Abstract
Polyhydroxyalkanoates (PHAs) are synthesized and assembled as PHA granules that undergo well-regulated formation in many microorganisms. However, this regulation remains unclear in haloarchaea. In this study, we identified a PHA granule-associated regulator (PhaR) that negatively regulates the expression of both its own gene and the granule structural gene phaP in the same operon (phaRP) in Haloferax mediterranei. Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) assays demonstrated a significant interaction between PhaR and the phaRP promoter in vivo. Scanning mutagenesis of the phaRP promoter revealed a specific cis-element as the possible binding position of the PhaR. The haloarchaeal homologs of the PhaR contain a novel conserved domain that belongs to a swapped-hairpin barrel fold family found in AbrB-like proteins. Amino acid substitution indicated that this AbrB-like domain is critical for the repression activity of PhaR. In addition, the phaRP promoter had a weaker activity in the PHA-negative strains, implying a function of the PHA granules in titration of the PhaR. Moreover, the H. mediterranei strain lacking phaR was deficient in PHA accumulation and produced granules with irregular shapes. Interestingly, the PhaR itself can promote PHA synthesis and granule formation in a PhaP-independent manner. Collectively, our results demonstrated that the haloarchaeal PhaR is a novel bifunctional protein that plays the central role in the regulation of PHA accumulation and granule formation in H. mediterranei.
INTRODUCTION
Polyhydroxyalkanoates (PHAs) are biodegradable polyesters synthesized by most genera of bacteria (1, 2) and some archaea (3–5). PHAs are accumulated as storage compounds of energy and carbon under imbalanced growth conditions (i.e., when nutrients such as nitrogen, phosphorus, or oxygen are limited but the carbon sources are in excess) (6).
PHAs are often deposited in the cytoplasm as water-insoluble inclusions that are called PHA granules (6). Native PHA granules are found to be composed of 97.5% PHA, 2% proteins, and likely some amount of lipids (7). At least four types of proteins were found to be the PHA granule-associated proteins (PGAPs) in bacteria: PHA synthases, PHA depolymerases, regulators, and structural proteins (phasins [PhaPs]) (8, 9). In recent years, increasing new roles have been found for the PGAPs. Besides the classical phasin role of preventing PHA granules from coalescing, two distinct phasin-like proteins, PhaM and PhaF, have also been characterized as being crucial for granule distribution during cell division (10, 11).
The PGAPs play important roles in PHA synthesis, PHA utilization, and granule formation and distribution (8, 9, 12), among which the regulatory proteins are responsible for ensuring the proper formation of PHA granules by influencing the expression of both phasins and themselves (13–17). A classic regulation model was presented in a poly(3-hydroxybutyrate) (PHB [a type of PHA])-accumulating bacterium, Ralstonia eutropha H16 (9). Briefly, the cytoplasmic regulator PhaR could bind to the promoter of phaP as well as the promoter of its own gene to repress their transcription. When cells start accumulating PHA, PhaR attaches to the PHA granules, which results in a lower cytoplasmic PhaR level. The block of the expression of phaP and phaR is released, and the cells start synthesizing more PhaP and PhaR to coat the growing PHA granules. PhaP is usually more abundant than PhaR and possesses a higher hydrophobic affinity to PHA granules. When the PHA granules reach a proper size, there is no more room on PHA granules for the excess PhaR to attach. The cytoplasmic PhaR concentration returns to a higher level to resume the repression of the transcription of both phaP and phaR. This tight regulation by PhaR ensures a well-organized granule formation process, in which sufficient PhaP proteins are produced to coat the newly synthesized PHAs, with few free PhaP present in the cytoplasm (9).
Unlike bacterial PGAPs, there has been little study of the archaeal PGAPs until recently. In our previous studies, five PGAPs were identified in a haloarchaeon, Haloferax mediterranei, which accumulates poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV [a type of PHA]) and shows potential for industrial applications (18–20). Besides the PHA synthase subunits (PhaC and PhaE) and a putative enoyl coenzyme A (enoyl-CoA) hydratase (MaoC), two conserved hypothetical proteins (encoded by HFX_5218 and HFX_5219) were also separated from the PHA granules of H. mediterranei. The protein encoded by HFX_5219 was identified to be the major phasin (PhaP) that could prevent the aggregation of PHA granules (20). HFX_5218 encodes a small protein that was temporarily named GAP12 (12.0 kDa). The gap12 gene was revealed to be cotranscribed with phaP, but its function is still unknown (20). Characterization of the GAP12 separated from PHA granules might provide important hints for the exploration of the regulation of PHA biosynthesis and granule formation in haloarchaea.
In this study, using a combined approach of gene expression, gene knockout, promoter activity analysis, and a chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) assay, the GAP12 protein was identified as a regulator and renamed PhaR, which directly binds to the promoter of phaRP and negatively regulates this operon. In addition, the cis-elements of the phaRP promoter were identified by site-directed mutagenesis, and the effects of PhaR on the PHA accumulation and granule formation were further demonstrated by gas chromatography and electron microscopy analyses. Therefore, the identification and characterization of the haloarchaeal type of phasin regulator PhaR, which is phylogenetically distinct from the bacterial counterpart, have provided new insights into the regulation of PHA synthesis in haloarchaea.
MATERIALS AND METHODS
Strains and culture conditions.
The strains used in this study are listed in Table 1. Escherichia coli JM109 was used for cloning procedures and was grown in lysogeny broth (LB) medium at 37°C (21). H. mediterranei DF50, a uracil-auxotrophic (ΔpyrF) strain of H. mediterranei ATCC 33500 (22), and its derivative mutants were cultivated at 37°C in nutrient-rich AS-168L medium (20). H. mediterranei strains carrying expression plasmids were cultivated in AS-168SYL medium (with yeast extract omitted from AS-168L) (20). For PHA accumulation analysis, the culture procedures were similar to those described previously (20). Briefly, H. mediterranei was first grown in AS-168L for 2 days and then was inoculated into a modified PHA production medium, named MGF medium, containing (per liter) 110 g NaCl, 9.6 g MgCl2, 14.4 g MgSO4, 5 g KCl, 1 g CaCl2, 3 g yeast extract, 2 g NH4Cl, 0.0375 g KH2PO4, 10 g glucose, 15 g PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 0.008 g NH4+-Fe(III) citrate, and 1 ml trace element solution SL-6 (pH 7.2) (18). For AS-168SYL seed cultures, yeast extract was also omitted from the MGF medium. When needed, ampicillin, uracil, and 5-fluoroorotic acid (5-FOA) were added to the medium at final concentrations of 100, 50, and 250 mg/liter, respectively.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | recA1 supE44 endA1 hsdR7 gyrA96 relA1 thi | 21 |
| BL21(DE3) | F− ompT hsdSB(rB− mB−) dcm gal (DE3) | Novagen |
| H. mediterranei | ||
| DF50 | pyrF deletion mutant of H. mediterranei ATCC 33500 | 22 |
| ΔphaP mutant | phaP deletion mutant of H. mediterranei DF50 | 20 |
| ΔphaR mutant | phaR deletion mutant of H. mediterranei DF50 | This study |
| ΔphaRP mutant | phaRP deletion mutant of H. mediterranei DF50 | This study |
| ΔphaEC mutant | phaEC deletion mutant of H. mediterranei DF50 | This study |
| ΔphaPEC mutant | phaPEC deletion mutant of H. mediterranei DF50 | This study |
| ΔphaRPEC mutant | phaRPEC deletion mutant of H. mediterranei DF50 | This study |
| E24A | DF50 strain with PhaR carrying E24A mutation | This study |
| Q28A | DF50 strain with PhaR carrying Q28A mutation | This study |
| Q30A | DF50 strain with PhaR carrying Q30A mutation | This study |
| R32A | DF50 strain with PhaR carrying R32A mutation | This study |
| K67A | DF50 strain with PhaR carrying K67A mutation | This study |
| R75A | DF50 strain with PhaR carrying R75A mutation | This study |
| E82A | DF50 strain with PhaR carrying E82A mutation | This study |
| R83A | DF50 strain with PhaR carrying R83A mutation | This study |
| Plasmids | ||
| pHFX | 4.0-kb integration vector containing pyrF and its native promoter, Ampr | 22 |
| pWL502 | 7.8-kb expression vector containing pyrF and its native promoter, Ampr | 20 |
| pSCM307 | 8.2-kb shuttle vector containing promoter of hsp5 of Halobacterium sp. strain NRC-1, Ampr | 26 |
| pJAM1020 | 10.7-kb expression plasmid containing smRSGFP gene, Ampr | 24 |
| pM1915 | 8.8-kb expression vector pWL502 containing smRSGFP gene and mutated promoter of PTS | 28 |
| pGEM-T Easy | 3.0-kb cloning vector, Ampr | Promega |
| pET-28a | 5.4-kb IPTG-inducible expression vector with His6 tag | Novagen |
| pDR | 5.6-kb integration vector of pHFX for knockout of phaR | This study |
| pDRP | 5.6-kb integration vector of pHFX for knockout of phaRP | This study |
| pDEC | 5.3-kb integration vector of pHFX for knockout of phaEC | This study |
| pDPEC | 5.4-kb integration vector of pHFX for knockout of phaPEC | This study |
| pDRPEC | 5.4-kb integration vector of pHFX for knockout of phaRPEC | This study |
| pR-IN | 5.2-kb integration vector of pHFX for knock-in of phaR | This study |
| pRF | 8.7-kb expression vector pWL502 containing smRSGFP gene and the phaRP promoter (−151 to +17) | This study |
| pEF | 8.7-kb expression vector pWL502 containing smRSGFP gene and phaEC promoter | This study |
| pWLR | 8.4-kb expression vector pWL502 containing phaR and phaRP promoter | This study |
| pWLP | 8.5-kb expression vector pWL502 containing phaP and phaRP promoter | 20 |
| pWLRP | 8.8-kb expression vector pWL502 containing phaRP and phaRP promoter | This study |
| pHP | 8.4-kb expression vector pWL502 containing phaP and hsp5 promoter | This study |
| pHRRF | 9.2-kb pRF-derived vector for additional expression of phaR under hsp5 promoter | This study |
| pRmyc | 8.3-kb pWL502 derived vector, expressing phaR-myc under mutated PTS promoter from pM1915 | This study |
| pT-Rpro | 2.9-kb pGEM-T Easy-derived cloning vector of phaRP promoter | This study |
| pM1 to pM16 | 8.7-kb pRF-derived vectors with mutations introduced into phaRP promoter | This study |
| pD41 | 8.6-kb pRF-derived vector with phaRP promoter truncated (−41 to +17) | This study |
| pD86 | 8.6-kb pRF-derived vector with phaRP promoter truncated (−86 to +17) | This study |
| pET-28aR | 5.7-kb pET-28a-derived vector, expressing phaR-His6 | This study |
| pET-28aP | 5.8-kb pET-28a derived vector, expressing phaP-His6 | This study |
| pR-IN-E24A | 5.2-kb integration vector of pHFX for knock-in of phaR with E24A mutation | This study |
| pR-IN-Q28A | 5.2-kb integration vector of pHFX for knock-in of phaR with Q28A mutation | This study |
| pR-IN-Q30A | 5.2-kb integration vector of pHFX for knock-in of phaR with Q30A mutation | This study |
| pR-IN-R32A | 5.2-kb integration vector of pHFX for knock-in of phaR with R32A mutation | This study |
| pR-IN-K67A | 5.2-kb integration vector of pHFX for knock-in of phaR with K67A mutation | This study |
| pR-IN-R75A | 5.2-kb integration vector of pHFX for knock-in of phaR with R75A mutation | This study |
| pR-IN-E82A | 5.2-kb integration vector of pHFX for knock-in of phaR with E82A mutation | This study |
| pR-IN-R83A | 5.2-kb integration vector of pHFX for knock-in of phaR with R83A mutation | This study |
Construction of mutants.
The plasmids and primers used in this study are listed in Tables 1 and 2, respectively. The in-frame gene deletion and gene expression in H. mediterranei were carried out as previously described (20, 22, 23).
TABLE 2.
Oligonucleotides used in this study
| Primer | 5′→3′ sequencea |
|---|---|
| Gene knockout and knock-in | |
| phaR-DF1 | ATAGGTACCCGGTGTCACCTGGATT |
| phaR-DR1 | ATAGGATCCGTCGTTCGTCATCTCCT |
| phaR-DF2 | TATGGATCCAGTGAACAAGCCAACCC |
| phaR-DR2 | ATACTGCAGGGTCTCCTCTATCTCCTGT |
| phaRP-DF1 | GATGGTACCACCATCGGCGTTCGTAA |
| phaRP-DR1 | GCAGGATCCCTCCTAACTCGGTGTTGT |
| phaRP-DF2 | TCTGGATCCCTACAGGAGATAGAGGAG |
| phaRP-DR2 | CGACAAGCTTCTTCGTTTGGGGTTTTGC |
| phaEC-DF1 | GATGGTACCCGATGGGTGACTTCC |
| phaEC-DR1 | TCTGGATCCCCGACAGACTACTCCG |
| phaEC-DF2 | TTAGGATCCCGTGGGTTGAACAGG |
| phaEC-DR2 | GCCGAAGCTTGATAGCACAGCGAAA |
| phaPEC-DF1 | ATAGGTACCCCTCGTCTCCGTCCAGTC |
| phaPEC-DR1 | GAGGGATCCTCACTCATTTGAATCACC |
| R-IN-F | ATAGGTACCCGAGTCGTCGTAGGCA |
| R-IN-R | ATAGGATCCACTACTCCGGCGTGTC |
| Gene complementary expression | |
| phaR-ex-F | GCAGGTACCCTTATGTACTTCGGTATGTG |
| phaR-ORF-R | GTCGGATCCTCACTCATTTGAATCACCAC |
| phaP-ORF-R | TATGGATCCTCTCGGGCGGGCTAAA |
| Gene overexpression | |
| phaR-ORF-F | GTACTCGCATATGACGAACGACTCAAACGATGC |
| phaR-ORF-R | GTCGGATCCTCACTCATTTGAATCACCAC |
| phaP-ORF-F | GTACTCGCATATGAGTGAACAAGCCAACCC |
| phaP-ORF-R | TATGGATCCTCTCGGGCGGGCTAAA |
| Promoter GFP fusion reporter | |
| phaR-Pro-F | CAGGGTACCCCCAACTTATGTACTTCG |
| phaR-Pro-R | CGCAAGCTTGTCGTTCGTCATCTCCT |
| gfp-ORF-F | CCCAAGCTTAGTAAAGGAGAAGAACTTTTCAC |
| gfp-ORF-R | CGGGATCCTTATTTGTATAGTTCATCCATGC |
| phaE-Pro-F | CTAGGTACCGAGGAGAACGCAGACG |
| phaE-Pro-R | CGCAAGCTTTTGTGACATGGGCATA |
| PhaR-His6 and PhaP-His6 expression | |
| phaR-28a-F | GTACTCGCATATGACGAACGACTCAAACG |
| phaR-28a-R | GGACTCGAGTCACTCATTTGAATCACCA |
| phaP-28a-F | GTACTCGCATATGAGTGAACAAGCCAACC |
| phaP-28a-R | AGACTCGAGCTACTCCGGCGTGTCTGGT |
| PhaR-Myc fusion expression | |
| M1915-Pro-F | CGGGGTACCCGAGGTAACCACTGTACG |
| M1915-Pro-R | CATGCCATGGCATAGTGTTGCCAACCCTCTGC |
| phaR-ORF-F2 | CATGCCATGGACGAACGACTCAAACGAT |
| phaR-ORF-R2 | CGCAAGCTTCTCATTTGAATCACCACG |
| myc1-ct-F | AGCTTGAGCAGAAGCTCATCAGCGAGGAGGATCTGTGAG |
| myc1-ct-R | GATCCTCACAGATCCTCCTCGCTGATGAGCTTCTGCTCA |
| Northern blot probe | |
| phaP-NB-F | ACAAGCCAACCCATTCA |
| phaP-NB-R | CCAGGTCTGTTCGGTCAT |
| 7S-NB-F | TAGGTCGGGCAGTTA |
| 7S-NB-R | GCGACGCACGTCCGATGGT |
| CR-RT-PCR and qRT-PCR | |
| phaRP-CRRT-F | CCTGGGATGTCATGGAAG |
| phaRP-CRRT-R | GCTGTCTGAAACACCCGTAC |
| 16S-qF | CGTCCGCAAGGATGAAA |
| 16S-qR | CAGCGTCGTGGTAAGGT |
| phaR-Pro-qF | CCCAACTTATGTACTTCGG |
| phaR-Pro-qR | GTCGTTCGTCATCTCCTA |
| glpR-Pro-qF | CCGTTTCTCGTTCAGTTTC |
| glpR-Pro-qR | CCTCGTTAGGTGGATGGTA |
| Truncation of phaRP promoter | |
| phaR-Pro-41F | CAGGGTACCCGAAGGGAACATATATG |
| phaR-Pro-86F | CAGGGTACCCGGCTTCTACACCATAC |
| phaR-Pro-R | CGCAAGCTTGTCGTTCGTCATCTCCT |
| Site-directed mutation of phaRP promoterb | |
| M1-F | CGAAGGGAACATATATGTTACTGACCGTACAACACCGAGTTAGGAG |
| M2-F | CGAAGGGAACATATATTGGACTGCAGGTACAACAC |
| M3-F | TGTCGAAGGGAACATAGCGTGGACTGCAGGTACAACAC |
| M4-F | CCACTAAATGGTGTCGCATGTCACATATATGTTACTGCAG |
| M5-F | CCATCTGATACCACTAACGCTGTGCGAAGGGAACATATA |
| M6-F | GATACCATCTGATACCAAGCCATGGTGTCGAAGGGA |
| M7-F | CCATACGATACCATCTGCTCAACCTAAATGGTGTCGAAG |
| M8-F | ACACCATACGATACCAGAGTATACCACTAAATGGTGT |
| M9-F | GCTTCTACACCATACGCGCAACTCTGATACCACTAAATG |
| M10-F | CGGCTTCTACACCATCATATACCATCTGATACCAC |
| M11-F | GATTTTTGCCGGCTTCTCACAACGACGATACCATCTGATAC |
| M13-F | GGGGATTTTTGCCGGCGGAGACACCATACGATACC |
| M14-F | CAGGCAGGGGATTTTTGACTGATTCTACACCATACGATACC |
| M15-F | TGCCAGGCAGGGGCTGTGTGCCGGCTTCTACAC |
| M16-F | ACTGAGTGCCAGGCCGTGTATTTTTGCCGGCTTCT |
| PhaR point mutationb | |
| E24A-F | GATGCAGAAAGCCAGCGCTGAGTTCACCCAACAAC |
| Q28A-F | CAGCGAAGAGTTCACCGCTCAACAACTCCGTCTGT |
| Q30A-F | AGAGTTCACCCAACAAGCACTCCGTCTGTTCGA |
| R32A-F | CACCCAACAACAACTCGCTCTGTTCGAACAACTG |
| K67A-F | CAAACCGCGGTCTTCGCAACGCGCGTGCAGA |
| R75A-F | TGCAGAGTGGGGGGGCTATCAGCATCCCCGAC |
| E82A-F | CATCCCCGACGCTGCGCGCGATGCCCTC |
| R83A-F | ATCCCCGACGCTGAGGCTGATGCCCTCGACATC |
Sequences representing restriction sites are underlined.
The substituted nucleotides in the sense primers (M1-F to M16-F and E24A-F to R83A-F) are indicated by boldface letters. The corresponding antisense primers are not listed because they are exactly the reverse complements of the sense primers.
For construction of the green fluorescent protein (GFP) reporter plasmid pRF, a 168-bp DNA fragment upstream of the phaR open reading frame (ORF) (the promoter of the phaRP operon, named PphaRP) was linked with the coding region of a soluble modified red-shifted green fluorescent protein (smRSGFP [simply named “GFP” here]) (24), and was cloned into the expression plasmid pWL502 (20). For another reporter plasmid, pEF, the PphaRP fragment in pRF was replaced by a 189-bp DNA fragment upstream of the phaE ORF. To introduce mutations into DNA fragments, the site-directed mutagenesis was performed using a DpnI-mediated method as described previously (25). Briefly, the 168-bp PphaRP fragment was cloned into the pGEM-T Easy vector (Promega), and the resultant plasmid, pT-Rpro, was used as the PCR template for the site-directed mutagenesis of PphaRP. The PphaRP fragments with desired mutations together with the gfp fragment were subcloned into pWL502 to generate the plasmids pD41, pD86, and pM1 to pM16, respectively.
For the amino acid residue substitutions in PhaR, a knock-in plasmid, pR-IN, which possesses a 1.2-kb DNA fragment containing the phaR ORF and its upstream and downstream regions, was used as the template. The substitutions were introduced independently into each plasmid by DpnI-mediated site-directed mutagenesis. The resultant plasmids (e.g., pR-IN-E24A) were, respectively, transferred into the H. mediterranei strain with phaR deleted and integrated into the chromosome to generate the corresponding strains, which express the desired PhaR mutants (e.g., PhaRE24A).
For the overexpression of phaR or phaP, each ORF region was inserted into the pSCM307 plasmid (26). Then, the corresponding fragments containing both the hsp5 promoter region and the ORF region of phaP (or phaR) were subcloned into the plasmid pWL502 (or pRF), resulting in plasmid pHP (or pHRRF). For the complementary expression of phaR or phaRP, a fragment containing the region of the native promoter and the ORF of phaR (or the ORFs of phaRP) was cloned into pWL502 to generate plasmid pWLR (or pWLRP).
All PCR-amplified sequences were verified by DNA sequencing. The plasmid transformation of H. mediterranei was performed by a polyethylene glycol-mediated transformation method (27).
Promoter activity assays.
H. mediterranei strains harboring the GFP reporter plasmids were cultured at 37°C in AS-168SYL medium. Cells (100 μl/well) from certain growth phases were transferred into 96-well plate to measure the turbidity (optical density at 600 nm [OD600]) and the fluorescence intensity (excitation, 488 nm; emission, 509 nm) using a Synergy H4 hybrid microplate reader (BioTek Instruments, Inc., Winooski, VT) (24, 28), with AS-168SYL medium serving as the blank control. The fluorescence intensity was normalized against the cell density (per OD600 of 0.1) and expressed as relative fluorescence units (RFU). At least three independent biological replicates were performed.
RNA extraction, CR-RT-PCR, and Northern blot analysis.
The total RNA of H. mediterranei cells in late-exponential phase was extracted with TRIzol reagent (Invitrogen) (29).
For the identification of the transcription start site (TSS), the circularized RNA reverse transcription-PCR (CR-RT-PCR) method was carried out as previously described (30). After the reverse transcription of self-ligated RNA with random hexamer primers, the cDNA was used as the PCR template. The PCR products amplified with the primer pair phaRP-CRRT-F/phaRP-CRRT-R were cloned into the pGEM-T Easy vector to determine the TSS by DNA sequencing.
For Northern blot analysis, 4 μg of each RNA sample was separated on a 4% denaturing polyacrylamide gel (7 M urea, 0.5× Tris-borate-EDTA [TBE] buffer) and transferred onto the nylon membranes using a semidry transfer cell (Bio-Rad). The probes used to detect the expression of phaP and the internal control 7S RNA were amplified by the primer pairs phaP-NB-F/phaP-NB-R and 7S-NB-F/7S-NB-R, respectively. The probes were labeled with biotin-11-dUTP (R0081; Thermo Scientific) by PCR. After the cross-linking of RNA onto the membranes by UV, the membranes were hybridized with the labeled probes. The prehybridization, hybridization, and washing procedures were performed as previously described (29). The biotin was detected using the Pierce chemiluminescent nucleic acid detection module (Thermo Scientific, Rockford, IL).
Protein expression and purification, antiserum preparation, and Western blot analysis.
The coding regions of phaR and phaP amplified by the primer pairs PhaR-28a-F/PhaR-28a-R and PhaP-28a-F/PhaP-28a-R, respectively, were inserted into the vector pET-28a (Novagen). The resultant expression plasmids, pET-28aR and pET-28aP, were transferred into E. coli BL21(DE3), respectively. Expression and purification of the PhaR-His6 and PhaP-His6 proteins, as well as the preparation of the corresponding antisera were performed as previously described (31).
For Western blot analysis, the cells cultivated in MGF medium were collected in the stationary phase (after cultivation for approximately 3 days). The cell pellets were dissolved in 8 M urea buffer and homogenized by ultrasonication. Cell debris was removed by centrifugation. The protein concentrations in the extracts were measured with a bicinchoninic acid protein assay kit (Applygen Technologies, Inc., Beijing, China). For each set of experiments, the same amount of total proteins (30 μg for detection of PhaP and 100 μg for detection of PhaR and Myc-tag) was resolved by 14% SDS-PAGE and analyzed by Western blotting with antibodies against PhaR, PhaP, or Myc tag (M20002; Abmart), performed as previously described (31).
ChIP and qPCR assays.
For chromatin immunoprecipitation (ChIP) analysis, a C-terminal tagged PhaR-Myc protein was expressed in a phaR knockout mutant. The cells from the late-exponential-phase culture of H. mediterranei were used for ChIP analysis according to Wilbanks et al. (32). Briefly, cells were fixed in 1% (vol/vol) formaldehyde for 10 min and then treated with 125 mM glycine for 5 min and washed twice with TBSL buffer (20). Approximately 1010 cells were resuspended in 700 μl cold lysis buffer (32) containing 1× protease inhibitor cocktail (PIC [Sigma]). The lysate was then sonicated (4 s on/5 s off, 4-min cycles, 20% power setting) using a JY92-IIDN ultrasonic homogenizer (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) to shear the DNAs to lengths between 200 and 1,000 bp. After centrifugation (at 16,000 × g at 4°C) for 3 min, 50 μl of the supernatant was saved as the input sample. The rest of the supernatant was mixed with 2 μl of anti-Myc antibody and 30 to ∼40 μl of protein A Sepharose CL-4B beads (GE Healthcare), which were preblocked with 5 mg/ml bovine serum albumin (BSA) in phosphate-buffered saline. The mixture was incubated overnight at 4°C. The following bead-washing steps and the elution step (with 50 μl of elution buffer) were performed exactly like the protocol reported by Wilbanks et al. (32). Tris-EDTA (TE)-SDS (32) (180 μl) was added to the eluates (20 μl) and the input sample (20 μl), respectively, to reverse the cross-linking by incubation overnight at 65°C. The DNA was purified with an E.Z.N.A. Cycle-Pure kit (Omega Bio-Tek).
The ChIP DNA fractions were analyzed by using quantitative real-time PCR (qPCR) to measure the enrichment of genomic DNA regions of interest with the primers shown in Table 2. The qPCR was performed on Rotor-Gene Q real-time cycler (Qiagen, Valencia, CA) with Kapa SYBR Fast qPCR master mix (2×) (KM4101; Kapa Biosystems) using a three-step PCR procedure (including initial denaturation at 95°C for 5 min followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 54°C for 20 s, and synthesis at 72°C for 30 s). Product specificity was confirmed by melting curve analysis. A 16S rRNA gene region (positions+836 to +980 relative to the TSS) was used to normalize the qPCR results of each ChIP sample. The enrichment of DNA fragments was analyzed with the input DNA samples serving as controls. Samples were analyzed in triplicate from three independent ChIP assays, and representative results from one biological replicate are presented.
PHA accumulation assay and TEM studies.
After a 3-day's cultivation in MGF medium, the cells in the stationary phase were harvested for the PHA accumulation assay or for transmission electron microscopy (TEM) analysis. The PHA content and PHA composition analyses using gas chromatography were performed as previously described (31). The PHA granule morphology was observed through TEM analysis with a workflow in accordance with the procedure described previously (20, 33). Photomicrographs were taken with a Philips JEOL-1400 electron microscope.
Bioinformatics analysis.
The codon adaptation index (CAI) was analyzed on the CAIcal server (http://genomes.urv.es/CAIcal/).
RESULTS
Identification of the AbrB-like protein PhaR and the promoter of the phaRP operon in H. mediterranei.
We have previously reported a small protein GAP12 (110 amino acids [aa]), which was present in abundance on the PHA granule of H. mediterranei, with its coding gene cotranscribed with the phasin gene phaP (20). The gap12-phaP operon is located upstream of the phaEC operon that encodes the two subunits of PHA synthase (Fig. 1A). Conserved domain search analysis at NCBI revealed that the C-terminal portion of GAP12 has a putative DNA-binding domain consisting of swapped-hairpin barrel fold, which shows low homology (with an E value of 1.82e−05) to the corresponding domain of the AbrB (antibiotic resistance protein B) superfamily of regulators (34), implying a regulatory-related function of GAP12. Thus, the GAP12 was renamed PhaR, and the gap12-phaP operon was renamed the phaRP operon (Fig. 1A).
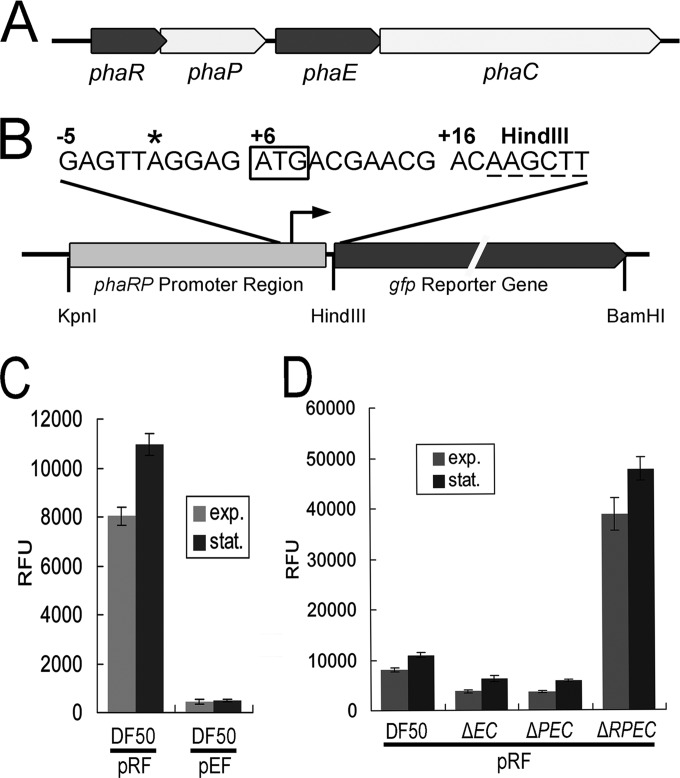
FIG 1.
Expression-level analyses of phaRP using the gfp reporter system in strains with pha genes mutated. (A) Genetic organization of the pha gene cluster (phaRP and phaEC) in H. mediterranei. The arrows indicate the direction of gene transcription. (B) Schematic representation (not to scale) of the PphaRP-gfp fusion in the reporter plasmid pRF. The transcription start site of the phaRP operon identified by CR-RT-PCR is indicated with an asterisk. The translation start codon of phaR (boxed) and the HindIII restriction site (dashed) are also shown. (C) Comparison of the promoter activities of the phaRP operon (pRF) and phaEC operon (pEF) in H. mediterranei strain DF50. (D) Comparison of the GFP expression levels of the H. mediterranei DF50, ΔphaEC (ΔEC), ΔphaPEC (ΔPEC), and ΔphaRPEC (ΔRPEC) strains harboring plasmid pRF. The promoter activities in both the exponential (exp.) phase and the stationary (stat.) phase are shown. Error bars show standard deviations (n = 3). RFU, relative fluorescence units.
To explore the function of PhaR and its expression profiles, a GFP-based reporter system was constructed to conveniently monitor the activity of the phaRP promoter (PphaRP). First, the TSS of the phaRP cotranscript was analyzed using a CR-RT-PCR approach. An adenine residue at 5 bp upstream of the initiator ATG codon was determined as the TSS of phaRP, which revealed an extended 5′-untranslated region (5′-UTR) with the sequence AGGAG (Fig. 1B). A 168-bp region (positions −151 to +17 relative to the TSS of phaRP) joined with the gfp gene was used to construct the PphaRP-gfp-fused reporter plasmid pRF (Fig. 1B and Fig. 2A). After plasmid pRF had been transferred into H. mediterranei DF50 (22), GFP could be visualized by fluorescence microscopy (see Fig. S1 in the supplemental material). The activity of the PphaRP promoter was evaluated by quantifying the fluorescence signal with a fluorescence microplate reader.
FIG 2.
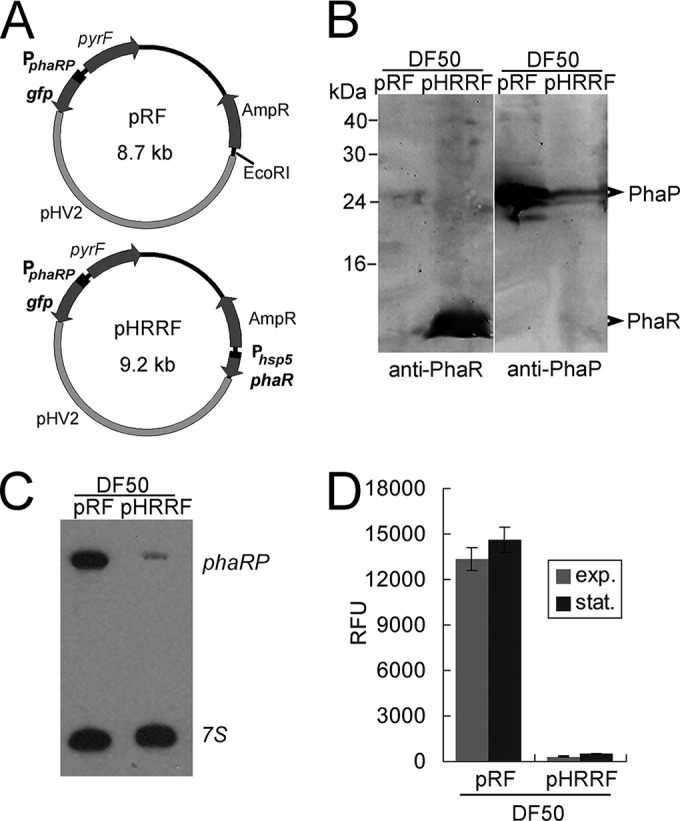
Expression-level analyses of phaRP in a phaR overexpression strain. (A) Schematic representation of the reporter plasmids pRF and pHRRF. The ampicillin resistance gene (AmpR), the pyrF gene, and the pHV2 replicon are labeled. The key genetic elements for the reporter system, including the phaRP promoter and the gfp ORF, are shown in both pRF and pHRRF. The hsp5 promoter and the phaR ORF, which are elements further introduced at the EcoRI restriction site of pRF, are indicated in pHRRF. (B) Western blot analyses of PhaR and PhaP expression levels in the DF50/pRF and DF50/pHRRF strains. Cells were collected at the stationary phase. To detect PhaP and PhaR, crude extracts of 30 μg and 100 μg, respectively, were loaded for SDS-PAGE. (C) Northern blot analysis of the phaRP transcript in the DF50/pRF and DF50/pHRRF strains. The 7S transcript served as an internal control. (D) Comparison of the GFP expression levels of the DF50/pRF and DF50/pHRRF strains. The phaRP promoter activities in both the exponential (exp.) phase and the stationary (stat.) phase are shown. Error bars show standard deviations (n = 3).
The promoter activities of the PphaRP were monitored during cell growth. As PHA is actively accumulated in the cells in the stationary phase and the promoter activity of PphaRP is more stable at this phase, we primarily show or discuss the data obtained in the stationary phase. Remarkable fluorescence intensity (reaching values of over 10,000 RFU) was detected in the DF50/pRF strain, while the promoter of the phaEC operon only showed a weaker signal (up to approximately 500 RFU in the DF50/pEF strain) (Fig. 1C). These results reveal that PphaRP exhibits a strong activity that is consistent with the high abundance of the PhaP protein.
Modulation of phaRP expression and its promoter activity by PhaR and PHA accumulation.
Since PhaR is associated with the PHA granules, first the effect of PHA production on the phaRP expression level was analyzed. The PHA-accumulating haloarchaea possess a conserved pha gene cluster (phaR-phaP-phaE-phaC), including two operons, phaRP and phaEC, in H. mediterranei (Fig. 1A) (20). The deletion of the PHA synthase operon (phaEC) makes cells incapable of synthesizing PHA (35). The pRF plasmid was transferred to three PHA-negative mutants, including the ΔphaEC, ΔphaPEC, and ΔphaRPEC strains, and the expression levels of GFP were evaluated. The ΔphaEC and ΔphaPEC mutants both showed an approximately 2-fold decrease in the activity of the PphaRP promoter compared with that of the same promoter in DF50 (Fig. 1D), implying that the expression of phaRP could be activated by the presence of PHA. In contrast, the further deletion of the phaR gene in ΔphaPEC, which resulted in the third PHA-negative mutant ΔphaRPEC strain, caused an increase of more than 4-fold in the activity of PphaRP (Fig. 1D). These results suggest that when cells do not synthesize PHA, the expression of phaRP is suppressed, whereas the knockout of the phaR gene could relieve this suppression effect. Thus, the PhaR could be a negative regulator of the phaRP operon.
To further explore the function of PhaR, we also carried out an overexpression of phaR using a plasmid-based method. The overexpression plasmid pHRRF was derived from pRF, with phaR driven by a strong hsp5 promoter at the EcoRI site of pRF (Fig. 2A). The plasmid pHRRF was transferred into the DF50 strain to generate the DF50/pHRRF strain, and the influence of PhaR on the expression of phaP was investigated. The enhanced expression of phaR in DF50/pHRRF strain was revealed by Western blotting using anti-PhaR antibody, whereas the expression level of the major phasin PhaP was analyzed with anti-PhaP antibody (Fig. 2B). As shown in the Western blot results, overproduced PhaR in the cells strongly reduced the amount of PhaP (Fig. 2B). Further Northern blot analysis with a phaP-specific probe showed that in contrast to the high abundance of phaRP transcript in the DF50/pRF strain, only a negligible amount of phaRP mRNA was detected in the phaR overexpression strain DF50/pHRRF (Fig. 2C). These data indicate that PhaR controls the amount of the PhaP protein by inhibiting the expression of phaRP at the transcriptional level. Concomitant with these observations, in the DF50/pHRRF strain, the fluorescence signal driven by the PphaRP promoter was strongly decreased to be a faint signal (200 to 300 RFU) by the larger amount of the PhaR protein (Fig. 2D), confirming the repression role of PhaR.
In conclusion, these results reveal that absence of PhaR could enhance the expression of phaRP, while excess of PhaR could reduce the expression, demonstrating that PhaR is a transcriptional repressor of both itself and phaP. In addition, the presence of PHA also could active the expression of phaRP, indicating that a PhaR titration effect of PHA granules plays an important role in the fine modulation of phaRP expression.
Direct binding of the regulator PhaR to the promoter of the phaRP operon in vivo.
The above results demonstrated that overexpression of PhaR severely inhibited the transcription of phaRP operon. To determine whether the repression effect by PhaR occurs by interacting directly with the promoter region of the phaRP operon, we further performed a ChIP-qPCR assay.
First, we expressed a PhaR protein that was Myc tagged at its C terminus in a phaR knockout strain. The expression of the PhaR-Myc was driven by a strong constitutive promoter that is a mutated promoter of the phosphotransferase system (PTS) gene cluster from plasmid pM1915 (28). The successful expression of Myc-tagged PhaR was confirmed via Western blot analysis with anti-PhaR and anti-Myc antibodies, respectively (Fig. 3A). After using monoclonal anti-Myc antibodies to immunoprecipitate the Myc-tagged PhaR protein, the coprecipitated DNA was analyzed. Both the ChIP-extracted DNA and the input DNA were examined by qPCR. A 145-bp region, F16S, from the 16S rRNA gene was used as an inner control to normalize all qPCR results. For the PphaRP promoter, the qPCR product, FphaR, was designed to span a region from −151 to +17 relative to the TSS of phaRP. A promoter region (FglpR; −148 to −12 relative to the translation start site of glpR) of the glpR gene, which is functionally irrelevant to the PHA biosynthesis as shown in the microarray data of the ΔphaEC mutant (36), was chosen to be a distal/negative-control region. As shown in Fig. 3B, the fragment FphaR was remarkably enriched (14.0-fold) by PhaR-Myc, while no significant enrichment (1.1-fold) of the distal/negative-control fragment FglpR to PhaR-Myc was observed, demonstrating that PhaR interacts specifically with the phaRP promoter in vivo. These data indicated that PhaR regulates phaRP expression directly through its interaction with the promoter of the phaRP operon.
FIG 3.
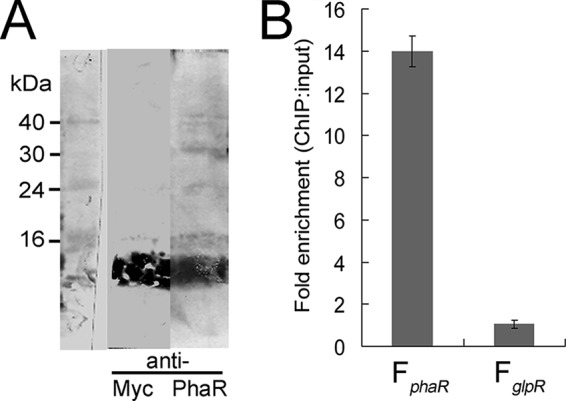
ChIP-qPCR assay for analysis of binding of PhaR with the promoter region of the phaRP operon. (A) Detection of PhaR-Myc fusion protein by Western blotting assay with anti-Myc and anti-PhaR antibodies, respectively. (B) DNA enrichment assay of the PhaR-Myc fusion protein. The fold enrichment of the phaR promoter region (FphaR; 168 bp) and the glpR promoter region (FglpR; 137 bp) is shown. The relative abundance of each region in both the ChIP and the input DNA samples was calculated by normalization to the abundance of the inner control region. Error bars show standard deviations (n = 3).
Identification of a specific negative cis-element in the promoter of phaRP operon.
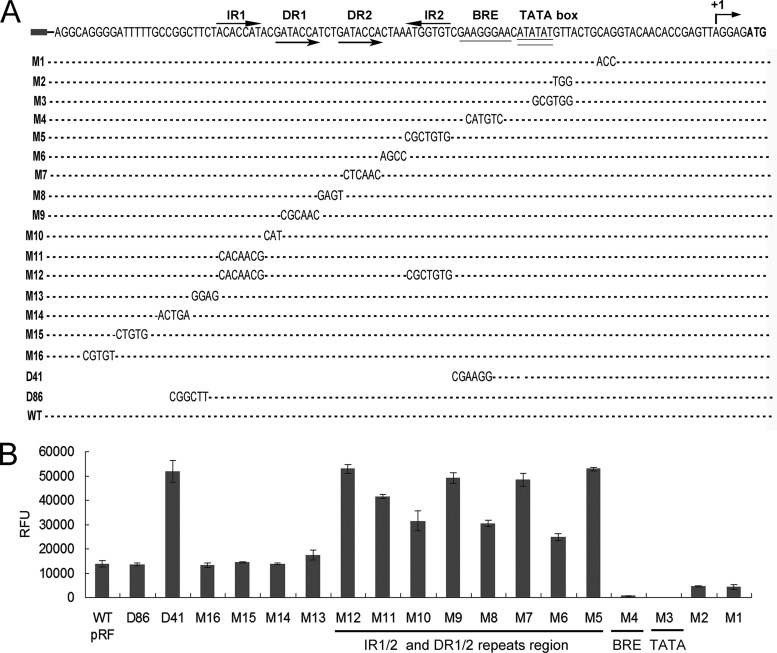
To seek the cis-elements important for the inhibition of phaRP expression, we introduced mutations into the promoter PphaRP and fused these mutated promoters with the gfp reporter gene. After transferring the promoter-gfp-fused plasmids (pD41, pD86, and pM1 to pM16) into the DF50 strain, the GFP reporter activities were measured. The PphaRP in the plasmid pRF was taken as the wild-type (WT) promoter (Fig. 1 and 2).
First, the putative core promoter elements, the TATA box and BRE, were found 25 bp upstream of the TSS (Fig. 4). Mutation of the putative TATA box (M3) completely abolished the promoter activity, while mutation of the putative BRE (M4) also caused a significant reduction in the promoter activity (Fig. 4), thus confirming the functional TATA box and BRE of the PphaRP promoter. Interestingly, a truncation mutant, D41, in which only the core promoter portion was left, displayed a remarkably enhanced (approximately 4-fold) promoter activity (Fig. 4). As deletion of the upstream sequence (in D41) significantly weakened or completely abolished the repressive effect to the promoter, this indicated that the negative regulatory elements are located upstream of the core promoter portion.
FIG 4.
Mapping the core promoter elements and cis-elements of the phaRP promoter. (A) The sequence of the promoter region of phaRP operon is shown on the top. The transcription start site (+1) and the translation start codon are indicated by a bent arrow and boldface letters, respectively. The BRE (single underlined) and TATA box (double underlined) identified by mutagenesis assays are also indicated. The inverted repeat sequences (IR1 and IR2) and the direct repeat sequences (DR1 and DR2) are indicated by arrows. For phaRP promoter scanning mutants (M1 to M16), the mutated bases are shown in letters, while the unaltered nucleotides are represented by dashes. The truncation sites in the 5′ deletion mutants (D41 and D86) are shown. The phaRP promoter in pRF is defined as the wild-type (WT) promoter. (B) The activities of these mutated promoters of phaRP were measured by detecting the fluorescent signal of the reporter GFP. The GFP expression levels of each mutant showed similar increase/decrease profiles (compared with the wild type) between the exponential phase and the stationary phase. For a clear view, only the data collected at the stationary phase are shown. Error bars show standard deviations (n = 3).
After analyzing the sequence upstream of the core promoter portion, four repeated sequences were found arrayed in tandem in a region with a length of approximately 40 bp (Fig. 4A). These four repeats were composed of a pair of perfect inverted repeats (IR1 and IR2) as well as a pair of direct repeats (DR1 and DR2). The pair of direct repeats (underlined) (GATACCAN3GATACCA) is flanked by the pair of inverted repeats (underlined) (ACACCATN23ATGGTGT). A truncation mutant, D86, with all four of these repeats reserved exhibited a wild-type promoter activity (Fig. 4). Moreover, the mutation of each repeat induced an increase of 3- to 4-fold in the expression level of PphaRP, whereas the mutation of the spacer sequences between the repeats caused an increase of approximately 2-fold (Fig. 4). Other mutations introduced into the sequence upstream of this region had almost no effect on the promoter activity (Fig. 4). These results show that each of these four repeated sequences is essential for the repression regulation of phaRP, suggesting that the region containing these repeats (IR1/IR2 and DR1/DR2) is the negative cis-element and would be the binding position of the transcriptional repressor.
Since PhaR was identified to be a transcriptional repressor of the phaRP operon and to interact directly with its promoter in vivo, it is most likely that these four repeated sequences of the negative cis-element serve as the binding sites of PhaR. To further support this hypothesis, the reporter plasmids with mutations on these four repeats, including pM5, pM7, pM9, pM11, and pD41, were transferred into the ΔphaRPEC strains, in which the PhaR was absent, and the promoter activities were measured. As shown in Fig. 5, mutations of those repeats all resulted in high levels of promoter activity similar to that of the wild-type (WT) promoter in pRF. Small increases in the promoter activity were observed in the ΔphaRPEC strains containing the plasmid pM5 or pD41, which might be caused by the alteration of the sequence of IR2 that is adjacent to the BRE region. This might promote the recruitment of TFB to BRE and thereby slightly enhanced the transcriptional activity. The mutations of the IR1/IR2 and DR1/DR2 sequences did not cause a significant further enhancement of the expression of phaRP in the absence of PhaR, indicating that the four repeated units of the cis-element might be the binding sites of PhaR.
FIG 5.
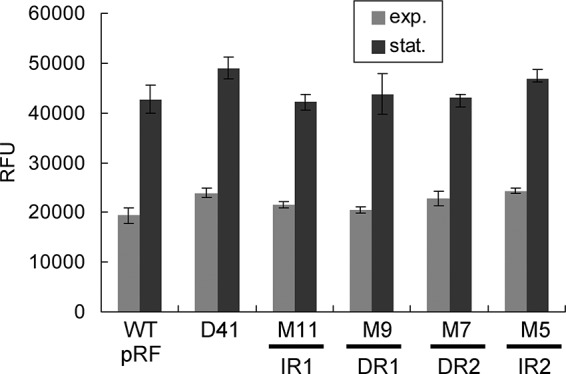
Activities of the representative mutated promoters of the phaRP operon in the ΔphaRPEC strain. The corresponding mutated regions (IR1/2 and DR1/2) are indicated at the bottom. The phaRP promoter in pRF is defined as the wild-type (WT) promoter. The promoter activities in both the exponential (exp.) phase and the stationary (stat.) phase are shown. Error bars show standard deviations (n = 3).
The conserved AbrB-like region is critical for the transcriptional repression activity of PhaR.
More than 50 homologs of the H. mediterranei PhaR were detected by NCBI BLASTp. They were all annotated as conserved hypothetical proteins and were significantly similar, with identities of approximately 50%. The SSDB Gene Cluster Search on KEGG revealed that the phaR homologs are located in a similar gene cluster or context at least in 19 species (data from before September 2014), in which only one species, Natronomonas pharaonis, lacks the PHA synthase genes and may not accumulate PHA. Interestingly, all 19 species belong to the phylum Euryarchaeota of the domain Archaea, including one thermophilic archaeon, Ferroglobus placidus, and 18 halophilic archaea (see Fig. S2 in the supplemental material). A multiple-alignment analysis of the amino acid sequences of these PhaR homologs revealed that the AbrB-like domain is highly conserved in the C-terminal portion of PhaR homologs (see Fig. S2), indicating an evolutionarily conserved PHA granule-associated regulator unique to archaea.
The high conservation of the AbrB-like domain in PhaR homologs indicated the importance of this domain for PhaR. Several of the highly conserved or charged amino acid residues, E24, Q28, Q30, R32, K67, R75, E82, and R83, in the PhaR protein were mutated, respectively, to alanine to examine the influence of these residues on the repressor function of PhaR (Fig. 6A). These substitution mutations were introduced into the phaR gene at its native locus in the genome through homologous recombination. The reporter vector pRF was then introduced into these mutants to assess the repressor activity of these mutated PhaR proteins.
FIG 6.
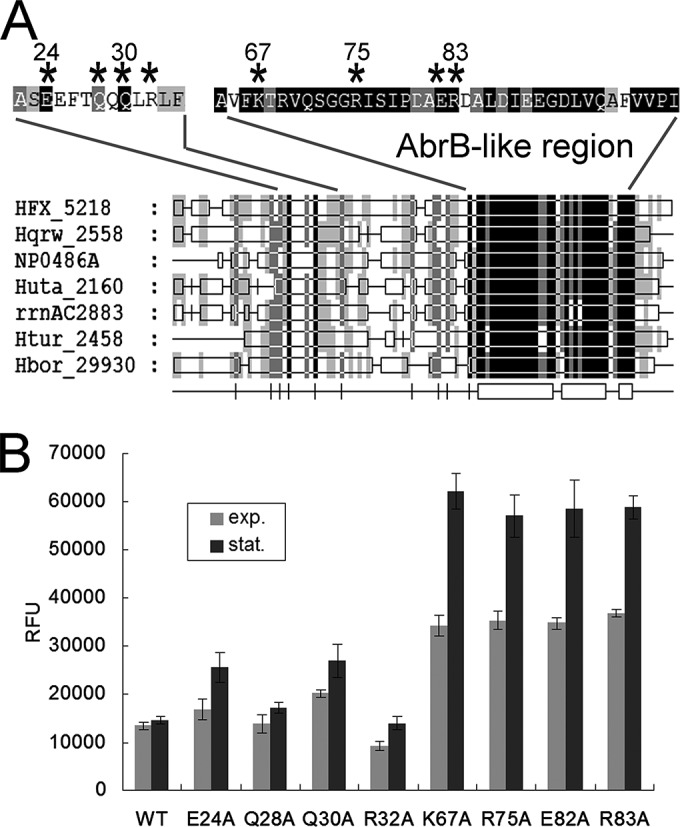
Mutational analysis of several conserved polar amino acid residues of the PhaR protein. (A) Summary view of the multiple alignments of amino acid sequences of PhaR homologs from seven representative haloarchaea species: H. mediterranei (HFX), Haloquadratum walsbyi (Hqrw), Natronomonas pharaonis (NP), Halorhabdus utahensis (Huta), Haloarcula marismortui (rrnAC), Haloterrigena turkmenica (Htur), and Halogeometricum borinquense (Hbor). The AbrB-like region is indicated. The residues to be mutated are marked with asterisks. (B) phaRP promoter activity assays of mutants with PhaR bearing amino acid substitutions. The residues are indicated in the one-letter amino acid code (E, glutamate; Q, glutamine; R, arginine; K, lysine; and A, alanine). The DF50 strain is indicated as the wild-type (WT) control. The promoter activities in both the exponential (exp.) phase and the stationary (stat.) phase are shown. Error bars show standard deviations (n = 3).
The reporter activities were examined and are shown in Fig. 6B. E24A, Q28A, Q30A, R32A, K67A, R75A, E82A, and R83A refer to the strains with PhaR protein bearing the corresponding amino acid mutations (i.e., E24A is the strain that has the PhaR protein with glutamate replacement with alanine at position 24). The substitutions of alanine for the glutamate, glutamine, and arginine residues in the N-terminal portion of PhaR in the E24A, Q28A, Q30A, and R32A strains only slightly affected the PphaRP activity. In contrast, the K67A, R75A, E82A, and R83A mutants, in which mutations occurred in the C-terminal conserved domain of PhaR, all showed a remarkable increase (2- to 4-fold) in PphaRP activity. These results revealed that PhaR mutants with amino acid substitution in the N-terminal portion still exhibited the capability to suppress the corresponding promoter, whereas, the mutation of the AbrB-like domain of PhaR could significantly weaken or abolish the repressor function of PhaR. These results show that the AbrB-like domain is crucial for the repressor role of PhaR.
It is noteworthy that the expression of the mutated PhaR was driven by the native promoter; thus, the mutant strains might possess different quantities of the mutated PhaR proteins. However, this would not affect our above conclusions on the crucial amino acids of the PhaR. For example, the K67A mutant, which had a high promoter activity of phaRP operon, would also produce more PhaRK67A. Nevertheless the overproduced PhaRK67A did not result in an enhanced repressive effect on the activity of the PphaRP in the reporter system, further indicating the weakened repressor effect of PhaRK67A.
Effect of PhaR on PHA accumulation and PHA granule morphology in H. mediterranei.
In order to assess the influence of PhaR in vivo on the PHA accumulation of H. mediterranei, the PHA production capability was investigated in the presence or absence of PhaR. The results showed that when both the phasin PhaP and the phasin regulator PhaR were absent, the H. mediterranei strain (ΔphaRP) was more deficient in PHA accumulation than the ΔphaP strain. The PHA production of the ΔphaRP mutant decreased to only approximately 15% to 25% of that of the DF50 strain (Table 3), whereas the ΔphaP mutant could maintain a PHA accumulation level of approximately 60% to 70% of that of the DF50 strain (20). The further decrease in the PHA synthesis level was caused by the further knockout of the phaR gene, suggesting that PhaR is also very important for PHA synthesis. A similar phenomenon has been reported in R. eutropha, in which the PhaR protein was proposed to promote PHA synthesis at least partially through a PhaP-independent pathway (14).
TABLE 3.
PHA accumulation in H. mediterranei strainsa
| Strain | PHBV content (% [wt/wt]) | 3HV fraction (mol%) | Cell dry wt (g/liter) | PHBV concn (g/liter) |
|---|---|---|---|---|
| DF50 | 41.79 ± 0.42 | 8.15 ± 0.21 | 4.56 ± 0.37 | 1.9 ± 0.14 |
| ΔphaRP mutant | 11.89 ± 0.29 | 5.27 ± 0.06 | 4.01 ± 0.1 | 0.48 ± 0.01 |
| ΔphaRP/pWL502 mutant | 11.05 ± 0.18 | 3.58 ± 0.15 | 3.16 ± 0.03 | 0.35 ± 0 |
| ΔphaRP/pWLR mutant | 41.93 ± 1.31 | 6.35 ± 0.17 | 6.24 ± 0.47 | 2.62 ± 0.17 |
| ΔphaRP/pWLP mutant | 33.49 ± 2.36 | 5.94 ± 0.21 | 5.91 ± 0.35 | 1.97 ± 0.06 |
| ΔphaRP/pWLRP mutant | 42.82 ± 3.2 | 6.32 ± 0.34 | 5.97 ± 0.72 | 2.54 ± 0.13 |
| DF50/pWL502 mutant | 49.81 ± 0.52 | 6.96 ± 0.3 | 5.02 ± 0.09 | 2.5 ± 0.07 |
Cells were cultured at 37°C for 3 days. Data are shown as means ± standard deviations (n = 3).
The complementary expression experiments were also performed. The complementary expression of the phaRP operon in the ΔphaRP strain can restore its PHA accumulation level to that of the wild-type control strain (DF50/pWL502) (Table 3). In addition to the coexpression of phaRP, the phaR and phaP genes were also separately expressed under the control of their native promoter in the plasmids pWLR and pWLP, respectively. The decreased PHA production in the ΔphaRP strain was partly restored by the expression of the phaP gene, whereas it was surprising that the expression of phaR fully restored the high PHA accumulation (Table 3). The results indicated that both PhaP and PhaR could independently promote PHA synthesis.
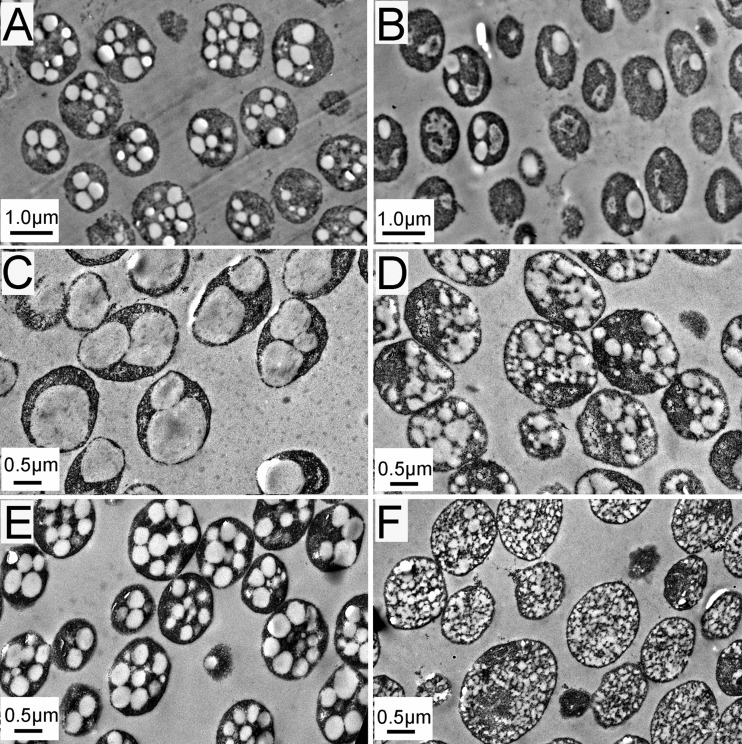
The effects of PhaR and PhaP on PHA granule morphology were further investigated by TEM (Fig. 7). In the wild-type strains DF50 and DF50/pWL502, most cells harbored multiple moderate-size PHA granules (Fig. 7A and E), whereas in the ΔphaRP strain, the cells usually produced only one or two medium-size granules (Fig. 7B). When the phaR gene was complementarily expressed in the ΔphaRP strain, one or two giant granules were produced in most cells (Fig. 7C), reminiscent of the phenotype of the ΔphaP strain (20), indicating that although the PGAP PhaR can recover the high PHA accumulation level and enlarge the PHA granule, PhaR cannot facilitate granule segregation like the major structural protein PhaP. On the other hand, in the ΔphaRP/pWLP strain, several small- or medium-size granules with an irregular shape were observed (Fig. 7D). A similar phenotype was exhibited by the ΔphaRP/pHP strain, in which phaP was overexpressed under the hsp5 promoter (Fig. 7F). This indicated that the expression of phaP without the control of PhaR would cause a disorder in PHA granule formation.
FIG 7.
Transmission electron micrographs of PHA granules in H. mediterranei strains. (A) H. mediterranei DF50 strain; (B) H. mediterranei ΔphaRP strain; (C) H. mediterranei ΔphaRP/pWLR strain; (D) H. mediterranei ΔphaRP/pWLP strain; (E) H. mediterranei DF50/pWL502 strain; (F) H. mediterranei DF50/pHP strain. The cells were cultivated in MGF medium with uracil added (A and B) or with yeast extract omitted (C to F). The cells were collected during the stationary phase (after cultivation for about 3 days).
Thus, besides acting as a key transcriptional regulator that controls the amount of PhaP to ensure the formation of regular PHA granules and to facilitate the segregation of granules, the PhaR protein itself is also very important for PHA accumulation and granule formation.
DISCUSSION
To achieve economic production of PHA, increasing studies involved in the metabolic pathways of PHA biosynthesis in haloarchaea have been performed (25, 36), and close attention has also been paid to the global regulation of PHA metabolism, which was explored through proteomic and transcriptomic approaches (37). In this study, we focused on the elucidation of the role of a specific regulator, PhaR, which was identified previously as a small PGAP (12.0 kDa) of H. mediterranei (20). Here we demonstrated in vivo that PhaR could bind its own promoter specifically and exert an inhibitory effect on the transcription of phaRP, suggesting PhaR as the repressor of the phasin gene phaP as well as its own gene. In addition, the weaker activity of PphaRP observed in PHA-negative strains implied that the expression level of phaRP was also modulated by PHA biosynthesis. Although a similar PHA-sensing regulation strategy was also used by bacteria, H. mediterranei has developed a different regulation pattern to achieve such a general smart regulation strategy, with a distinct AbrB-like regulator, PhaR.
First, the regulation pattern of phaP by PhaR in H. mediterranei is subtly different from that of the bacterial counterpart. In bacteria, the phaP gene usually has its own promoter, which is bound by the autoregulator (PhaR) to repress the expression of phaP (13, 38, 39). In contrast, in the haloarchaeon H. mediterranei, the phaP gene overlaps that of the phaR gene by 8 bp and the phaP is cotranscribed with phaR (20). Evidence also showed that there is no independent promoter (20) or independent transcript for phaP (Fig. 2C), and PhaR regulates both its own gene and the phaP gene simultaneously by binding to their common promoter. These results imply a new regulation pattern for PhaR and PhaP in haloarchaea. A similar regulation pattern was proposed previously in the phaQ/phaP system in Bacillus megaterium. However, a likely phaP transcript was observed, and the phasin regulator gene phaQ is located far upstream (168 bp) of the phaP gene (40). Since 2004, there has been no additional convincing evidence to refute the suggestion presented in 1999 that B. megaterium phaP (phaPBm) was also transcribed from a separate promoter (41).
Taking into account the differences in protein level (i.e., that the amount of PhaP is much higher than that of PhaR) and the cotranscriptional expression patterns of the phaR and phaP genes in H. mediterranei, additional regulation might be occurring at the posttranscription levels, such as differences in translational efficiency or protein stability. The codon usage bias analysis provided some insight. For example, all of the three lysine residues of PhaR utilized the lower-frequency codon (AAA), while all of the five lysine residues in PhaP are encoded by the higher-frequency codon (AAG). The codon adaptation index of phaR (0.571) is lower than that of phaP (0.647). Moreover, the 5′-UTR (AGGAG) of phaR is a Shine-Dalgarno (SD)-like sequence, while SD-like sequences (GAGGAAGGAGA) were also found 50 bp upstream of the ATG codon of the phaP gene. As reported in chloroplasts, a downstream cistron (atpE) of dicistronic mRNAs possesses its own cis-element for efficient translation (42); it is likely that phaP might also be translated independently, even though there is an overlap (ATGAGTGA) of the phaR ORF and the phaP ORF. Further research would also be dedicated to the exploration of this multilevel regulation between phaR and phaP in haloarchaea.
Second, unlike the bacterial phasin regulators PhaR and PhaQ (13, 40, 43), which are predicted to have a helix-turn-helix motif for DNA binding, the H. mediterranei PhaR was shown to have a putative DNA-binding motif similar to that of the N-terminal domain of AbrB. AbrB is a small protein (10.2 to 10.6 kDa) that functions as a transition state regulator in Bacillus species (44). The N-terminal domains of the AbrB dimmer form a novel fold that was previously called the “looped-hinge helix” (45) and renamed later as the “swapped-hairpin barrel” (34). In addition to various bacteria, this AbrB-like DNA recognition fold is present in archaea, such as the putative chromatin-associated protein Sso7c4 protein in Sulfolobus solfataricus (46). The AbrB-like domain of H. mediterranei PhaR is highly conserved in its archaeal homologs. Several charged residues in this domain, including the positively charged Lys67, Arg75, and Arg83 residues, were identified to be crucial, which indicated that these positively charged residues might contribute to DNA binding by interacting with the negatively charged DNA and demonstrated a critical involvement of the AbrB-like domain in the negative regulatory role of the PhaR in H. mediterranei. AbrB and AbrB-like proteins usually function as dimers or tetramers (44, 47). In bacteria, PhaR was also shown to form a tetramer in vitro (43). In the promoter region of the phaRP operon, we identified a specific negative cis-element composed of four repeat sequences in tandem (Fig. 4), which are very likely the binding sites of H. mediterranei PhaR. In combination with the oligomer character of bacterial PhaR and AbrB, it is implied that the AbrB-like protein PhaR might also function as an oligomer, such as a dimer or a tetramer, to bind the four repeat DNA sequences. Therefore, although they have different DNA-binding motifs, both the haloarchaeal and bacterial PhaRs may regulate the phasin genes by a similar dose-dependent mechanism.
Notably, in addition to the regulation mechanism, the central role of PhaR in PHA accumulation and granule formation in H. mediterranei was further addressed in this study. When both of the two major PGAPs PhaR and PhaP were absent, there would be a deficiency in the protection layer between the PHA granule and the cytoplasm, and therefore the cells of the ΔphaRP strain could only accumulate a small amount of PHA (Table 3 and Fig. 7B). When only phaR was expressed in the ΔphaRP strain, PHA accumulation returned to a wild-type level, and the cells synthesized granules larger than those of the ΔphaRP strain (Table 3 and Fig. 7C). It is speculated that PhaR might facilitate PHA synthesis in a PhaP-independent mechanism, such as by promoting PHA synthase activity or by acting as the major protein to form a boundary to protect both the PHA and the cytoplasmic protein from unspecific binding. The reason why the ΔphaRP/pWLR strain had a higher PHA accumulation level than the ΔphaP strain might be the larger amount of PhaR proteins produced by the increased phaR gene copy number, as the complementary expression was carried out through a plasmid-based method that increased the gene copy number. When the repressor PhaR was absent, the transcriptional repression effect was released. The sole expression of phaP under its native promoter would produce excess PhaP protein. The disordered PHA granule morphology displayed in the ΔphaRP/pWLP strain and the DF50/pHP strain (Fig. 7D and F) indicates that the proper amount of PhaP is critical to the formation of regular PHA granules and that it is important to keep the expression of phaP under the control of PhaR. Therefore, H. mediterranei PhaR, which is essential for the control of the expression of phaP, plays a very important role in maintaining proper granule formation.
In summary, our results reveal a novel phasin regulator, PhaR, with a novel regulation pattern in haloarchaea. We demonstrated that in addition to acting as a phasin regulator to control PHA granule morphology, H. mediterranei PhaR can also facilitate PHA biosynthesis in a PhaP-independent manner. It is noteworthy that mutation of the promoter of the phaRP operon has also generated several very strong promoters that would have potential application in genetic engineering in haloarchaea. Therefore, this study has provided not only new insights into the regulation of PHA synthesis and granule formation in H. mediterranei but also the tools and targets for the further exploration and engineering of PHA metabolism in haloarchaea.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jingnan Liang (Institute of Microbiology, Chinese Academy of Sciences) for technical assistance in the transmission electron microscopy experiments.
This work was supported by grants 31330001 and 31370096 from the National Natural Science Foundation of China.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02878-14.
REFERENCES
- 1.Lee SY. 1996. Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14. doi:. [DOI] [PubMed] [Google Scholar]
- 2.Steinbüchel A, Füchtenbusch B. 1998. Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427. doi: 10.1016/S0167-7799(98)01194-9. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Castillo R, Rodriguez-Valera F, Gonzalez-Ramos J, Ruiz-Berraquero F. 1986. Accumulation of poly(beta-hydroxybutyrate) by halobacteria. Appl Environ Microbiol 51:214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J, Hou J, Liu H, Cai S, Feng B, Zhou J, Xiang H. 2010. Wide distribution among halophilic archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl Environ Microbiol 76:7811–7819. doi: 10.1128/AEM.01117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legat A, Gruber C, Zangger K, Wanner G, Stan-Lotter H. 2010. Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species. Appl Microbiol Biotechnol 87:1119–1127. doi: 10.1007/s00253-010-2611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AJ, Dawes EA. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griebel R, Smith Z, Merrick JM. 1968. Metabolism of poly-beta-hydroxybutyrate. I. Purification, composition, and properties of native poly-beta-hydroxybutyrate granules from Bacillus megaterium. Biochemistry 7:3676–3681. [DOI] [PubMed] [Google Scholar]
- 8.Jendrossek D, Pfeiffer D. 2014. New insights in formation of polyhydroxyalkanoate (PHA) granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate) (PHB). Environ Microbiol 16:2357–2373. doi: 10.1111/1462-2920.12356. [DOI] [PubMed] [Google Scholar]
- 9.Pötter M, Steinbüchel A. 2005. Poly(3-hydroxybutyrate) granule-associated proteins: impacts on poly(3-hydroxybutyrate) synthesis and degradation. Biomacromolecules 6:552–560. doi: 10.1021/bm049401n. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer D, Wahl A, Jendrossek D. 2011. Identification of a multifunctional protein, PhaM, that determines number, surface to volume ratio, subcellular localization and distribution to daughter cells of poly(3-hydroxybutyrate), PHB, granules in Ralstonia eutropha H16. Mol Microbiol 82:936–951. doi: 10.1111/j.1365-2958.2011.07869.x. [DOI] [PubMed] [Google Scholar]
- 11.Galán B, Dinjaski N, Maestro B, de Eugenio LI, Escapa IF, Sanz JM, García JL, Prieto MA. 2011. Nucleoid-associated PhaF phasin drives intracellular location and segregation of polyhydroxyalkanoate granules in Pseudomonas putida KT2442. Mol Microbiol 79:402–418. doi: 10.1111/j.1365-2958.2010.07450.x. [DOI] [PubMed] [Google Scholar]
- 12.Rehm BH. 2006. Genetics and biochemistry of polyhydroxyalkanoate granule self-assembly: the key role of polyester synthases. Biotechnol Lett 28:207–213. doi: 10.1007/s10529-005-5521-4. [DOI] [PubMed] [Google Scholar]
- 13.Pötter M, Madkour MH, Mayer F, Steinbüchel A. 2002. Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 148:2413–2426. [DOI] [PubMed] [Google Scholar]
- 14.York GM, Stubbe J, Sinskey AJ. 2002. The Ralstonia eutropha PhaR protein couples synthesis of the PhaP phasin to the presence of polyhydroxybutyrate in cells and promotes polyhydroxybutyrate production. J Bacteriol 184:59–66. doi: 10.1128/JB.184.1.59-66.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian JM, He AM, Lawrence AG, Liu PH, Watson N, Sinskey AJ, Stubbe J. 2005. Analysis of transient polyhydroxybutyrate production in Wautersia eutropha H16 by quantitative Western analysis and transmission electron microscopy. J Bacteriol 187:3825–3832. doi: 10.1128/JB.187.11.3825-3832.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada M, Yamashita K, Wakuda A, Ichimura K, Maehara A, Maeda M, Taguchi S. 2007. Autoregulator protein PhaR for biosynthesis of polyhydroxybutyrate [P(3HB)] possibly has two separate domains that bind to the target DNA and P(3HB): functional mapping of amino acid residues responsible for DNA binding. J Bacteriol 189:1118–1127. doi: 10.1128/JB.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pötter M, Müller H, Steinbüchel A. 2005. Influence of homologous phasins (PhaP) on PHA accumulation and regulation of their expression by the transcriptional repressor PhaR in Ralstonia eutropha H16. Microbiology 151:825–833. doi: 10.1099/mic.0.27613-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhao D, Cai L, Wu J, Li M, Liu H, Han J, Zhou J, Xiang H. 2013. Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei. Appl Microbiol Biotechnol 97:3027–3036. doi: 10.1007/s00253-012-4415-3. [DOI] [PubMed] [Google Scholar]
- 19.Koller M, Hesse P, Bona R, Kutschera C, Atlić A, Braunegg G. 2007. Potential of various archae- and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol Biosci 7:218–226. doi: 10.1002/mabi.200600211. [DOI] [PubMed] [Google Scholar]
- 20.Cai S, Cai L, Liu H, Liu X, Han J, Zhou J, Xiang H. 2012. Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl Environ Microbiol 78:1946–1952. doi: 10.1128/AEM.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 22.Liu H, Han J, Liu X, Zhou J, Xiang H. 2011. Development of pyrF-based gene knockout systems for genome-wide manipulation of the archaea Haloferax mediterranei and Haloarcula hispanica. J Genet Genomics 38:261–269. doi: 10.1016/j.jgg.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Krebs MP, Mollaaghababa R, Khorana HG. 1993. Gene replacement in Halobacterium halobium and expression of bacteriorhodopsin mutants. Proc Natl Acad Sci U S A 90:1987–1991. doi: 10.1073/pnas.90.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuter CJ, Maupin-Furlow JA. 2004. Analysis of proteasome-dependent proteolysis in Haloferax volcanii cells, using short-lived green fluorescent proteins. Appl Environ Microbiol 70:7530–7538. doi: 10.1128/AEM.70.12.7530-7538.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou J, Feng B, Han J, Liu HL, Zhao DH, Zhou J, Xiang H. 2013. Haloarchaeal-type beta-ketothiolases involved in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthesis in Haloferax mediterranei. Appl Environ Microbiol 79:5104–5111. doi: 10.1128/AEM.01370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao D, Sun C, Xiang H. 2009. Construction and application of a novel shuttle expression vector based on haloarchaeal plasmid pSCM201. Wei Sheng Wu Xue Bao 49:1040–1047. (In Chinese.) [PubMed] [Google Scholar]
- 27.Cline SW, Lam WL, Charlebois RL, Schalkwyk LC, Doolittle WF. 1989. Transformation methods for halophilic archaebacteria. Can J Microbiol 35:148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- 28.Cai L, Cai SF, Zhao DH, Wu JH, Wang L, Liu XQ, Li M, Hou J, Zhou J, Liu JF, Han J, Xiang H. 2014. Analysis of the transcriptional regulator GlpR, promoter elements, and posttranscriptional processing involved in fructose-induced activation of the phosphoenolpyruvate-dependent sugar phosphotransferase system in Haloferax mediterranei. Appl Environ Microbiol 80:1430–1440. doi: 10.1128/AEM.03372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Q, Han J, Zhou L, Coker JA, DasSarma P, DasSarma S, Xiang H. 2008. Dissection of the regulatory mechanism of a heat-shock responsive promoter in haloarchaea: a new paradigm for general transcription factor directed archaeal gene regulation. Nucleic Acids Res 36:3031–3042. doi: 10.1093/nar/gkn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn J, Binder S. 2002. RT-PCR analysis of 5′ to 3′-end-ligated mRNAs identifies the extremities of cox2 transcripts in pea mitochondria. Nucleic Acids Res 30:439–446. doi: 10.1093/nar/30.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Lu Q, Zhou L, Zhou J, Xiang H. 2007. Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl Environ Microbiol 73:6058–6065. doi: 10.1128/AEM.00953-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilbanks EG, Larsen DJ, Neches RY, Yao AI, Wu CY, Kjolby RAS, Facciotti MT. 2012. A workflow for genome-wide mapping of archaeal transcription factors with ChIP-seq. Nucleic Acids Res 40:e74. doi: 10.1093/nar/gks063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian J, Sinskey AJ, Stubbe J. 2005. Kinetic studies of polyhydroxybutyrate granule formation in Wautersia eutropha H16 by transmission electron microscopy. J Bacteriol 187:3814–3824. doi: 10.1128/JB.187.11.3814-3824.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coles M, Djuranovic S, Söding J, Frickey T, Koretke K, Truffault V, Martin J, Lupas AN. 2005. AbrB-like transcription factors assume a swapped hairpin fold that is evolutionarily related to double-psi beta barrels. Structure 13:919–928. doi: 10.1016/j.str.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Lu QH, Han J, Zhou LG, Zhou J, Xiang H. 2008. Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei. J Bacteriol 190:4173–4180. doi: 10.1128/JB.00134-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han J, Hou J, Zhang F, Ai GM, Li M, Cai SF, Liu HL, Wang L, Wang ZJ, Zhang SL, Cai L, Zhao DH, Zhou J, Xiang H. 2013. Multiple propionyl coenzyme A-supplying pathways for production of the bioplastic poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Haloferax mediterranei. Appl Environ Microbiol 79:2922–2931. doi: 10.1128/AEM.03915-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Luo Y, Han J, Wu J, Wu Z, Feng D, Cai S, Li M, Liu J, Zhou J, Xiang H. 2013. Proteome reference map of Haloarcula hispanica and comparative proteomic and transcriptomic analysis of polyhydroxyalkanoate biosynthesis under genetic and environmental perturbations. J Proteome Res 12:1300–1315. doi: 10.1021/pr300969m. [DOI] [PubMed] [Google Scholar]
- 38.Maehara A, Taguchi S, Nishiyama T, Yamane T, Doi Y. 2002. A repressor protein, PhaR, regulates polyhydroxyalkanoate (PHA) synthesis via its direct interaction with PHA. J Bacteriol 184:3992–4002. doi: 10.1128/JB.184.14.3992-4002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou ME, Yang MK. 2010. Analyses of binding sequences of the PhaR protein of Rhodobacter sphaeroides FJ1. FEMS Microbiol Lett 302:138–143. doi: 10.1111/j.1574-6968.2009.01836.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee TR, Lin JS, Wang SS, Shaw GC. 2004. PhaQ, a new class of poly-beta-hydroxybutyrate (PHB)-responsive repressor, regulates phaQ and phaP (phasin) expression in Bacillus megaterium through interaction with PHB. J Bacteriol 186:3015–3021. doi: 10.1128/JB.186.10.3015-3021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCool GJ, Cannon MC. 1999. Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol 181:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki H, Kuroda H, Yukawa Y, Sugiura M. 2011. The downstream atpE cistron is efficiently translated via its own cis-element in partially overlapping atpB-atpE dicistronic mRNAs in chloroplasts. Nucleic Acids Res 39:9405–9412. doi: 10.1093/nar/gkr644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maehara A, Doi Y, Nishiyama T, Takagi Y, Ueda S, Nakano H, Yamane T. 2001. PhaR, a protein of unknown function conserved among short-chain-length polyhydroxyalkanoic acids producing bacteria, is a DNA-binding protein and represses Paracoccus denitrificans phaP expression in vitro. FEMS Microbiol Lett 200:9-15. doi: 10.1111/j.1574-6968.2001.tb10685.x. [DOI] [PubMed] [Google Scholar]
- 44.Chumsakul O, Takahashi H, Oshima T, Hishimoto T, Kanaya S, Ogasawara N, Ishikawa S. 2011. Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation. Nucleic Acids Res 39:414–428. doi: 10.1093/nar/gkq780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaughn JL, Feher V, Naylor S, Strauch MA, Cavanagh J. 2000. Novel DNA binding domain and genetic regulation model of Bacillus subtilis transition state regulator abrB. Nat Struct Biol 7:1139–1146. doi: 10.1038/81999. [DOI] [PubMed] [Google Scholar]
- 46.Hsu CH, Wang AH. 2011. The DNA-recognition fold of Sso7c4 suggests a new member of SpoVT-AbrB superfamily from archaea. Nucleic Acids Res 39:6764–6774. doi: 10.1093/nar/gkr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao F, Strauch MA. 2005. Independent and interchangeable multimerization domains of the AbrB, Abh, and SpoVT global regulatory proteins. J Bacteriol 187:6354–6362. doi: 10.1128/JB.187.18.6354-6362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.