditions examined. Therefore, we constructed a BCCT quadruple mutant that lacked all four BCCTs and four different triple mutants that each contained a single BCCT. Comparisons of the shifts in lag phases between the quadruple and triple mutants demonstrated that VP1456 transports GB, proline, and choline and is the sole transporter of ectoine. These data also demonstrated that VP1723 is an important transporter of GB, choline, and proline, whereas VP1905 and VPA0356 transported only GB, and VP1905 did so much more efficiently than VPA0356. The ability of the V. parahaemolyticus BCCT-negative strain to survive at high salinity similarly to the wild type may reflect the fact that two functional CS synthesis systems and two additional CS transporters are present in the genome (17). Heterologous gene expression analysis of E. coli MKH13 also demonstrated that all four BCCTs could transport GB. Thus, the ability of all four BCCTs to take up GB is not too surprising given that GB is the most abundant CS present in the environment and is one of the most effective CSs. Complementation of E. coli MKH13 with pVP1456 confirmed that this transporter had the broadest substrate range. Our triple mutant data indicate that VP1723 could take up choline; however, this was not shown in the E. coli MKH13 NMR studies. This difference probably reflects the different conditions under which the experiments were performed; the NMR choline uptake assay with E. coli was a 1-h assay, compared to a 24-h assay for the V. parahaemolyticus mutant strains. Our data showed that not all BCCTs are equal and that VP1456 and VP1905 had the highest affinities for GB, with affinity constants in the range of what was previously described for other bacterial species (8, 9, 36). The lower energetic cost associated with the uptake than with the synthesis of CSs provides a rationale for the evolutionary acquisition and retention of the multiple transport systems in V. parahaemolyticus. Having the ability to take up CS at low and high concentrations by using different transporters could be an important adaptive strategy to grow at different salt concentrations, such as those encountered in marine environments and in host species. Having multiple CS transporters may also be critical for the organism to adapt to other environmental stresses, such as temperature fluctuations and, perhaps, acid stress conditions. For example, we have previously shown that growth at high salinity preadapts V. parahaemolyticus to additional osmotic and acid stresses; we found that a short preadaptation to high salt concentrations increased the survival of the wild-type strain under lethal acid conditions (37). It will be of interest to determine whether these BCCT systems are required under other stress conditions.
ACKNOWLEDGMENTS
We thank Megan R. Carpenter, Brandy Haines-Menges, Sai Siddarth Kalburge, J. B. Lubin, and Abish Regmi for their careful review and helpful discussion of the manuscript. We thank Steve Bai at the Nuclear Magnetic Resonance Core Facility, Department of Chemistry and Biochemistry, University of Delaware, for help and assistance with NMR analysis and the Center of Biomedical Research Excellence (COBRE) in Membrane Protein Production and Characterization at the University of Delaware.
Research on Vibrio stress response mechanisms was supported by National Science Foundation grant IOS-0918429 to E.F.B.
REFERENCES
- 1.DePaola A, Nordstrom JL, Bowers JC, Wells JG, Cook DW. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl Environ Microbiol 69:1521–1526. doi: 10.1128/AEM.69.3.1521-1526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L. 2000. Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis 181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 3.Cabanillas-Beltran H, Llausas-Magana E, Romero R, Espinoza A, Garcia-Gasca A, Nishibuchi M, Ishibashi M, Gomez-Gil B. 2006. Outbreak of gastroenteritis caused by the pandemic Vibrio parahaemolyticus O3:K6 in Mexico. FEMS Microbiol Lett 265:76–80. doi: 10.1111/j.1574-6968.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- 4.Colwell RR, Kaper J, Joseph SW. 1977. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394–396. doi: 10.1126/science.910135. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko T, Colwell RR. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J Bacteriol 113:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneko T, Colwell RR. 1975. Incidence of Vibrio parahaemolyticus in Chesapeake Bay. Appl Microbiol 30:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krantz GE, Colwell RR, Lovelace E. 1969. Vibrio parahaemolyticus from the blue crab Callinectes sapidus in Chesapeake Bay. Science 164:1286–1287. doi: 10.1126/science.164.3885.1286. [DOI] [PubMed] [Google Scholar]
- 8.Csonka LN. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galinski EA. 1995. Osmoadaptation in bacteria. Adv Microb Physiol 37:272–328. [PubMed] [Google Scholar]
- 10.Oren A. 2008. Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:2. doi: 10.1186/1746-1448-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventosa A, Nieto JJ, Oren A. 1998. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pflüger K, Müller V. 2004. Transport of compatible solutes in extremophiles. J Bioenerg Biomembr 36:17–24. doi: 10.1023/B:JOBB.0000019594.43450.c5. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler C, Bremer E, Kramer R. 2010. The BCCT family of carriers: from physiology to crystal structure. Mol Microbiol 78:13–34. doi: 10.1111/j.1365-2958.2010.07332.x. [DOI] [PubMed] [Google Scholar]
- 14.Reen FJ, Almagro-Moreno S, Ussery D, Boyd EF. 2006. The genomic code: inferring Vibrionaceae niche specialization. Nat Rev Microbiol 4:697–704. doi: 10.1038/nrmicro1476. [DOI] [PubMed] [Google Scholar]
- 15.Boyd EF, Cohen AL, Naughton LM, Ussery DW, Binnewies TT, Stine OC, Parent MA. 2008. Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus. BMC Microbiol 8:110. doi: 10.1186/1471-2180-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naughton LM, Blumerman SL, Carlberg M, Boyd EF. 2009. Osmoadaptation among Vibrio species and unique genomic features and physiological responses of Vibrio parahaemolyticus. Appl Environ Microbiol 75:2802–2810. doi: 10.1128/AEM.01698-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ongagna-Yhombi SY, Boyd EF. 2013. Biosynthesis of the osmoprotectant ectoine, but not glycine betaine, is critical for survival of osmotically stressed Vibrio parahaemolyticus cells. Appl Environ Microbiol 79:5038–5049. doi: 10.1128/AEM.01008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. 2009. Molecular basis of transport and regulation in the Na+/betaine symporter BetP. Nature 458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 19.Kappes RM, Kempf B, Bremer E. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol 178:5071–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peter H, Burkovski A, Kramer R. 1996. Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J Bacteriol 178:5229–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapfhammer D, Karatan E, Pflughoeft KJ, Watnick PI. 2005. Role for glycine betaine transport in Vibrio cholerae osmoadaptation and biofilm formation within microbial communities. Appl Environ Microbiol 71:3840–3847. doi: 10.1128/AEM.71.7.3840-3847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malek AA, Chen C, Wargo MJ, Beattie GA, Hogan DA. 2011. Roles of three transporters, CbcXWV, BetT1, and BetT3, in Pseudomonas aeruginosa choline uptake for catabolism. J Bacteriol 193:3033–3041. doi: 10.1128/JB.00160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Beattie GA. 2008. Pseudomonas syringae BetT is a low-affinity choline transporter that is responsible for superior osmoprotection by choline over glycine betaine. J Bacteriol 190:2717–2725. doi: 10.1128/JB.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamark T, Kaasen I, Eshoo MW, Falkenberg P, McDougall J, Strom AR. 1991. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol Microbiol 5:1049–1064. doi: 10.1111/j.1365-2958.1991.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 25.Wargo MJ. 2013. Choline catabolism to glycine betaine contributes to Pseudomonas aeruginosa survival during murine lung infection. PLoS One 8:e56850. doi: 10.1371/journal.pone.0056850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wargo MJ. 2013. Homeostasis and catabolism of choline and glycine betaine: lessons from Pseudomonas aeruginosa. Appl Environ Microbiol 79:2112–2120. doi: 10.1128/AEM.03565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wargo MJ, Szwergold BS, Hogan DA. 2008. Identification of two gene clusters and a transcriptional regulator required for Pseudomonas aeruginosa glycine betaine catabolism. J Bacteriol 190:2690–2699. doi: 10.1128/JB.01393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitaker WB, Parent MA, Naughton LM, Richards GP, Blumerman SL, Boyd EF. 2010. Modulation of responses of Vibrio parahaemolyticus O3:K6 to pH and temperature stresses by growth at different salt concentrations. Appl Environ Microbiol 76:4720–4729. doi: 10.1128/AEM.00474-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makino K, Oshima K, Kurokawa K, Yokoyama K, Uda T, Tagomori K, Iijima Y, Najima M, Nakano M, Yamashita A, Kubota Y, Kimura S, Yasunaga T, Honda T, Shinagawa H, Hattori M, Iida T. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 30.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 31.Haardt M, Kempf B, Faatz E, Bremer E. 1995. The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol Gen Genet 246:783–786. doi: 10.1007/BF00290728. [DOI] [PubMed] [Google Scholar]
- 32.Peters JE, Thate TE, Craig NL. 2003. Definition of the Escherichia coli MC4100 genome by use of a DNA array. J Bacteriol 185:2017–2021. doi: 10.1128/JB.185.6.2017-2021.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casadaban MJ. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol 104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 34.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Kempf B, Bremer E. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 170:319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- 37.Kalburge SS, Whitaker WB, Boyd EF. 2014. Salinity adaptation of the human pathogen Vibrio parahaemolyticus to lethal environmental conditions. J Food Prot 77:246–253. doi: 10.4315/0362-028X.JFP-13-241. [DOI] [PubMed] [Google Scholar]
- 38.Philippe N, Alcaraz JP, Coursange E, Geiselmann J, Schneider D. 2004. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51:246–255. doi: 10.1016/j.plasmid.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Kovach ME, Phillips RW, Elzer PH, Roop RM II, Peterson KM. 1994. pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16:800–802. [PubMed] [Google Scholar]