Abstract
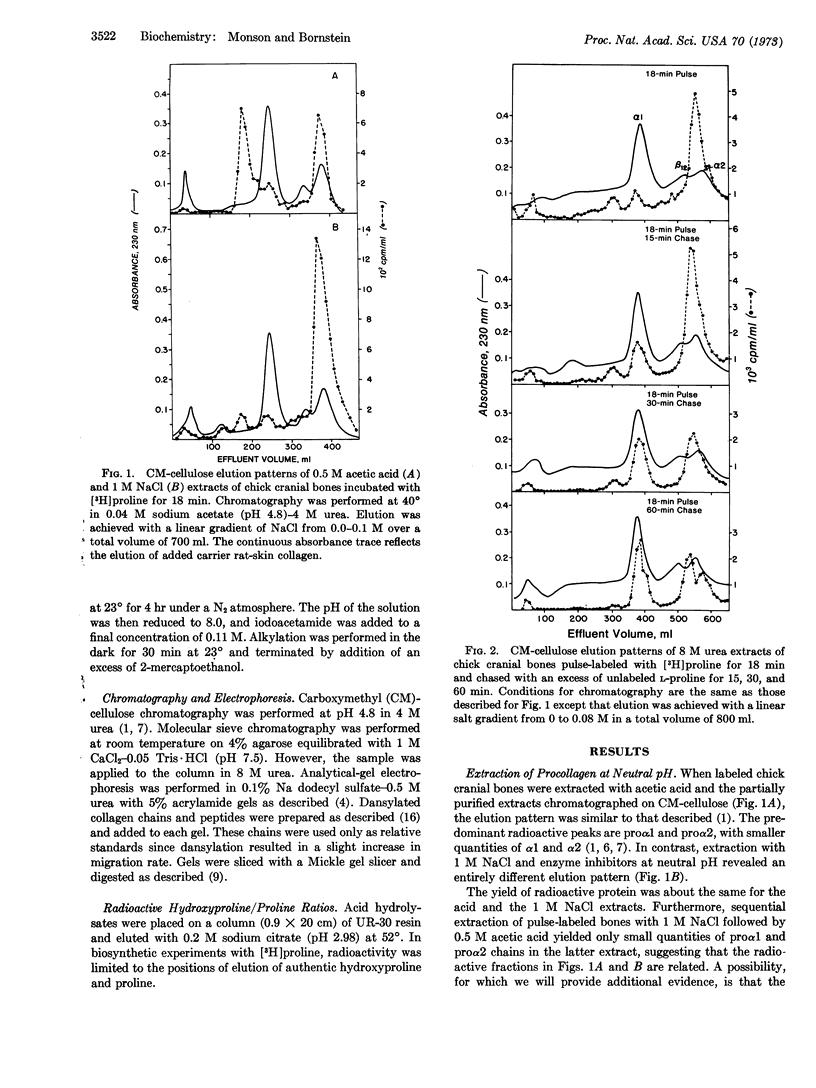
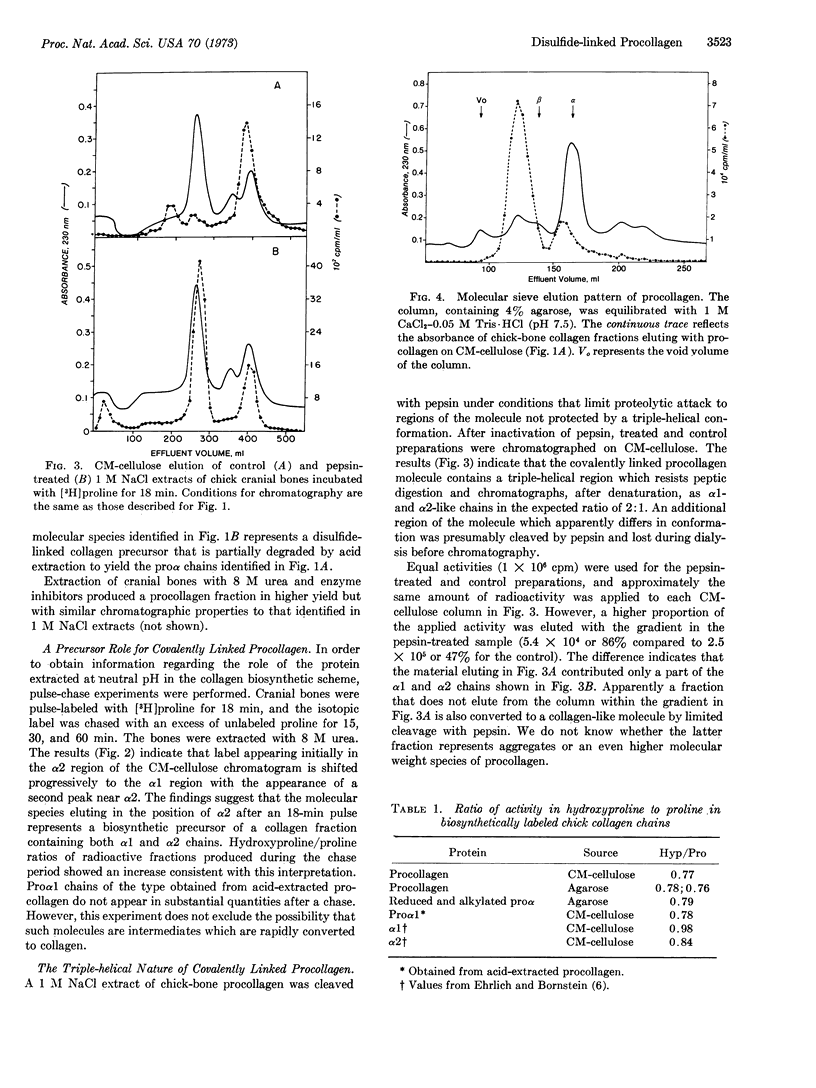
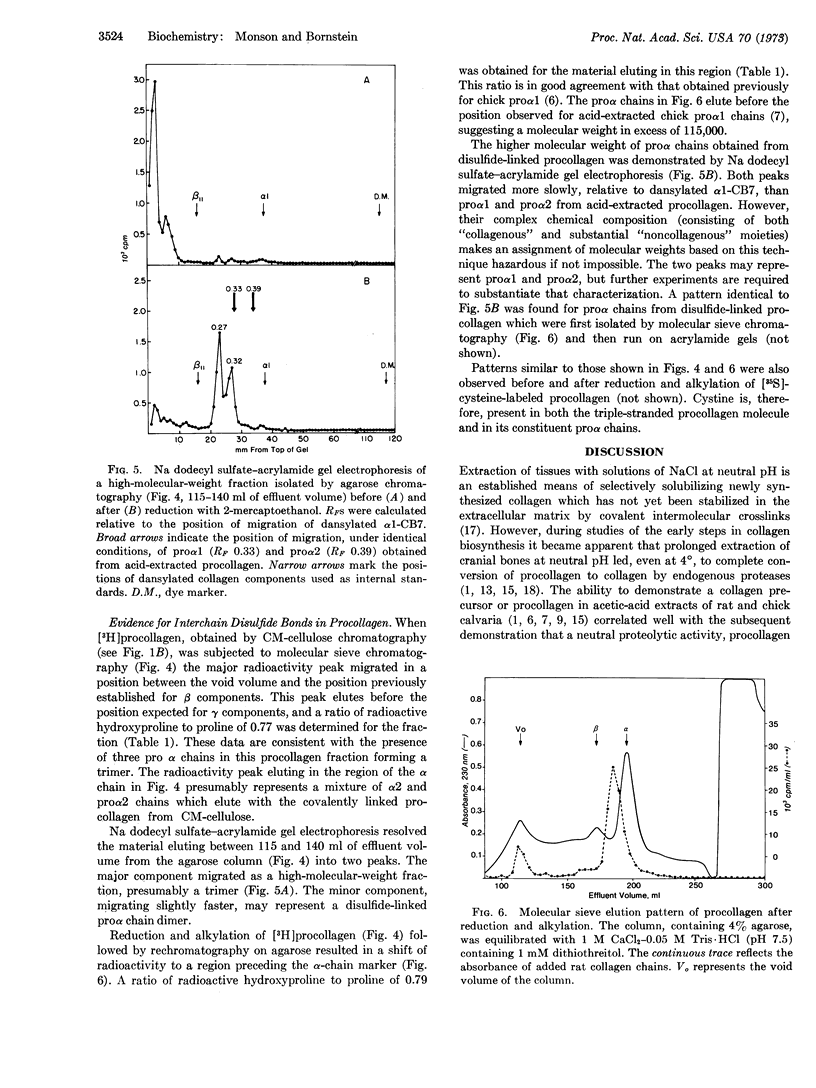
Chick cranial-bone procollagen, extracted at neutral pH in the presence of inhibitors of proteolytic enzymes, exists as a triple-stranded protein with disulfide bonds linking all three chains. The biosynthetic precursor function of this procollagen was demonstrated by pulsechase experiments. The ratio of radioactive hydroxyproline to proline in proα chains obtained by reduction and alkylation of the disulfide-bonded precursor was similar to the value determined for proα1 from acid-extracted procollagen. However, the molecular weight of these chains was higher than that previously determined for proα1 and proα2, suggesting that extraction of tissue at low pH results in a selective loss of disulfide-bonded regions from procollagen. These findings reconcile several apparently conflicting reports on the nature of procollagen identified in bone and in the medium of cultured fibroblasts and indicate that in both systems procollagen is synthesized as a disulfidelinked protein.
Keywords: carboxymethyl cellulose chromatography, molecular sieve chromatography
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates C. J., Bailey A. J., Prynne C. J., Levene C. I. The effect of ascorbic acid on the synthesis of collagen precursor secreted by 3T6 mouse fibroblasts in culture. Biochim Biophys Acta. 1972 Sep 29;278(2):372–390. doi: 10.1016/0005-2795(72)90241-3. [DOI] [PubMed] [Google Scholar]
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Ehrlich H. P., Wyke A. W. Procollagen: conversion of the precursor to collagen by a neutral protease. Science. 1972 Feb 4;175(4021):544–546. doi: 10.1126/science.175.4021.544. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Burgeson R. E., Wyke A. W., Fessler J. H. Collagen synthesis by cells II: secretion of a disulfide linked material. Biochem Biophys Res Commun. 1972 Aug 21;48(4):892–897. doi: 10.1016/0006-291x(72)90692-4. [DOI] [PubMed] [Google Scholar]
- Church R. L., Pfeiffer S. E., Tanzer M. L. Collagen biosynthesis: synthesis and secretion of a high molecular weight collagen precursor (procollagen). Proc Natl Acad Sci U S A. 1971 Nov;68(11):2638–2642. doi: 10.1073/pnas.68.11.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehm P., Jimenez S. A., Olsen B. R., Prockop D. J. A transport form of collagen from embryonic tendon: electron microscopic demonstration of an NH 2 -terminal extension and evidence suggesting the presence of cystine in the molecule (chick embryo-tropocollagen-gel filtration). Proc Natl Acad Sci U S A. 1972 Jan;69(1):60–64. doi: 10.1073/pnas.69.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Bornstein P. Further characterization of procollagen: the identification of a pro- 2 chain. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1750–1756. doi: 10.1016/0006-291x(72)90046-0. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol. 1972 Aug 30;238(87):257–260. doi: 10.1038/newbio238257a0. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Evidence for a previously undetected sequence at the carboxyterminus of the 1 chain of chicken bone collagen. Biochem Biophys Res Commun. 1972 Aug 7;48(3):720–727. doi: 10.1016/0006-291x(72)90408-1. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Epstein E. H., Jr, Sherr C. J. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3655–3659. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Sherr C. J. Secretion and extracellular processing of procollagen by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1973 Feb;70(2):361–365. doi: 10.1073/pnas.70.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Lapière C. M., Lenaers A., Kohn L. D. Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3054–3058. doi: 10.1073/pnas.68.12.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Rauterberg J., Fietzek P., Rexrodt F., Becker U., Stark M., Kühn K. The amino acid sequence of the carboxyterminal nonhelical cross link region of the alpha 1 chain of calf skin collagen. FEBS Lett. 1972 Mar;21(1):75–79. doi: 10.1016/0014-5793(72)80167-4. [DOI] [PubMed] [Google Scholar]
- Smith B. D., Byers P. H., Martin G. R. Production of procollagen by human fibroblasts in culture. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3260–3262. doi: 10.1073/pnas.69.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman P. T. Proposed mechanism for the biological assembly of collagen triple helix. Nature. 1971 Jan 22;229(5282):241–243. doi: 10.1038/229241a0. [DOI] [PubMed] [Google Scholar]
- Stark M., Lenaers A., Lapiere C., Kühn K. Electronoptical studies of procollagen from the skin of dermatosparaxic calves. FEBS Lett. 1971 Nov 1;18(2):225–227. doi: 10.1016/0014-5793(71)80450-7. [DOI] [PubMed] [Google Scholar]
- Veis A., Anesey J. R., Garvin J. E., Dimuzio M. T. High molecular weight collagen: a long-lived intermediate in the biogenesis of collagen fibrils. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1404–1411. doi: 10.1016/0006-291x(72)90869-8. [DOI] [PubMed] [Google Scholar]
- Von der Mark K., Bornstein P. Characterization of the pro 1 chain of procollagen. Isolation of a sequence unique to the precursor chain. J Biol Chem. 1973 Apr 10;248(7):2285–2289. [PubMed] [Google Scholar]
- Vuust J., Piez K. A. A kinetic study of collagen biosynthesis. J Biol Chem. 1972 Feb 10;247(3):856–862. [PubMed] [Google Scholar]



