Abstract
Corrosion of iron occurring under anoxic conditions, which is termed microbiologically influenced corrosion (MIC) or biocorrosion, is mostly caused by microbial activities. Microbial activity that enhances corrosion via uptake of electrons from metallic iron [Fe(0)] has been regarded as one of the major causative factors. In addition to sulfate-reducing bacteria and methanogenic archaea in marine environments, acetogenic bacteria in freshwater environments have recently been suggested to cause MIC under anoxic conditions. However, no microorganisms that perform acetogenesis-dependent MIC have been isolated or had their MIC-inducing mechanisms characterized. Here, we enriched and isolated acetogenic bacteria that induce iron corrosion by utilizing Fe(0) as the sole electron donor under freshwater, sulfate-free, and anoxic conditions. The enriched communities produced significantly larger amounts of Fe(II) than the abiotic controls and produced acetate coupled with Fe(0) oxidation prior to CH4 production. Microbial community analysis revealed that Sporomusa sp. and Desulfovibrio sp. dominated in the enrichments. Strain GT1, which is closely related to the acetogen Sporomusa sphaeroides, was eventually isolated from the enrichment. Strain GT1 grew acetogenetically with Fe(0) as the sole electron donor and enhanced iron corrosion, which is the first demonstration of MIC mediated by a pure culture of an acetogen. Other well-known acetogenic bacteria, including Sporomusa ovata and Acetobacterium spp., did not grow well on Fe(0). These results indicate that very few species of acetogens have specific mechanisms to efficiently utilize cathodic electrons derived from Fe(0) oxidation and induce iron corrosion.
INTRODUCTION
Corrosion of iron structures causes great economic losses and environmental pollution. Iron corrosion is an electrochemical process involving oxidation of metallic iron [Fe(0)] to Fe(II) (anodic reaction; equation 1) and reduction of external electron acceptors (cathodic reaction):
| (1) |
where −0.47 V references the standard hydrogen electrode (SHE). The cathodic reaction consists of oxygen reduction in the presence of air (equation 2), while it usually consists of proton reduction (H2 evolution) under anoxic conditions (equation 3):
| (2) |
| (3) |
where +0.82 V and −0.41 V reference the SHE.
The cathodic hydrogen evolution on iron surfaces is usually a particularly slow reaction under neutral pH conditions, because proton availability is limited and the reaction has low electrode potential and high overpotential. Hence, theoretically, iron corrosion in anoxic environments should not be a serious problem. However, iron corrosion in anoxic environments has been reported often and, in most cases, it is thought to be mediated by metabolic activities of microorganisms therein (1–3). Iron corrosion that occurs in this manner is termed microbiologically influenced corrosion (MIC) or biocorrosion. Diverse kinds of microorganisms, including sulfate-reducing bacteria (SRB), Fe(II) oxidizers, Fe(III) reducers, fermenting bacteria, and methanogens, have been reported as contributing to MIC (4–9). These microorganisms induce MIC in a number of ways, including formation of redox/chemical gradient on the iron surfaces, production of corrosive chemicals (e.g., H2S and organic acids), degradation of protective coatings on iron surfaces, and acceleration of cathodic reactions (10).
Among such diverse MIC mechanisms, acceleration of cathodic reactions, often referred to as cathodic depolarization, was first proposed in the 1930s and has been considered one of the most prominent causes of MIC (3). Cathodic depolarization is induced by microorganisms utilizing electrons in Fe(0) as the electron donor, resulting in acceleration of the cathodic reaction and hence the overall corrosion process. Microorganisms consuming H2 abiotically generated on iron surfaces (equation 3) coupled with sulfate reduction (equation 4) or methanogenesis (equation 5) have long been considered agents of cathodic depolarization (11, 12):
| (4) |
| (5) |
where −0.22 V and −0.24 V reference the SHE.
However, recent studies demonstrated that only microorganisms with special mechanisms to take up electrons from Fe(0) have the ability to induce MIC. Dinh et al. reported that novel SRB and methanogens enriched and isolated with Fe(0) as the sole electron donor reduced sulfate and produced methane, respectively, at much higher rates than were seen with abiotic H2 production from Fe(0), while authentic H2 scavengers did not show such activities (13). After that, two different methanogenic strains with an increased ability to induce MIC compared to the closely related hydrogenotrophic strains were isolated (7, 14). Physiological and electrochemical analyses have disclosed that the MIC-inducible SRB strains appear to take up electrons directly from Fe(0) rather than consuming abiotically generated H2, although the molecular mechanisms remain unknown (15–17). The MIC induced by direct consumption of cathodic electrons was specially termed electrical MIC (EMIC) and was considered the major causal factor of cathodic depolarization (15–17).
All EMIC-inducing microorganisms isolated so far were from marine environments (7, 13, 14). Although MIC is also problematic in freshwater and terrestrial soil environments (12, 18–21), knowledge on MIC-inducible freshwater microorganisms has been limited. In addition to methanogens, microorganisms that conserve energy through acetogenesis (equation 6) have been proposed to engage in EMIC in freshwater environments (22):
| (6) |
where −0.28 V references the SHE.
Acetogenesis-dependent MIC was recently demonstrated to induce biocorrosion in sulfate-free freshwater environment under anoxic conditions (23). However, acetogenesis-dependent MIC has been observed in cultures of complex microbial communities. Pure cultures that perform acetogenesis-dependent MIC have not been isolated, and the mechanisms for acetogenesis-dependent MIC remain unknown.
In the present study, we enriched for MIC-inducing microorganisms by culturing rice paddy field soil microbial communities in a sulfate-free freshwater medium with Fe(0) granules as the sole electron donor. Further enrichment was made in the presence of methanogen inhibitor with the hope of observing MIC concomitantly with acetogenesis. We found that acetogenesis was coupled with MIC and eventually isolated the organisms responsible for acetogenesis-dependent MIC.
MATERIALS AND METHODS
Enrichment cultures of iron-corroding microbial communities.
Microbial communities were enriched in serum bottles (68 ml in capacity) filled with 20 ml of a freshwater basal medium containing the following ingredients (per liter): 0.3 g of KH2PO4; 1 g of NH4Cl; 0.1 g of MgCl2·7H2O; 0.08 g of CaCl2·7H2O; 0.6 g of NaCl; 2 g of KHCO3; 9.52 g of HEPES; 0.03 g of Na2S·9H2O; 0.1 g yeast extract; and 10 ml each of trace metal solution and vitamin solution (24). The medium pH was adjusted to 7.0 and kept constant during incubation (pH 7.0 ± 0.2). For the enrichments of the iron-corroding and H2-oxidizing microbial community, 1 g of iron granules (Alfa Aesar) (1 to 2 mm in diameter, 99.98% purity) and 200 kPa of H2:CO2 (80:20), respectively, were supplemented as the sole electron donor. Approximately 50 mg (wet weight) of rice paddy field soil was inoculated as a source of microorganisms. Unless stated otherwise, the cultivations were conducted at 30°C under an atmosphere of N2:CO2 (80:20) without shaking. When methanogenesis reached a plateau, 0.5 ml of culture solution was transferred to the fresh media. After five passages, the enrichment cultures were subjected to chemical and phylogenetic analyses.
Isolation of acetogenic bacteria.
Cultures of Fe(0) and Fe(0)-plus-2-bromoethanesulfonate [Fe(0)+BES] enrichments were subjected to vigorous vortex mixing to detach microbial cells from iron granules. The culture solution was serially diluted with the freshwater basal medium and spread onto the same medium solidified with 0.6% (wt/vol) gellan gum. Lactate (10 mM) or ethanol (10 mM) or methanol (10 mM) or H2:CO2 (80:20) was supplemented as the carbon and energy source(s). All procedures were conducted in an anaerobic chamber filled with N2 gas. The inoculated plates were incubated under anaerobic conditions using an AnaeroPack pouch bag (Mitsubishi Gas Chemical) with an AnaeroPack oxygen absorber at 30°C. Grown colonies were further purified by repetitive plating. The purity of isolates was confirmed by microscopic observation.
Pure culture experiments for iron corrosion.
Sporomusa sphaeroides DSM2875, Sporomusa ovate DSM2662, Acetobacterium carbinolicum DSM2925, and Acetobacterium woodii DSM1030 were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). These strains and isolated acetogens were routinely cultivated in the freshwater basal medium supplemented with 20 mM trimethylglycine under anaerobic conditions at 30°C. For the test of biocorrosion, all strains were cultivated in the freshwater basal medium supplemented with 1 g of iron granules as the sole electron donor.
Phylogenetic analyses.
Microbial DNA was extracted with a Fast DNA Spin kit for soil (MP Biomedicals) according to the manufacturer's instructions. The whole lengths of 16S rRNA gene sequences of isolated strains were determined by direct sequencing of the DNA fragment amplified by PCR with primer pair 27F and 1492R as described previously (25). PCR amplification of 16S rRNA gene fragments for clone library analyses was conducted as described previously (26), with primer pair U515f and U1492r for bacteria and primer pair A25f and A958r for archaea (27). The sequences obtained were assigned to each phylotype with a cutoff value of 97% identity using FastGroup II software (28). Classification of phylotypes was performed using the Classifier program in the Ribosomal Database Project database (29). The sequence of each phylotype was compared with those in the GenBank nucleotide sequence database using the BLAST program (30) to infer the most closely related species.
Chemical analyses.
The partial pressure of H2 and CH4 was determined using a gas chromatograph (GC-2014; Shimadzu) as described previously (31). The concentration of acetate was determined using a high-performance liquid chromatography system (D-2000 LaChrom Elite HPLC system; Hitachi) equipped with an Aminex HPX-87H ion exclusion column (Bio-Rad) and an L2400 UV detector (Hitachi). Ferrous iron in the whole cultures, including iron granules and microbiologically produced mineral particles, was dissolved by 0.67 M HCl (containing 0.67 M hexamethylenetetramine to avoid dissolution of metallic iron) and quantified colorimetrically using a ferrozine method as described elsewhere (32).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this study have been submitted to GenBank under accession no. AB854327 to AB854356 and AB981438.
RESULTS AND DISCUSSION
Enrichment of MIC-inducing microorganisms with Fe(0) as the sole electron donor.
Iron-corroding microorganisms were enriched from rice paddy field soil using a sulfate-free freshwater medium supplemented with Fe(0) granules as the sole electron donor under an N2/CO2 gas atmosphere [here designated Fe(0) enrichments]. The medium contained only CO2 and H+ as available electron acceptors. After several weeks of cultivation, CH4 generation was observed, the surface of the iron granules turned a dull-gray color, and grayish precipitate was formed (Fig. 1). CH4 production and corrosion products were not observed in the abiotic controls (without inoculation of soil) and cultures without iron granules. These observations demonstrated that growth of microorganisms utilizing Fe(0) as the sole electron donor occurred in the Fe(0) enrichments and that most of the reducing equivalents for the CH4 generation were derived from Fe(0) oxidation.
FIG 1.
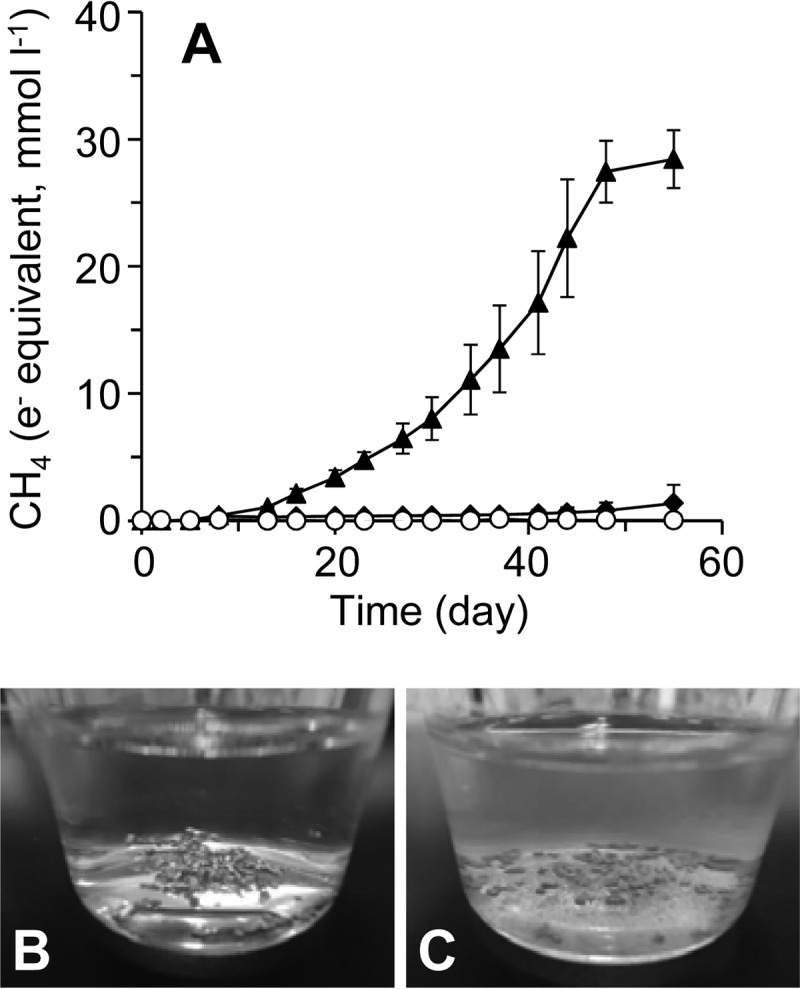
Corrosion and methanogenesis in the cultures of the soil microbial community with Fe(0) granules. (A) Generation of CH4 was monitored during the incubation of a paddy soil microbial community in the presence (filled triangles) and absence (filled diamonds) of Fe(0) granules. The data of the abiotic controls (inoculated with sterilized soil) are also shown (open circles). Data are presented as the means of the results of three independent cultures, and error bars represent standard deviations. mmoll−1, millimoles per liter. (B and C) The photographs were taken after 50 days of incubation of the abiotic control (B) and paddy soil microbial community (C) in the presence of Fe(0) granules.
Metabolic profiles of the enrichment cultures.
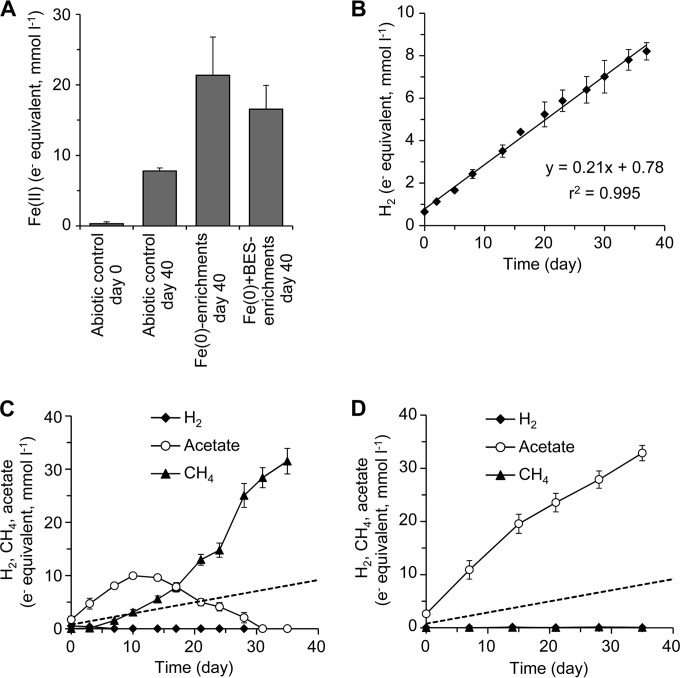
After five successive subcultures, the metabolic products in the Fe(0) enrichments were analyzed. For ease of comparison, the amounts of all metabolites were represented as electron equivalents in the unit of mmol e− per liter of culture medium, using the respective half-reaction formulas (equations 1 to 6). To confirm the occurrence of MIC, the amounts of Fe(II) were measured after the cultivation of the Fe(0) enrichments (Fig. 2A). The amount of HCl-extractable Fe(II) in the Fe(0) enrichment cultures was significantly greater than that of the Fe(II) generation in the abiotic control, indicating that microorganisms in the Fe(0) enrichments have the ability to induce MIC. It should be noted that the value of Fe(II) generation in the Fe(0) enrichment cultures may be underestimated, since some grayish precipitate remained even after the acid treatments.
FIG 2.
Metabolites generated in the enrichment cultures. (A) Generation of Fe(II) from Fe(0) granules by the enrichment cultures and in the abiotic controls. (B) Generation of H2 in the abiotic controls with Fe(0) granules. The approximation curve estimated by the least-squares method is given. (C and D) Acetate, CH4, and H2 production in the Fe(0) (C) and Fe(0)+BES (D) enrichments. The dashed lines represent the abiotic corrosion rate calculated from the abiotic H2 production rate shown in panel B. Data are presented as the means of the results of three independent cultures, and error bars represent standard deviations.
The amounts of H2, CH4, and organic compounds were measured to infer the microbial metabolisms causing MIC. Acetate was the only organic compound detected from the enrichment cultures throughout this study. Continuous generation of H2 was observed during incubation of the abiotic control (Fig. 2B). Since the reducing equivalents for the H2 generation should be derived from chemical oxidation of Fe(0), the abiotic corrosion rate under the experimental conditions was calculated to be 0.21 ± 0.01 mmol e− equivalent liter−1 day−1.
In contrast, instead of H2 generation, production of CH4 and acetate was observed in the Fe(0) enrichment cultures (Fig. 2C). The amount of acetate gradually increased and peaked at day 10, followed by a gradual decrease. CH4 was produced after acetate accumulation. The acetate production rate during the first 10 days (0.83 ± 0.04 mmol e− equivalent liter−1 day−1) was nearly 4 times higher than the abiotic corrosion rate. These observations imply that acetate production with Fe(0) as the electron donor was the main metabolic process occurring in the Fe(0) enrichments during at least the first 10 days.
The Fe(0) enrichments were further enriched in the presence of a specific inhibitor of methanogens to confirm a role of acetogenesis for MIC [here designated Fe(0)+BES enrichments]. While methanogenesis was inhibited completely in the presence of 5 mM BES, the acetate concentration gradually increased (Fig. 2D). The acetate production rate (1.13 ± 0.12 mmol e− equivalent liter−1 day−1) was more than 5 times higher than the abiotic corrosion rate. The amount of Fe(II) generated by the Fe(0)+BES enrichments after 40 days of incubation was also significantly higher than that seen with the abiotic control (Fig. 2A). These results clearly demonstrated that acetogenesis with Fe(0) as the electron donor is the main causative factor for MIC observed in the enrichment cultures.
Microbial community analysis of the enrichment cultures.
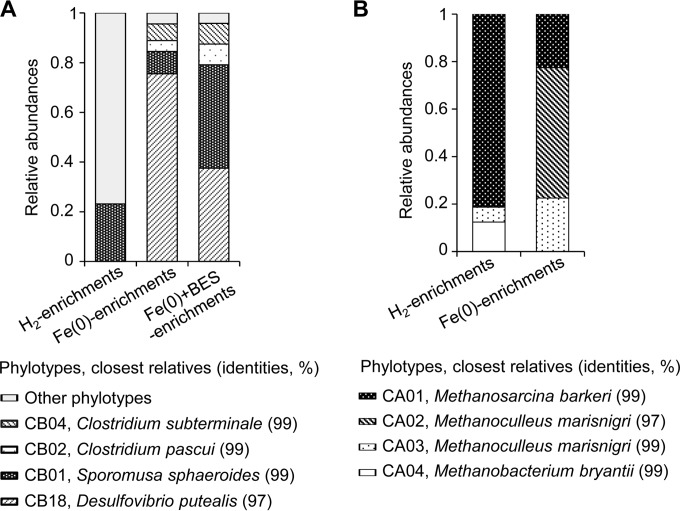
Microbial community analysis was conducted to identify the microbial species contributing to MIC. As a control, enrichment cultures with H2 as the sole electron donor, designated H2 enrichments, were separately constructed with microbes derived from rice paddy field soil, and consumption of H2 and concomitant generation of both CH4 and acetate were observed (see Fig. S1 in the supplemental material). The 16S rRNA gene fragments were amplified by PCR from genomic DNA extracted from the H2, Fe(0), and Fe(0)+BES enrichments using universal primers targeting either the bacterial or archaeal counterparts, cloned, sequenced, and subjected to phylogenetic analysis. The summarized features of the bacterial and archaeal community analyses are presented in Fig. 3. All phylotypes detected from each enrichment culture are listed in Tables S1 and S2 in the supplemental material.
FIG 3.
Phylogenetic distribution of bacterial (A) and archaeal (B) 16S rRNA gene clones recovered from the H2, Fe(0), and Fe(0)+BES enrichments.
Relatively simple bacterial populations were found in the Fe(0) and Fe(0)+BES enrichments, in which most of the phylotypes are affiliated with either the phylum Proteobacteria or the phylum Firmicutes. All Proteobacteria clones recovered from the Fe(0) and Fe(0)+BES enrichments were classified into the CB18 phylotype, whose closest relative is Desulfovibrio putealis (97% identity) (Fig. 3A; see also Table S1 in the supplemental material). This phylotype was not detected from the H2 enrichments. D. putealis has been reported to be a typical SRB and has not been examined for acetogenic activity (33). The enrichment cultures were sulfate free, and the major energy metabolism occurring in the Fe(0) and Fe(0)+BES enrichments was most likely Fe(0)-dependent acetogenesis. Some autotrophic SRB, including Desulfovibrio spp., harbor the enzymatic system for the Wood-Ljungdahl pathway, which is required for acetogenesis, as a carbon fixation pathway (34, 35). Also, Desulfovibrio spp. have often been detected as the major microbial constituent in sulfate-free environments with H2 or cathodic electrodes as the sole electron donor (36–38). These reports support the possibility that the D. putealis-like phylotype contributes to the acetogenesis-dependent MIC observed in the Fe(0) and Fe(0)+BES enrichments.
The most abundant Firmicutes phylotype detected from the Fe(0) and Fe(0)+BES enrichments was the CB01 phylotype, which is closely related to Sporomusa sphaeroides (99% identity) (Fig. 3A; see also Table S1 in the supplemental material). Since Sporomusa spp. are typical acetogenic bacteria (39), the CB01 phylotype is also assumed to engage in acetogenesis-dependent MIC. The other major Firmicutes phylotypes detected from the Fe(0) and Fe(0)+BES enrichments, phylotypes CB02 and CB04, were affiliated with Clostridium cluster I and are closely related to Clostridium pascui (99% identity) and Clostridium subterminale (99% identity), respectively (Fig. 3A; see also Table S1). While C. pascui and C. subterminale are nonsaccharolytic, amino-acid-fermenting bacteria and their acetogenic activity has not been reported (40), Clostridium cluster I contains some acetogenic species such as Clostridium magnum and Clostridium drakei (41). Also, stable-isotope-labeling studies of H2/13CO2-fed soil microbial communities demonstrated that Clostridium cluster I bacteria function as dominant acetogens in natural environments (42). While the Clostridium-like phylotypes detected in this study are assumed to grow on amino acids derived from supplemented yeast extract and/or produced by acetogenic bacteria, the possibility of their contribution to acetogenesis-dependent MIC cannot be excluded.
Only 4 archaeal phylotypes were recovered from the H2 and Fe(0) enrichments (Fig. 3B; see also Table S2 in the supplemental material). The CA01 phylotype (99% identity to Methanosarcina barkeri) was the only phylotype classified as an aceticlastic methanogen. This phylotype dominated in the H2 and Fe(0) enrichments and was the phylotype most likely to contribute to conversion of acetate into CH4. In the Fe(0) enrichments, the ratio of hydrogenotrophic and aceticlastic methanogens was significantly higher than in the H2 enrichments (Fig. 3B). The CA02 phylotype (97% identity to Methanoculleus marinigri) dominated in the Fe(0) enrichments, while this phylotype was not detected in the H2 enrichments. This observation suggests that the CA02 phylotype contributed to methanogenesis-dependent MIC in the Fe(0) enrichments. Further studies needed to be done to confirm the assumption.
Isolation of acetogenic bacteria from the enrichment cultures.
After enrichment, acetogenic bacteria were isolated from the enrichment cultures. Samples of the Fe(0) and Fe(0)+BES enrichments were serially diluted and inoculated onto the gellan gum-solidified freshwater basal medium supplemented with lactate, ethanol, methanol, or H2:CO2 as the carbon and energy sources. Colonies appeared within 1 week of incubation with all tested substrates and were transferred to the same medium for further purification. After purification, the partial 16S rRNA gene sequences of >20 strains were determined. Among the isolates, 3 strains had nearly identical sequences (>99.5% identity to each other), and their sequences are closely related to those of both S. sphaeroides DSM2875 and the CB01 phylotype that dominated in the Fe(0) and Fe(0)+BES enrichment (>99.5% identity). One of the isolates, designated strain GT1, was selected as a representative strain for further experiments. The 16S rRNA gene sequence of strain GT1, containing a continuous stretch of 1,446 nucleotides (nt), was determined. The sequence-similarity calculations indicated that strain GT1 is closely related to S. sphaeroides DSM2875 (99.7% identity).
Acetogenesis-dependent MIC by the isolated strain and authentic acetogens.
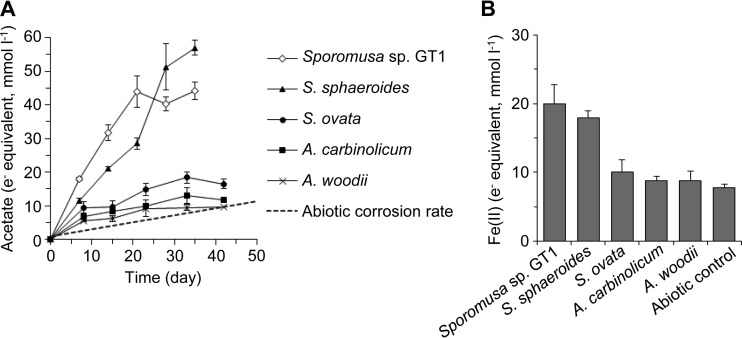
The isolate (Sporomusa sp. GT1) and type strains of known acetogenic bacteria (S. sphaeroides, S. ovata, A. carbinolicum, and A. woodii) were cultivated in the freshwater basal medium supplemented with Fe(0) granules as the sole electron donor to evaluate their ability for acetogenesis-dependent MIC (Fig. 4). The acetate production rates of pure cultures of Sporomusa sp. GT1 (2.08 ± 0.23 mmol e− equivalent liter−1 day−1) and its closest relative, S. sphaeroides (1.71 ± 0.11 mmol e− equivalent liter−1 day−1), were nearly 10 and 8 times higher than the abiotic corrosion rate, respectively (Fig. 4A). The amounts of Fe(II) generated by Sporomusa sp. GT1 and S. sphaeroides were also significantly higher than that of the abiotic control (Fig. 4B). These results clearly demonstrated that Sporomusa sp. GT1 and S. sphaeroides have the ability to induce corrosion by utilizing Fe(0) as the electron donor for acetogenesis.
FIG 4.
Acetate (A) and Fe(II) (B) production from Fe(0) granules by pure cultures of acetogenic bacteria. The dashed line in panel A represents the abiotic corrosion rate calculated from the abiotic H2 production rate shown in Fig. 2B. Data are presented as the means of the results of three independent cultures, and error bars represent standard deviations.
In contrast, the acetate production rates of pure cultures of the other acetogenic species (S. ovata, A. carbinolicum, and A. woodii) (0.27 to 0.48 mmol e− equivalent liter−1 day−1) were only slightly higher than the abiotic corrosion rate, and no significant differences were observed for Fe(II) generation compared to the abiotic control results (Fig. 4). Mand et al. reported that the type strain of A. woodii, a close relative of the dominant acetogens in their iron-corroding enrichment cultures, did not enhance corrosion by the pure culture (23). Mori et al. reported that 11 strains of H2-consuming acetogens isolated from oil facilities, mostly affiliated with the genus Acetobacterium, did not show significant enhancement of iron corrosion (14). Taking the data together, consumption of H2 derived from chemical oxidation of Fe(0) is not sufficient for acetogenesis-dependent MIC, as reported for sulfidogenesis- and methanogenesis-dependent MIC (7, 13–17).
It has been reported that typical H2-consuming SRBs and methanogens closely related to MIC-inducing strains do not enhance iron corrosion (7, 13, 14), indicating that MIC-inducing microorganisms have specific mechanisms to efficiently take up cathodic electrons derived from Fe(0) oxidation. The proposed mechanism is direct uptake of electrons from solid compounds, including Fe(0). Venzlaff et al. and Lohner et al. reported that a MIC-inducing SRB (Desulfobacterium corrodens) and a methanogen (Methanococcus maripaludis), respectively, appear to have the ability to directly take up electrons from polarized electrodes in a manner independent of abiotical H2 generation on the electrode surfaces (16, 43). However, the molecular mechanisms for direct electron uptake from solid compounds and its engagement in iron corrosion remained unclear. The present study demonstrated a similar phenomenon for acetogenesis-dependent MIC. Only limited strains of acetogens were MIC inducible, while other hydrogen-consuming acetogens were not. Corrosion-inducing acetogens found in this study should also have specific mechanisms for efficient uptake of electrons from insoluble materials such as Fe(0). Nevin et al. reported that some strains of genus Sporomusa, including the type strain of S. sphaeroides, generate acetate with poised cathodic electrodes as the sole electron donor (44), indicating the some Sporomusa spp. have the ability to effectively take up electrons from extracellular solid materials. Comparison of MIC-inducing and noninducing strains of Sporomusa spp. found in this study, with respect to genomics, transcriptomics, and electrochemistry, will shed light on the molecular mechanisms of electron transfer from solid compounds to acetogenic bacteria.
Ecological implications.
From the physiological and evolutionary viewpoints, utilization of Fe(0) as an energy source is an enigmatic ability, since almost all Fe(0) has been recently introduced into environments by human activities. One plausible explanation is that the utilization of electrons in Fe(0) is due to the promiscuous usage of other metabolic systems that take up electrons from naturally occurring solid materials. Our group recently demonstrated that naturally occurring, (semi)conductive iron minerals serve as an electron source and sink for some soil microbial species (26, 45–47). Furthermore, a novel process of microbial syntrophic metabolism, namely, electric syntrophy, in which electrons released by one microorganism are transferred to another through electric current flowing through biological and mineralogical solid compounds, has recently been demonstrated (48, 49). Previous reports suggested that diverse kinds of microorganisms, including nitrate reducers, ferric iron reducers, methanogens, and dehalorespirators, participate in the electron-consuming part of electric syntrophy (48–52). The present report proposes a probability that electron uptake from such naturally occurring conductive materials is an unidentified strategy by which acetogenic bacteria thrive in natural environments.
Conclusion.
This paper is the first to demonstrate acetogenesis-dependent MIC in pure cultures of acetogenic bacteria. The newly isolated acetogen Sporomusa sp. GT1 and its close relative S. sphaeroides enhanced iron corrosion by generating acetate with Fe(0) granules as the sole electron donor, while other acetogens did not. Further studies on the corrosion-inducing acetogens will shed light on the relevance of acetogenesis to iron corrosion in actual environments and the extracellular electron transfer mechanisms of acetogenic bacteria.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mia Terashima for critical reading of the manuscript and Hiromi Ikebuchi for technical assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02767-14.
REFERENCES
- 1.Hamilton WA. 2003. Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling 19:65–76. doi: 10.1080/0892701021000041078. [DOI] [PubMed] [Google Scholar]
- 2.Beech IB, Sunner J. 2004. Biocorrosion: towards understanding interactions between biofilms and metals. Curr Opin Biotechnol 15:181–186. doi: 10.1016/j.copbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Videla HA, Herrera LK. 2005. Microbiologically influenced corrosion: looking to the future. Int Microbiol 8:169–180. [PubMed] [Google Scholar]
- 4.Lee AK, Newman DK. 2003. Microbial iron respiration: impacts on corrosion processes. Appl Microbiol Biotechnol 62:134–139. doi: 10.1007/s00253-003-1314-7. [DOI] [PubMed] [Google Scholar]
- 5.Muthukumar N, Mohanan S, Maruthamuthu S, Subramanian P, Palaniswamy N, Raghavan M. 2003. Role of Brucella sp. and Gallionella sp. in oil degradation and corrosion. Electrochem Comm 5:422–427. doi: 10.1016/S1388-2481(03)00093-6. [DOI] [Google Scholar]
- 6.Zhu XY, Lubeck J, Kilbane JJ. 2003. Characterization of microbial communities in gas industry pipelines. Appl Environ Microbiol 69:5354–5363. doi: 10.1128/AEM.69.9.5354-5363.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchiyama T, Ito K, Mori K, Tsurumaru H, Harayama S. 2010. Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol 76:1783–1788. doi: 10.1128/AEM.00668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang H, Chen R, Wang L, Shao S, Dai L, Ye Y, Guo L, Huang G, Klotz MG. 2011. Molecular characterization of putative biocorroding microbiota with a novel niche detection of Epsilon- and Zetaproteobacteria in Pacific Ocean coastal seawaters. Environ Microbiol 13:3059–3074. doi: 10.1111/j.1462-2920.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 9.McBeth JM, Little BJ, Ray RI, Farrar KM, Emerson D. 2011. Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl Environ Microbiol 77:1405–1412. doi: 10.1128/AEM.02095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coetser SE, Cloete TE. 2005. Biofouling and biocorrosion in industrial water systems. Crit Rev Microbiol 31:213–232. doi: 10.1080/10408410500304074. [DOI] [PubMed] [Google Scholar]
- 11.King RA, Miller JDA. 1971. Corrosion by the sulphate-reducing bacteria. Nature 233:491–492. doi: 10.1038/233491a0. [DOI] [PubMed] [Google Scholar]
- 12.Daniels L, Belay N, Rajagopal BS, Weimer PJ. 1987. Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 237:509–511. doi: 10.1126/science.237.4814.509. [DOI] [PubMed] [Google Scholar]
- 13.Dinh HT, Kuever J, Mussmann M, Hassel AW, Stratmann M, Widdel F. 2004. Iron corrosion by novel anaerobic microorganisms. Nature 427:829–832. doi: 10.1038/nature02321. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, Tsurumaru H, Harayama S. 2010. Iron corrosion activity of anaerobic hydrogen-consuming microorganisms isolated from oil facilities. J Biosci Bioeng 110:426–430. doi: 10.1016/j.jbiosc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Enning D, Venzlaff H, Garrelfs J, Dinh HT, Meyer V, Mayrhofer K, Hassel AW, Stratmann M, Widdel F. 2012. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ Microbiol 14:1772–1787. doi: 10.1111/j.1462-2920.2012.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venzlaff H, Enning D, Srinivasan J, Mayrhofer K, Hassel AW, Widdel F, Stratmann M. 2013. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros Sci 66:88–96. doi: 10.1016/j.corsci.2012.09.006. [DOI] [Google Scholar]
- 17.Enning D, Garrelfs J. 2014. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol 80:1226–1236. doi: 10.1128/AEM.02848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao TS, Sairam N, Viswanathan B, Nair KVK. 2000. Carbon steel corrosion by iron oxidizing and sulphate reducing bacteria in a freshwater cooling system. Corros Sci 42:1417–1431. doi: 10.1016/S0010-938X(99)00141-9. [DOI] [Google Scholar]
- 19.Pitonzo BJ, Castro P, Amy PS, Southam G, Jones DA, Ringelberg D. 2004. Microbiologically influenced corrosion capability of bacteria isolated from Yucca Mountain. Corrosion 60:64–74. doi: 10.5006/1.3299233. [DOI] [Google Scholar]
- 20.Rajasekar A, Anandkumar B, Maruthamuthu S, Ting YP, Rahman PK. 2010. Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol 85:1175–1188. doi: 10.1007/s00253-009-2289-9. [DOI] [PubMed] [Google Scholar]
- 21.Usher KM, Kaksonen AH, Cole I, Marney D. 2014. Critical review: microbially influenced corrosion of buried carbon steel pipes. Int Biodeteriol Biodegr 93:84–106. doi: 10.1016/j.ibiod.2014.05.007. [DOI] [Google Scholar]
- 22.Suflita JM, Phelps TJ, Little B. 2008. Carbon dioxide corrosion and acetate: a hypothesis on the influence of microorganisms. Corros Sci 64:854–859. doi: 10.5006/1.3279919. [DOI] [Google Scholar]
- 23.Mand J, Park HS, Jack TR, Voordouw G. 2014. The role of acetogens in microbially influenced corrosion of steel. Front Microbiol 5:268. doi: 10.3389/fmicb.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H. 2000. Syntrophothermus lipocalidus gen. nov., sp. nov., a novel thermophilic, syntrophic, fatty-acid-oxidizing anaerobe which utilizes isobutyrate. Int J Syst Evol Microbiol 50(Pt 2):771–779. doi: 10.1099/00207713-50-2-771. [DOI] [PubMed] [Google Scholar]
- 25.Kato S, Haruta S, Cui ZJ, Ishii M, Yokota A, Igarashi Y. 2004. Clostridium straminisolvens sp. nov., a moderately thermophilic, aerotolerant and cellulolytic bacterium isolated from a cellulose-degrading bacterial community. Int J Syst Evol Microbiol 54:2043–2047. doi: 10.1099/ijs.0.63148-0. [DOI] [PubMed] [Google Scholar]
- 26.Kato S, Kai F, Nakamura R, Watanabe K, Hashimoto K. 2010. Respiratory interactions of soil bacteria with (semi)conductive iron-oxide minerals. Environ Microbiol 12:3114–3123. doi: 10.1111/j.1462-2920.2010.02284.x. [DOI] [PubMed] [Google Scholar]
- 27.Dojka MA, Hugenholtz P, Haack SK, Pace NR. 1998. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol 64:3869–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Breitbart M, McNairnie P, Rohwer F. 2006. FastGroupII: a web-based bioinformatics platform for analyses of large 16S rDNA libraries. BMC Bioinformatics 7:57. doi: 10.1186/1471-2105-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. [DOI] [PubMed] [Google Scholar]
- 31.Kato S, Sasaki K, Watanabe K, Yumoto I, Kamagata Y. 2014. Physiological and transcriptomic analyses of a thermophilic, aceticlastic methanogen Methanosaeta thermophila responding to ammonia stress. Microbes Environ 29:162–167. doi: 10.1264/jsme2.ME14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovley DR, Phillips EJ. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basso O, Caumette P, Magot M. 2005. Desulfovibrio putealis sp. nov., a novel sulfate-reducing bacterium isolated from a deep subsurface aquifer. Int J Syst Evol Microbiol 55:101–104. doi: 10.1099/ijs.0.63303-0. [DOI] [PubMed] [Google Scholar]
- 34.Schauder R, Preuβ A, Jetten MS, Fuchs G. 1988. Oxidative and reductive acetyl CoA/carbon monoxide dehydrogenase pathway in Desulfobacterium autotrophicum. Arch Microbiol 151:84–89. doi: 10.1007/BF00444674. [DOI] [Google Scholar]
- 35.Bergmann F, Selesi D, Weinmaier T, Tischler P, Rattei T, Meckenstock RU. 2011. Genomic insights into the metabolic potential of the polycyclic aromatic hydrocarbon degrading sulfate-reducing Deltaproteobacterium N47. Environ Microbiol 13:1125–1137. doi: 10.1111/j.1462-2920.2010.02391.x. [DOI] [PubMed] [Google Scholar]
- 36.Jeremiasse AW, Hamelers HV, Buisman CJ. 2010. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 78:39–43. doi: 10.1016/j.bioelechem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Parameswaran P, Torres CI, Lee HS, Rittmann BE, Krajmalnik-Brown R. 2011. Hydrogen consumption in microbial electrochemical systems (MXCs): the role of homo-acetogenic bacteria. Bioresour Technol 102:263–271. doi: 10.1016/j.biortech.2010.03.133. [DOI] [PubMed] [Google Scholar]
- 38.Pisciotta JM, Zaybak Z, Call DF, Nam JY, Logan BE. 2012. Enrichment of microbial electrolysis cell biocathodes from sediment microbial fuel cell bioanodes. Appl Environ Microbiol 78:5212–5219. doi: 10.1128/AEM.00480-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Möller B, Oßmer R, Howard BH, Gottschalk G, Hippe H. 1984. Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch Microbiol 139:388–396. doi: 10.1007/BF00408385. [DOI] [Google Scholar]
- 40.Wilde E, Collins MD, Hippe H. 1997. Clostridium pascui sp. nov., a new glutamate-fermenting sporeformer from a pasture in Pakistan. Int J Syst Bacteriol 47:164–170. doi: 10.1099/00207713-47-1-164. [DOI] [PubMed] [Google Scholar]
- 41.Schink B. 1984. Clostridium magnum sp. nov., a nonautotrophic homoacetogenic bacterium. Arch Microbiol 137:250–255. doi: 10.1007/BF00414553. [DOI] [Google Scholar]
- 42.Liu F, Conrad R. 2011. Chemolithotrophic acetogenic H2/CO2 utilization in Italian rice field soil. ISME J 5:1526–1539. doi: 10.1038/ismej.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohner ST, Deutzmann JS, Logan BE, Leigh J, Spormann AM. 2014. Hydrogenase-independent uptake and metabolism of electrons by the archaeon Methanococcus maripaludis. ISME J 8:1673–1681. doi: 10.1038/ismej.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, Snoeyenbos-West OL, Lovley DR. 2011. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microbiol 77:2882–2886. doi: 10.1128/AEM.02642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura R, Kai F, Okamoto A, Newton GJ, Hashimoto K. 2009. Self-constructed electrically conductive bacterial networks. Angew Chem Int Ed Engl 48:508–511. doi: 10.1002/anie.200804750. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura R, Okamoto A, Tajima N, Newton GJ, Kai F, Takashima T, Hashimoto K. 2010. Biological iron-monosulfide production for efficient electricity harvesting from a deep-sea metal-reducing bacterium. ChemBioChem 11:643–645. doi: 10.1002/cbic.200900775. [DOI] [PubMed] [Google Scholar]
- 47.Kato S, Hashimoto K, Watanabe K. 2013. Iron-oxide minerals affect extracellular electron-transfer paths of Geobacter spp. Microbes Environ 28:141–148. doi: 10.1264/jsme2.ME12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. 2010. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330:1413–1415. doi: 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- 49.Kato S, Hashimoto K, Watanabe K. 2012. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc Natl Acad Sci U S A 109:10042–10046. doi: 10.1073/pnas.1117592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morita M, Malvankar NS, Franks AE, Summers ZM, Giloteaux L, Rotaru AE, Rotaru C, Lovley DR. 2011. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2:e00159-11. doi: 10.1128/mBio.00159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato S, Hashimoto K, Watanabe K. 2012. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ Microbiol 14:1646–1654. doi: 10.1111/j.1462-2920.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 52.Aulenta F, Rossetti S, Amalfitano S, Majone M, Tandoi V. 2013. Conductive magnetite nanoparticles accelerate the microbial reductive dechlorination of trichloroethene by promoting interspecies electron transfer processes. ChemSusChem 6:433–436. doi: 10.1002/cssc.201200748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.