Abstract
Understanding how the body's natural defenses function to protect the oral cavity from the myriad of bacteria that colonize its surfaces is an ongoing topic of research that can lead to breakthroughs in treatment and prevention. One key defense mechanism on all moist epithelial linings, such as the mouth, gastrointestinal tract, and lungs, is a layer of thick, well-hydrated mucus. The main gel-forming components of mucus are mucins, large glycoproteins that play a key role in host defense. This study focuses on elucidating the connection between MUC5B salivary mucins and dental caries, one of the most common oral diseases. Dental caries is predominantly caused by Streptococcus mutans attachment and biofilm formation on the tooth surface. Once S. mutans attaches to the tooth, it produces organic acids as metabolic by-products that dissolve tooth enamel, leading to cavity formation. We utilize CFU counts and fluorescence microscopy to quantitatively show that S. mutans attachment and biofilm formation are most robust in the presence of sucrose and that aqueous solutions of purified human MUC5B protect surfaces by acting as an antibiofouling agent in the presence of sucrose. In addition, we find that MUC5B does not alter S. mutans growth and decreases surface attachment and biofilm formation by maintaining S. mutans in the planktonic form. These insights point to the importance of salivary mucins in oral health and lead to a better understanding of how MUC5B could play a role in cavity prevention or diagnosis.
INTRODUCTION
One of the body's key defense mechanisms on wet epithelial linings, such as the mouth, gastrointestinal tract, and lungs, is a layer of thick, well-hydrated mucus. The viscoelastic properties of mucus are attributed to mucins, large glycoproteins that play a key role in host defense and maintaining a healthy microbial environment (1–3). Defects in mucin production can lead to diseases such as ulcerative colitis when mucins are underproduced or cystic fibrosis and asthma when mucins are overproduced (4–6). In addition, studies have shown that mucins can interact with microbes, such as Helicobacter pylori, Haemophilus parainfluenzae, and human immunodeficiency virus (7–10). These diseases and microbial interactions highlight the necessity of mucins as one of the body's key natural defenses; however, few studies have focused specifically on the connection between MUC5B salivary mucins and oral diseases. This study fills this gap in understanding by exploring the connection between purified human MUC5B and the virulence of Streptococcus mutans, one of the main cavity-causing bacteria naturally found in the oral cavity (11). MUC7 is another salivary mucin, but MUC5B is the primary mucin component of the dental pellicle coating the soft and hard tissues in the oral cavity (12, 13). Importantly, the effects of MUC5B are characterized in a clinically relevant three-dimensional model that mimics the natural environment in the oral cavity; mucins are secreted into an aqueous phase as opposed to a two-dimensional surface coating, which can create artificially concentrated amounts of surface mucins (14–16). Suspending MUC5B in media allows polymer domains to interact, preventing collapse of the hydrogel structure (8, 17, 18).
Understanding the structure of MUC5B illustrates how this specific glycoprotein can play such a dominant role in maintaining oral health. There are several serotypes of mucins throughout the body, but MUC5B is the predominant polymeric mucin found in the oral cavity and female genital tract (19, 20). In the oral cavity, MUC5B is produced by goblet cells in the submandibular and sublingual glands (2). The peptide backbone is composed of a variable number of tandem repeats (VNTR) section that has repeating sequences rich in serine, threonine, and proline, which participate in O-glycosylation (1, 2, 21). Because of the extended VNTR region, MUC5B is composed of approximately 80% carbohydrate in the form of O-linked glycan chains and 20% protein, consisting of the peptide backbone (22, 23). MUC5B's complex structure allows it to interact with an array of different salivary proteins and microbes to maintain a healthy oral cavity (24, 25). The exact mechanisms through which MUC5B provides defense are not well understood, but it has been proposed that it acts as a physical protective barrier, provides lubrication, and has antimicrobial properties (13, 25, 26).
S. mutans is a biofilm-forming facultative anaerobic bacterium that produces three glucosyltransferase enzymes to synthesize glucans from dietary sugar (27–29). Glucans are sticky polymers that allow the cells to attach to the tooth surface and form an extracellular matrix that protects it from host defenses and mechanical removal (30, 31). Once S. mutans attaches to the tooth surface, organic acids, which are produced as metabolic by-products, become concentrated within the extracellular matrix and cause a drop in pH from neutral to 5 or below. This acidic environment begins dissolving tooth enamel, leading to cavity formation, and the high tolerance of S. mutans for acidic environments gives it an ecological advantage. Without proper hygiene and nutritional awareness, S. mutans can proliferate quickly, causing serious damage to the tooth structure. S. mutans biofilm formation is particularly problematic in the interproximal spaces between teeth, where mechanical removal is difficult.
Because S. mutans attachment and biofilm formation are critical steps in cavity formation, we use CFU counts and fluorescence microscopy to quantify the effects of supplemental sugar and purified human salivary MUC5B on these key stages of disease progression. We first validate our mucin studies by showing that S. mutans attachment and biofilm formation are most robust in the presence of sucrose as opposed to glucose. When supplemental MUC5B is added in the presence of sucrose, however, S. mutans attachment and biofilm formation are significantly decreased. Although the number of surface-attached bacteria decreases in the presence of MUC5B, we show that bacterial growth is unchanged in the presence of MUC5B and the observed effects are due to increased numbers of S. mutans cells in the planktonic form. These findings that link MUC5B with S. mutans virulence could significantly impact our understanding of the pathogenesis of cavity formation and aid in the development of novel oral diagnostic methods or strategies for disease prevention.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strain Streptococcus mutans UA159 was kindly given as a gift by Dan Smith (Forsyth Institute). For sucrose and glucose experiments, S. mutans was grown overnight in brain heart infusion (BHI) medium (Becton, Dickinson and Company) containing 1% (wt/vol) sucrose and BHI with 1% (wt/vol) glucose (Sigma). For experiments determining the effects of MUC5B, S. mutans was grown overnight in BHI with 1% sucrose. BHI with 1% sucrose and either 0.3% MUC5B or 0.3% (wt/vol) methylcellulose (Sigma) was used to resuspend S. mutans cells before inoculating them into the experiment. Hydroxyapatite disc (Clarkson Chromatography, Inc.) or chambered glass slide (LabTek) surfaces were used to test S. mutans attachment and biofilm formation. S. mutans was grown and incubated at 37°C with 5% CO2.
Saliva collection.
Submandibular saliva was collected from 10 volunteers using a custom vacuum pump setup. Specifically, two holes were cut into the cap of a 50-ml conical tube (Falcon); the vacuum line was inserted into one hole and a small-diameter Tygon collection tube was inserted into the other hole (Saint Gobain Performance Plastics). Cotton swabs were used to absorb the volunteers' parotid gland secretions. The collection tube was used to suck up pooled unstimulated submandibular gland secretions from under the tongue. The collection vessel was kept on ice at all times. Saliva from volunteers was pooled before MUC5B purification. Protocols involving the use of human subjects were approved by Massachusetts Institute of Technology's Committee on the Use of Humans as Experimental Subjects.
MUC5B purification.
Immediately after collection, saliva was diluted using 5.5 M sodium chloride containing 0.04% sodium azide so that the final concentration of sodium chloride was 0.16 M. The following antibacterial agents and protease inhibitors were then added at the indicated final concentrations: benzamidine HCl (5 mM), dibromoacetophenone (1 mM), phenylmethylsulfonyl fluoride (1 mM), and EDTA (5 mM, pH 7) (Sigma). The mucins in the saliva were solubilized overnight by gentle stirring at 4°C. Saliva was then centrifuged at 3,800 × g for 10 min in a swinging-bucket centrifuge to remove cellular debris. MUC5B was purified using a Bio-Rad NGC fast protein liquid chromatography (FPLC) system equipped with an XK 50 column packed with Sepharose CL-2B resin (GE Healthcare Bio-Sciences). Mucin-containing fractions were identified using a periodic acid-Schiff's reagent assay and analysis of UV absorbance at 280 nm from FPLC. Fractions were then combined, dialyzed, and concentrated using an ultrafiltration device and were then lyophilized for storage at −80°C.
Assay of CFU counts to evaluate S. mutans attachment and biofilm formation.
To test the effects of sucrose or glucose on S. mutans physiology, S. mutans was grown to mid-exponential phase in BHI with 1% sucrose and BHI with 1% glucose, and then equal numbers of bacteria (107) from each culture were seeded in triplicate into wells containing glass or hydroxyapatite surfaces. For experiments testing the effect of MUC5B, S. mutans was grown to mid-exponential phase in BHI with 1% sucrose and then seeded in triplicate into wells containing BHI with 1% sucrose and 0.3% MUC5B or control medium. For all experiments, attachment was evaluated at 20, 40, and 60 min and biofilm formation was evaluated at 6, 18, and 24 h. Attachment was defined to occur at time points up to 1 h because the doubling time of S. mutans is approximately 1.5 h. Biofilm formation was defined to occur at all time points after 1 h. At the time point being evaluated, the surface was washed with phosphate-buffered saline (PBS) to remove nonadherent cells, fresh PBS was added, and then adherent cells were lifted using a sterile pipette tip. The suspended bacteria were vigorously pipetted to individualize the cells. The suspension was diluted (10−1 to 10−7) and plated on BHI agar. The numbers of CFU were counted after 24 to 36 h of incubation. Statistically significant differences were determined using Student's t test, with P values of <0.02 considered significant.
Fluorescent staining and microscopy.
To visually assess the effects of sucrose, glucose, or MUC5B on S. mutans attachment and biofilm formation, fluorescent SYTO9 staining with light microscopy was used (Life Technologies). S. mutans was grown to mid-exponential phase, then seeded into a chambered glass slide with BHI containing 1% sucrose and 0.3% MUC5B or with control medium. At the time point being evaluated, the surface was washed with PBS to remove nonadherent cells, and then 200 μl SYTO9 (0.6 μl SYTO9/200 μl Milli-Q water) was added. The biofilm was incubated with SYTO9 in the dark for 30 min. After incubation, the biofilm was washed with Milli-Q water to remove excess dye and fresh Milli-Q water was added. A Zeiss Axio Observer Z1 fluorescence inverted microscope was used for imaging. All experiments were repeated in triplicate.
Assay to evaluate S. mutans growth.
Overnight cultures of S. mutans at an optical density at 600 nm (OD600) of 0.05 were seeded in triplicate into a 96-well polystyrene plate containing BHI medium supplemented with 1% sucrose and 0.3% MUC5B or control medium. Bacteria were incubated at 37°C with 5% CO2. At 1-h intervals, the cultures were mixed and the OD600 was recorded using a microplate reader. The averages for each time point were plotted, and a comparison of S. mutans' growth rates in the various media was evaluated within the estimated error.
Time-kill assay.
CFU counts were used to evaluate the effect of MUC5B on S. mutans viability at time points up to 24 h. S. mutans was grown to mid-exponential phase in BHI with 1% sucrose, and then equal numbers of bacteria (107) were seeded in triplicate into glass-bottom wells containing BHI with 1% sucrose and 0.3% MUC5B or control medium. The cultures were incubated for 6, 18, and 24 h. At the time point being evaluated, the contents of the wells were gently mixed and then the supernatant was removed and diluted (10−1 to 10−8). The remaining biofilm was then washed with PBS, scraped off with a sterile pipette tip, and diluted (10−1 to 10−6). Dilutions were plated on BHI agar. The numbers of CFU were counted after 24 to 36 h of incubation to quantify the number of viable bacteria.
RESULTS AND DISCUSSION
Sucrose enhances S. mutans attachment and biofilm formation.
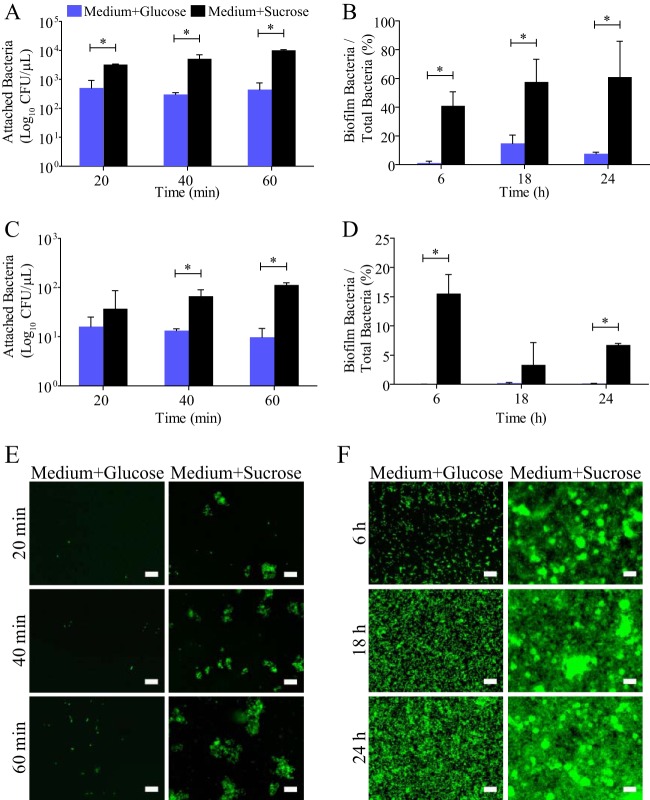
To determine the growth conditions where S. mutans attachment and biofilm formation are most robust, we investigated the effect of the addition of sucrose or glucose to BHI medium. S. mutans was inoculated into BHI containing 1% sucrose or 1% glucose. CFU counts were used to evaluate attachment at 20, 40, and 60 min and biofilm formation at 6, 18, and 24 h. Attachment was defined to occur at time points up to 60 min because the doubling time of S. mutans in exponential phase is approximately 1.5 h. Experiments were carried out on glass and hydroxyapatite discs because there are surface-specific effects on S. mutans attachment and biofilm formation (32). S. mutans attachment on glass and hydroxyapatite was increased by 15 and 6 times, respectively, when sucrose was present compared to when glucose was present (Fig. 1A and C). S. mutans biofilm formation in the presence of sucrose was increased by 45% on glass and 8% on hydroxyapatite compared to that in the presence of glucose (Fig. 1B and D). The number of S. mutans cells in the biofilm for each condition is represented as a fraction of the total number of bacteria in the well, because S. mutans' growth rate changes in the presence of sucrose compared with that in the presence of glucose (Fig. 2). The increase in S. mutans attachment and biofilm formation in the presence of sucrose is supported by a fluorescence microscopy time series using SYTO9 nucleic acid stain to visualize S. mutans attachment and biofilm formation in BHI containing 1% sucrose or 1% glucose on a glass surface (Fig. 1E and F). The role of sucrose in enhancing S. mutans biofilm formation has been well established using genetic analysis and biochemical assays studying biofilm architecture (33–36). Here we characterized this phenomenon using a quantitative method that directly evaluates the number of live S. mutans cells attached and producing biofilm on various surfaces. Our results support those of previous studies by quantitatively showing that the addition of 1% sucrose enhanced S. mutans attachment and biofilm formation at all time points compared to attachment and biofilm formation in the presence of 1% glucose. There was little or no growth in BHI without a sugar source, illustrating that protein alone cannot support S. mutans attachment or biofilm formation. Furthermore, our results showing that sucrose enhances S. mutans attachment and biofilm formation were consistent on hydroxyapatite and glass surfaces, indicating that the effect is not surface specific. These findings set the groundwork for our investigation of the role of MUC5B in S. mutans attachment and biofilm formation. When testing the effect of MUC5B on S. mutans physiology, 1% sucrose was added to BHI medium to challenge the effect of MUC5B by ensuring that S. mutans attachment and biofilm formation are most robust.
FIG 1.
Sucrose enhances S. mutans attachment and biofilm formation. The levels of S. mutans attachment (A) and biofilm formation (B) on glass are significantly enhanced at all time points when the bacteria are grown in BHI containing 1% sucrose (Medium+Sucrose) compared to the levels achieved in BHI containing 1% glucose (Medium+Glucose). S. mutans attachment (C) and biofilm formation (D) on hydroxyapatite are similarly increased in the presence of sucrose, illustrating that the effect of sucrose on S. mutans physiology is not surface specific. Fluorescence microscopy images verify the findings of the CFU count experiments by showing an increase in S. mutans attachment (E) and biofilm formation (F) on glass in the presence of sucrose. *, statistically significant difference determined by Student's t test (P < 0.02). Error bars represent SDs. Scale bars, 20 μm.
FIG 2.
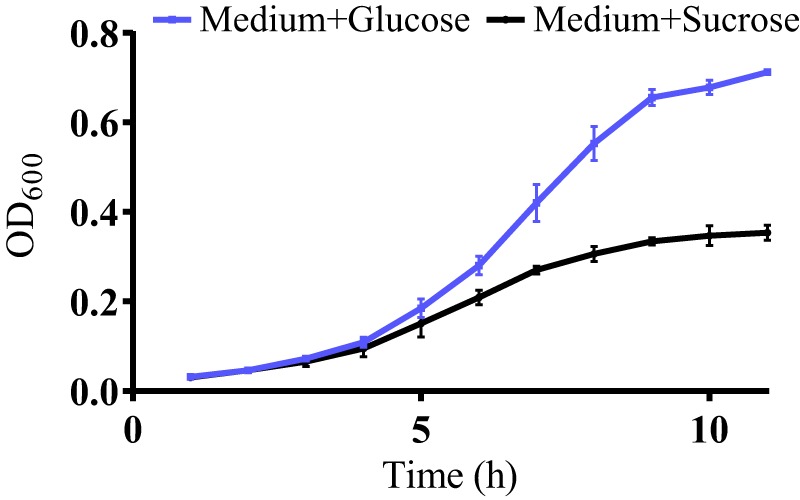
Supplemental sugar alters S. mutans growth. A growth curve of S. mutans in BHI with added 1% glucose (Medium+Glucose) or 1% sucrose (Medium+Sucrose) shows that S. mutans' growth rate changes based on the specific sugar present in the growth medium. Error bars represent SDs.
MUC5B decreases S. mutans attachment and biofilm formation.
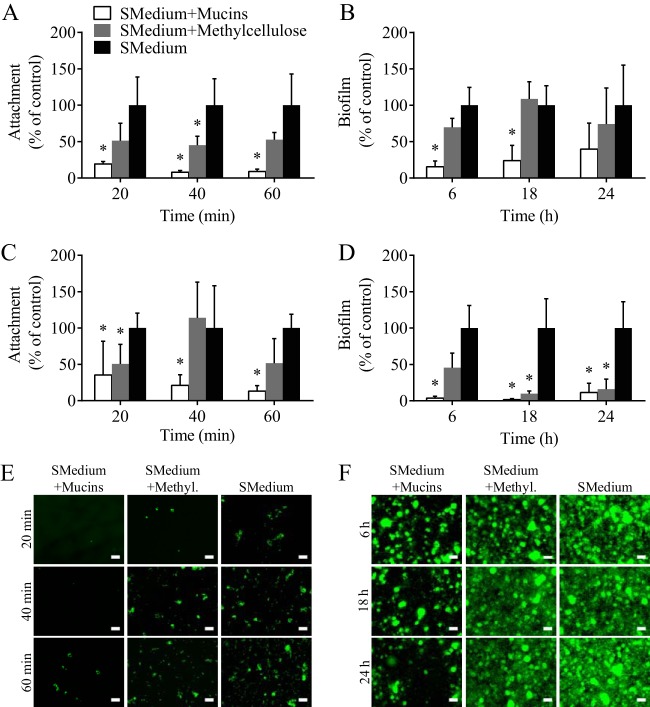
The effect of MUC5B on S. mutans attachment and biofilm formation was evaluated using CFU counts on various surfaces and fluorescence microscopy. S. mutans (107 bacteria) was grown in a chambered glass slide or on a hydroxyapatite disc in the presence of BHI with 1% sucrose and 0.3% MUC5B. BHI with 1% sucrose and 0.3% methylcellulose and BHI with no added polymer served as controls. Methylcellulose is a gel-forming compound that, like mucins, imparts viscosity but does not contain the complex, glycosylated structure that is characteristic of MUC5B. On glass, the addition of MUC5B to growth medium decreased S. mutans attachment by 88% and biofilm formation by 74% compared to the levels of attachment and biofilm formation in BHI with 1% sucrose (Fig. 3A and B). In comparison, the addition of methylcellulose reduced S. mutans attachment and biofilm formation on glass by 50% and 16%, respectively (Fig. 3A and B). When S. mutans was grown on hydroxyapatite discs in the presence of MUC5B, attachment was decreased by 77% and biofilm formation was decreased by 95% compared to the levels in BHI with 1% sucrose (Fig. 3C and D). In comparison, the presence of methylcellulose reduced S. mutans attachment by 27% and biofilm formation by 76% on hydroxyapatite discs (Fig. 3C and D). There was an overall decrease in biofilm formation at 18 and 24 h due to the dissolution of the hydroxyapatite discs. By evaluating the attachment and biofilm formation of S. mutans in medium containing methylcellulose, which simulates an environment that has physical properties similar to those of mucins, we can better understand if MUC5B is acting as a physical barrier to attachment most likely through increased viscosity or if the observed effect is due to specific MUC5B properties. We can conclude that the latter is most likely because S. mutans attachment and biofilm formation in the presence of MUC5B are significantly decreased at most time points compared to the levels in the presence of methylcellulose. There are at least three potential mechanisms by which MUC5B could protect the surface from bacterial colonization: (i) MUC5B could bind or agglutinate bacteria, which would allow planktonic bacteria to be swept out of the oral cavity with salivary flow but enhance bacterial attachment to surfaces coated with MUC5B (14, 25, 37–44), (ii) MUC5B could have the opposite effect, where its heterogeneous glycan chains repel bacteria, thereby preventing surface attachment (9, 11, 45–47), or (iii) MUC5B could directly downregulate S. mutans genes involved in attachment and biofilm formation. In our case, it appears that MUC5B is repelling S. mutans and/or directly influencing genetic modifications that protect the glass and hydroxyapatite surfaces from bacterial attachment and biofilm formation. The decrease in attachment caused by methylcellulose indicates that increased viscosity may also be playing some role in reducing attachment at early time points. Fluorescence microscopy experiments using SYTO9 staining confirm the findings obtained by CFU counts by showing a visually detectable decrease in the amount of S. mutans on glass and that the characteristic microcolony morphology of S. mutans biofilms is unchanged (Fig. 3E and F) (48, 49).
FIG 3.
Salivary mucins reduce S. mutans attachment and biofilm formation. The addition of 0.3% mucins to the control medium, BHI containing 1% sucrose (SMedium), significantly reduces the levels of S. mutans attachment and biofilm formation on glass (A, B) and hydroxyapatite (C, D) compared to the levels obtained with the control consisting of BHI with 1% sucrose. Similarly, the addition of 0.3% methylcellulose to BHI with 1% sucrose reduces S. mutans attachment and biofilm formation; however, the effect is not significant for the majority of time points studied. Fluorescence microscopy was used to visually assess S. mutans attachment (E) and biofilm formation (F) on glass when the bacteria are grown in BHI with 1% sucrose and 0.3% mucins, BHI with 1% sucrose and 0.3% methylcellulose (Methyl.), and BHI with 1% sucrose. *, statistically significant difference from BHI with 1% sucrose determined by Student's t test (P < 0.02). Error bars represent SDs. Scale bars, 20 μm.
MUC5B does not alter S. mutans growth.
To evaluate the effect of MUC5B on bacterial growth, S. mutans was grown in BHI medium containing 1% sucrose and 0.3% MUC5B. BHI with 1% sucrose and 0.3% methylcellulose and BHI with 1% sucrose were used as controls. These are the same media used in experiments that determine the effects of MUC5B on S. mutans attachment and biofilm formation. Optical density readings over the course of 12 h show that the addition of MUC5B or methylcellulose does not alter S. mutans growth compared to its growth in BHI containing 1% sucrose (Fig. 4). Because the growth of S. mutans is unchanged by MUC5B, we can conclude that the observed decrease in S. mutans attachment and biofilm formation in the presence of MUC5B is not due to slower growth but, rather, is due to the intrinsic properties of the MUC5B glycoprotein.
FIG 4.
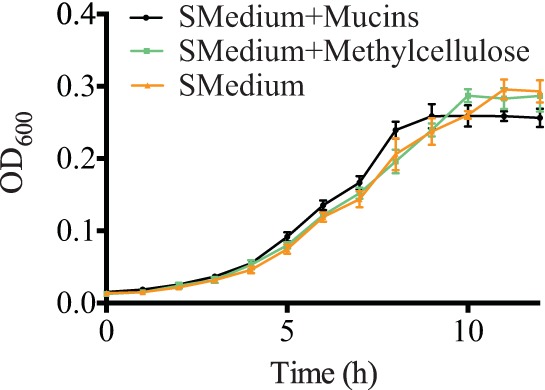
S. mutans growth is unaffected by the presence of salivary mucins. A growth curve of S. mutans in BHI with 1% sucrose (SMedium), BHI with 1% sucrose and 0.3% mucins, or BHI with 1% sucrose and 0.3% methylcellulose indicates that the presence of mucins and methylcellulose does not alter the growth of S. mutans. Error bars represent SDs.
MUC5B keeps S. mutans in planktonic form.
By quantifying the biofilm and supernatant bacteria in S. mutans cultures grown from 6 to 24 h, we determined the effect of MUC5B on S. mutans over time after stationary phase is reached. When the numbers of planktonic and biofilm S. mutans CFU are combined to determine the total number of viable S. mutans cells in a given experiment, results show that there is no significant difference between the total numbers of live bacteria in cultures containing 0.3% MUC5B and in control media (Fig. 5). Based on these findings, we show that the reduction in S. mutans attachment and biofilm formation on glass and hydroxyapatite in the presence of MUC5B (Fig. 3) is not due to bactericidal properties of MUC5B. The presence of MUC5B reduces S. mutans attachment and biofilm formation by maintaining bacteria in the planktonic phase. These findings point to the importance of MUC5B in establishing a healthy oral microbiome that allows species diversity but, at the same time, protects teeth from bacterial damage.
FIG 5.
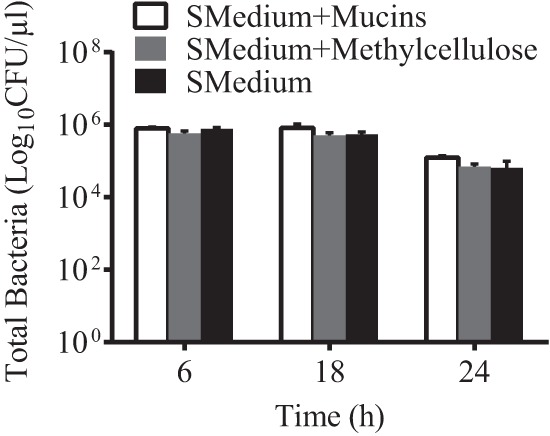
S. mutans survival is unaffected by salivary mucins. The graph represents the total number of viable S. mutans cells per well in the supernatant and biofilm in BHI with 1% sucrose (SMedium), BHI with 1% sucrose and 0.3% mucins, or BHI with 1% sucrose and 0.3% methylcellulose. Salivary mucins and methylcellulose show no bactericidal effects at time points up to 24 h. Error bars represent SDs.
Conclusion.
In summary, we used CFU counts and fluorescence light microscopy to quantitatively show that S. mutans attachment and biofilm formation are most robust when the organism is grown in the presence of sucrose and that the addition of purified human salivary MUC5B significantly decreases S. mutans attachment and biofilm formation even in the presence of sucrose. We determined that MUC5B does not alter S. mutans growth or lead to bacterial killing over 24 h but limits biofilm formation by maintaining S. mutans primarily in the planktonic form (Fig. 6). We speculate that the observed decrease in bacterial attachment and biofilm formation is due to a combination of genetic changes that decrease bacterial virulence and repulsion by MUC5B's heterogeneous glycans. S. mutans attachment and biofilm formation are key steps in the development of cavities; therefore, these findings have particularly important clinical implications. The presence or absence of MUC5B in the oral cavity could alter individuals' susceptibility to dental cavity formation, which could then be an easily accessible, highly predictable clinical diagnostic marker of disease. From a therapeutic standpoint, exogenous MUC5B could potentially be utilized as a treatment or preventative measure for cavities. These findings illustrate that MUC5B may help protect teeth from cavity formation, but further studies, such as those that use RNA sequencing or other genetic profiling techniques, are needed to fully characterize the mechanism underlying the observed decrease in S. mutans attachment and biofilm formation.
FIG 6.
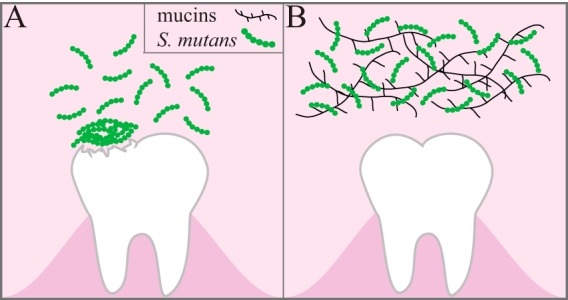
Summary of conclusions. S. mutans utilizes sucrose to form sticky extracellular polysaccharides that facilitate attachment to the tooth surface and subsequent biofilm formation. (A) In the biofilm, bacterial metabolism of sucrose causes a decrease in the local pH, leading to demineralization of the tooth structure. (B) The presence of mucins in sucrose-supplemented growth medium decreases S. mutans attachment and biofilm formation on the tooth surface by maintaining S. mutans in the planktonic state.
ACKNOWLEDGMENTS
We thank Nicole Kavanaugh for her critical review of the figures.
This work was made possible by generous funding support from Colgate-Palmolive (to K.R.) and Biological Sciences in Dental Medicine grants for tuition and a stipend (to E.S.F.).
REFERENCES
- 1.Brockhausen I, Schachter H, Stanley P. 2009. O-GalNAc glycans. In Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, Etzler M (ed), Essentials of glycobiology, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- 2.Perez-Vilar J, Mabolo R. 2007. Gel-forming mucins. Notions from in vitro studies. Histol Histopathol 22:455–464. [DOI] [PubMed] [Google Scholar]
- 3.Tabak LA. 1995. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol 57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 4.Van der Sluis M, De Koning B, De Bruijn A, Velcich A, Meijerink J, Van Goudoever J, Büller H, Dekker J, Van Seuningen I, Renes I, Einerhand A. 2006. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Rose MC, Voynow JA. 2006. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 6.Derrien M, van Passel MW, van de Bovenkamp JH, Schipper RG, de Vos WM, Dekker J. 2010. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 1:254–268. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habte HH, Mall AS, de Beer C, Lotz ZE, Kahn D. 2006. The role of crude human saliva and purified salivary MUC5B and MUC7 mucins in the inhibition of human immunodeficiency virus type 1 in an inhibition assay. Virol J 3:99. doi: 10.1186/1743-422X-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansil R, Turner BS. 2006. Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid Interface Sci 11:164–170. doi: 10.1016/j.cocis.2005.11.001. [DOI] [Google Scholar]
- 9.Veerman EC, Ligtenberg AJ, Schenkels LC, Walgreen-Weterings E, Nieuw Amerongen AV. 1995. Binding of human high-molecular-weight salivary mucins (MG1) to Hemophilus parainfluenzae. J Dent Res 74:351–357. doi: 10.1177/00220345950740011101. [DOI] [PubMed] [Google Scholar]
- 10.Bansil R, Celli JP, Hardcastle JM, Turner BS. 2013. The influence of mucus microstructure and rheology in Helicobacter pylori infection. Front Immunol 4:310. doi: 10.3389/fimmu.2013.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Hashimi I, Levine MJ. 1989. Characterization of in vivo salivary-derived enamel pellicle. Arch Oral Biol 34:289–295. doi: 10.1016/0003-9969(89)90070-8. [DOI] [PubMed] [Google Scholar]
- 13.Cárdenas M, Elofsson U, Lindh L. 2007. Salivary mucin MUC5B could be an important component of in vitro pellicles of human saliva: an in situ ellipsometry and atomic force microscopy study. Biomacromolecules 8:1149–1156. doi: 10.1021/bm061055h. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Cohen L, Hay DI. 1986. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun 52:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bushnak IA, Labeed FH, Sear RP, Keddie JL. 2010. Adhesion of microorganisms to bovine submaxillary mucin coatings: effect of coating deposition conditions. Biofouling 26:387–397. doi: 10.1080/08927011003646809. [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Ardehali R, Caldwell KD, Valint P. 2000. Mucin coating on polymeric material surfaces to suppress bacterial adhesion. Colloids Surf B Biointerfaces 17:229–239. doi: 10.1016/S0927-7765(99)00121-6. [DOI] [Google Scholar]
- 17.Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. 2010. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol 298:L15–L22. doi: 10.1152/ajplung.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakubov GE, Papagiannopoulos A, Rat E, Easton RL, Waigh TA. 2007. Molecular structure and rheological properties of short-side-chain heavily glycosylated porcine stomach mucin. Biomacromolecules 8:3467–3477. doi: 10.1021/bm700607w. [DOI] [PubMed] [Google Scholar]
- 19.Thornton DJ, Khan N, Mehrotra R, Howard M, Sheehan JK, Veerman E, Packer NH. 1999. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology 9:293–302. doi: 10.1093/glycob/9.3.293. [DOI] [PubMed] [Google Scholar]
- 20.Andersch-Björkman Y, Thomsson KA, Larsson JMH, Ekerhovd E, Hansson GC. 2007. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics 6:708–716. doi: 10.1074/mcp.M600439-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Gerken TA. 1993. Biophysical approaches to salivary mucin structure, conformation and dynamics. Crit Rev Oral Biol Med 4:261–270. [DOI] [PubMed] [Google Scholar]
- 22.Slomiany BL, Murty VLN, Piotrowski J, Slomiany A. 1996. Salivary mucins in oral mucosal defense. Gen Pharmacol Vasc Syst 27:761–771. doi: 10.1016/0306-3623(95)02050-0. [DOI] [PubMed] [Google Scholar]
- 23.Van Klinken BJ, Dekker J, Büller HA, Einerhand AW. 1995. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol 269:G613–G627. [DOI] [PubMed] [Google Scholar]
- 24.Iontcheva I, Oppenheim FG, Troxler RF. 1997. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich proteins, statherin, and histatins. J Dent Res 76:734–743. doi: 10.1177/00220345970760030501. [DOI] [PubMed] [Google Scholar]
- 25.Tabak LA, Levine MJ, Mandel ID, Ellison SA. 1982. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol 11:1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 26.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. 2008. Mucins in the mucosal barrier to infection. Mucosal Immunol 1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowen WH, Koo H. 2011. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res 45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano YJ, Kuramitsu HK. 1992. Mechanism of Streptococcus mutans glucosyltransferases: hybrid-enzyme analysis. J Bacteriol 174:5639–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krzyœciak W, Jurczak A, Koœcielniak D, Bystrowska B, Skalniak A. 2014. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis 33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross SE, Kreth J, Zhu L, Sullivan R, Shi W, Qi F, Gimzewski JK. 2007. Nanomechanical properties of glucans and associated cell-surface adhesion of Streptococcus mutans probed by atomic force microscopy under in situ conditions. Microbiology 153:3124–3132. doi: 10.1099/mic.0.2007/007625-0. [DOI] [PubMed] [Google Scholar]
- 31.Socransky SS, Haffajee AD. 2002. Dental biofilms: difficult therapeutic targets. Periodontol 2000 28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 32.Shemesh M, Tam A, Aharoni R, Steinberg D. 2010. Genetic adaptation of Streptococcus mutans during biofilm formation on different types of surfaces. BMC Microbiol 10:51. doi: 10.1186/1471-2180-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shemesh M, Tam A, Steinberg D. 2007. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J Med Microbiol 56:1528–1535. doi: 10.1099/jmm.0.47146-0. [DOI] [PubMed] [Google Scholar]
- 34.Koo H, Xiao J, Klein MI, Jeon JG. 2010. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol 192:3024–3032. doi: 10.1128/JB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duarte S, Klein MI, Aires CP, Cury JA, Bowen WH, Koo H. 2008. Influences of starch and sucrose on Streptococcus mutans biofilms. Oral Microbiol Immunol 23:206–212. doi: 10.1111/j.1399-302X.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 36.Kreth J, Zhu L, Merritt J, Shi W, Qi F. 2008. Role of sucrose in the fitness of Streptococcus mutans. Oral Microbiol Immunol 23:213–219. doi: 10.1111/j.1399-302X.2007.00413.x. [DOI] [PubMed] [Google Scholar]
- 37.McBride BC, Gisslow MT. 1977. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun 18:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Houte PJ. 1982. Bacterial adherence and dental plaque formation. Infection 10:252–260. doi: 10.1007/BF01666923. [DOI] [PubMed] [Google Scholar]
- 39.Levine MJ, Herzberg MC, Levine MS, Ellison SA, Stinson MW, Li HC, van Dyke T. 1978. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun 19:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein A, Carnoy C, Wieruszeski JM, Strecker G, Strang AM, Van Halbeek H, Roussel P, Lamblin G. 1992. The broad diversity of neutral and sialylated oligosaccharides derived from human salivary mucins. Biochemistry (Mosc) 31:6152–6165. doi: 10.1021/bi00141a028. [DOI] [PubMed] [Google Scholar]
- 41.Williams RC, Gibbons RJ. 1975. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun 11:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsson KA, Prakobphol A, Leffler H, Reddy MS, Levine MJ, Fisher SJ, Hansson GC. 2002. The salivary mucin MG1 (MUC5B) carries a repertoire of unique oligosaccharides that is large and diverse. Glycobiology 12:1–14. doi: 10.1093/glycob/12.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Gibbons RJ, Dankers I. 1981. Lectin-like constituents of foods which react with components of serum, saliva, and Streptococcus mutans. Appl Environ Microbiol 41:880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibbons RJ, Qureshi JV. 1978. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun 22:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray PA, Prakobphol A, Lee T, Hoover CI, Fisher SJ. 1992. Adherence of oral streptococci to salivary glycoproteins. Infect Immun 60:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amerongen AVN, Bolscher JGM, Veerman ECI. 1995. Salivary mucins: protective functions in relation to their diversity. Glycobiology 5:733–740. doi: 10.1093/glycob/5.8.733. [DOI] [PubMed] [Google Scholar]
- 47.Schenkels LCPM, Ligtenberg AJM, Veerman ECI, Van Nieum Amerongen A. 1993. Interaction of the salivary glycoprotein EP-GP with the bacterium Streptococcus salivarius HB. J Dent Res 72:1559–1565. doi: 10.1177/00220345930720120501. [DOI] [PubMed] [Google Scholar]
- 48.Xiao J, Koo H. 2010. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J Appl Microbiol 108:2103–2113. doi: 10.1111/j.1365-2672.2009.04616.x. [DOI] [PubMed] [Google Scholar]
- 49.Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR III, Heydorn A, Koo H. 2012. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog 8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]