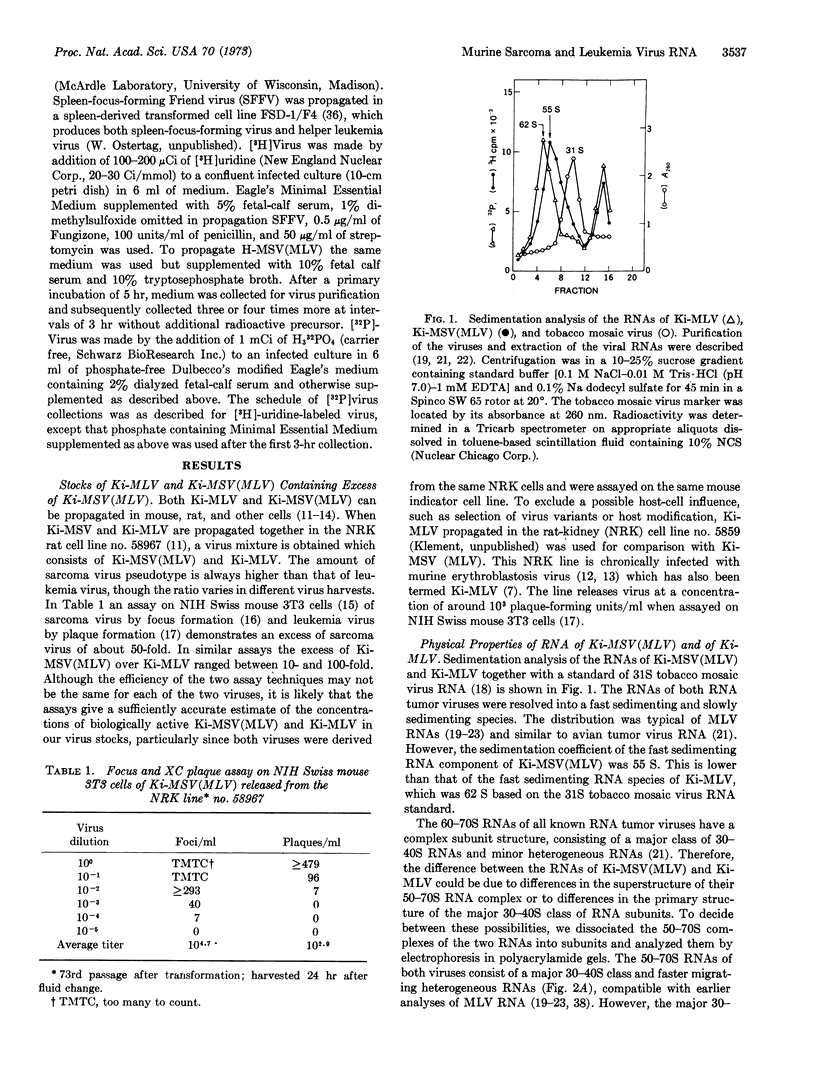
Abstract
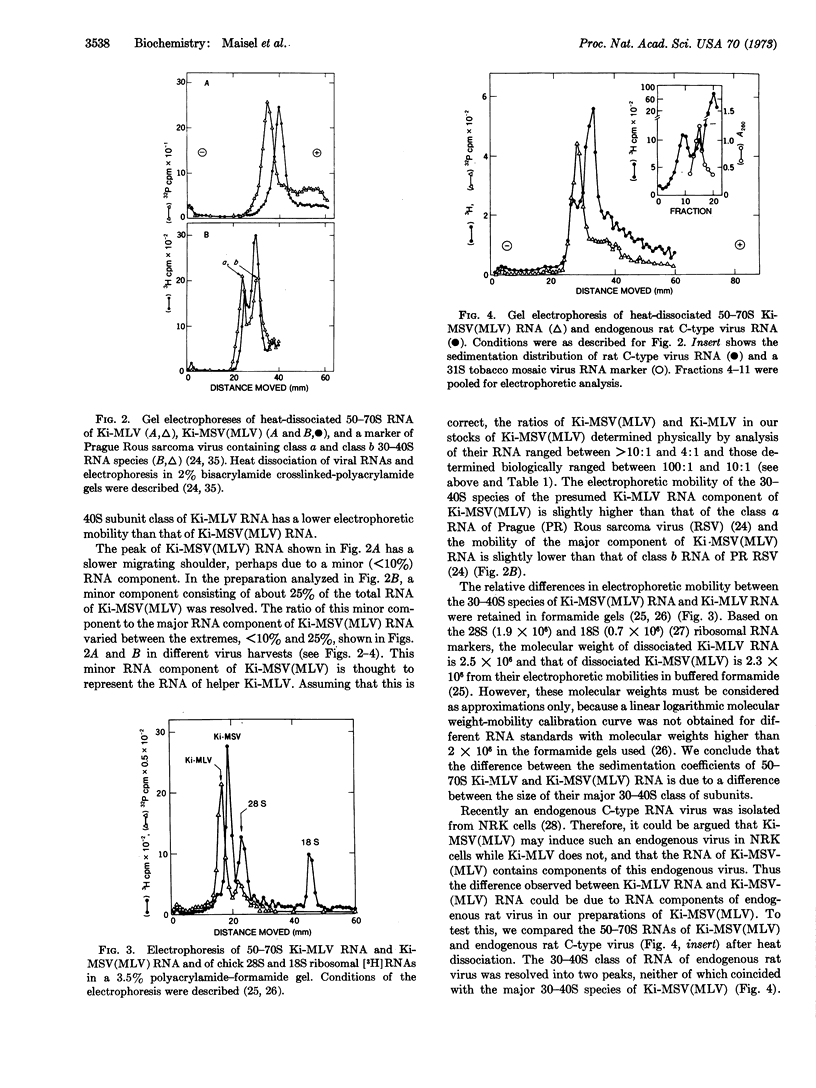
Defective Kirsten murine sarcoma virus was present as leukemia virus pseudotype [Ki-MSV(MLV)] in a 10- to 100-fold excess over its helper, Kirsten murine leukemia virus (Ki-MLV), when the two viruses were propagated in an NRK rat cell line. The sω,20 of the fastsedimenting RNA complex of Ki-MLV and of Ki-MSV-(MLV) was 62 S and 55 S, respectively. Gel electrophoresis in buffered aqueous or formamide solution of the dissociated 62S RNA complex of Ki-MLV showed a single major peak of molecular weight about 2.5 × 106. Dissociated 55S RNA of Ki-MSV(MLV) was resolved into a major component with a higher electrophoretic mobility than that of Ki-MLV RNA and molecular weight about 2.3 × 106. Occasionally, a minor component with the same electrophoretic mobility as Ki-MLV RNA was observed in Ki-MSV(MLV) RNA; it is thought to be the RNA of Ki-MLV present as helper virus in our stocks of Ki-MSV(MLV). The RNA of an endogenous rat C-type RNA virus was electrophoretically different from both Ki-MLV RNA and Ki-MSV(MLV) RNAs. Oligonucleotide fingerprinting of the RNAs digested with RNase T1 indicated that the RNAs of Ki-MSV(MLV) and Ki-MLV are different. However, the extent of the difference between the two RNAs could not be estimated by this method.
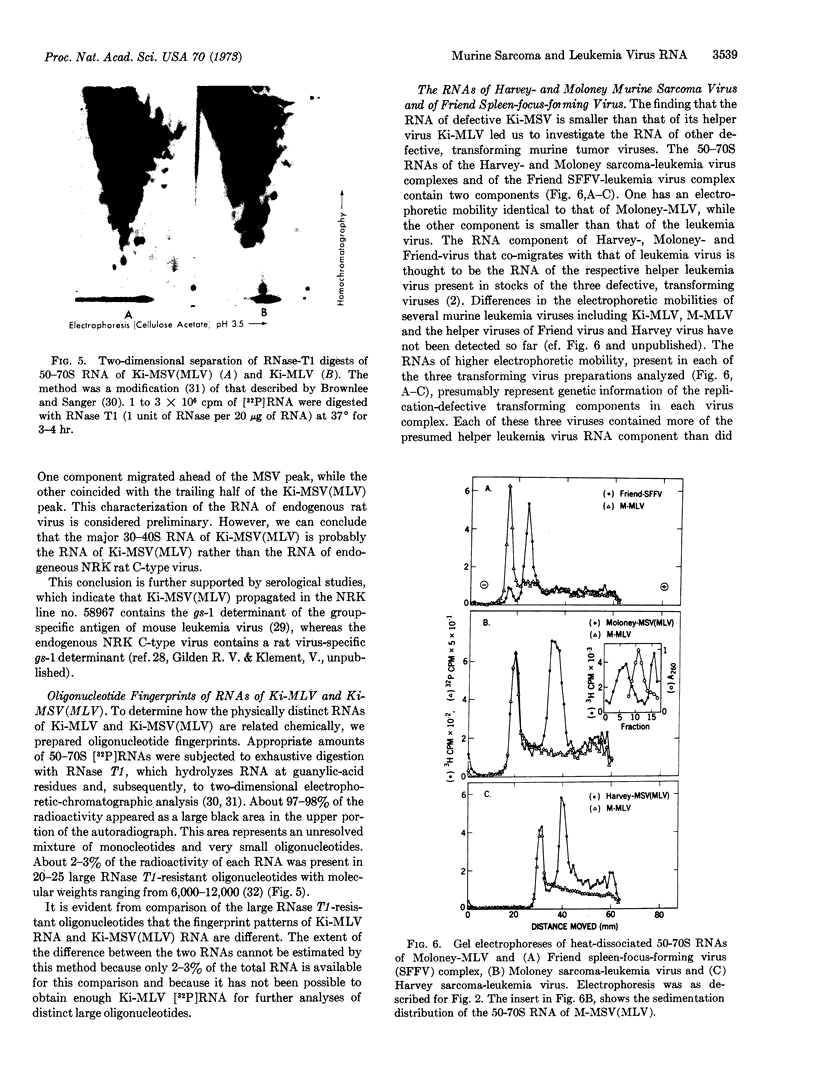
The heat-dissociated 50-70S RNAs of two other defective murine sarcoma-leukemia viruses; Harvey-MSV(MLV) and Moloney-MSV(MLV) and of defective spleen-focus-forming Friend virus were resolved electrophoretically into two components. The larger components had the same electrophoretic mobility as the RNA of Ki-MLV or Moloney MLV. The smaller were not present in leukemia virus. It is suggested that the small RNA components of the two murine viruses and of Friend virus represent specific genetic information of these replication-defective transforming viruses.
Possible relationships between the RNAs of murine leukemia viruses and replication-defective murine sarcoma and Friend viruses are discussed.
Keywords: NRK rat cells, fingerprinting, gel electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Transformation and virus growth by murine sarcoma viruses in human cells. Nature. 1970 Jan 31;225(5231):458–459. doi: 10.1038/225458a0. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Duesberg P. H. Structure of Rauscher mouse leukaemia virus RNA. Nature. 1968 Oct 26;220(5165):396–399. doi: 10.1038/220396a0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. On the structure of RNA tumor viruses. Curr Top Microbiol Immunol. 1970;51:78–104. [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Nucleic acid and proteins isolated from the Rauscher mouse leukemia virus (MLV). Proc Natl Acad Sci U S A. 1966 Jan;55(1):219–227. doi: 10.1073/pnas.55.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. J., East J. The murine sarcoma virus (MSV). Int Rev Exp Pathol. 1971;10:265–360. [PubMed] [Google Scholar]
- Horst J., Content J., Mandeles S., Fraenkel-Conrat H., Duesberg P. Distinct oligonucleotide patterns of distinct influenza virus RNA's. J Mol Biol. 1972 Aug 21;69(2):209–215. doi: 10.1016/0022-2836(72)90226-4. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten W. H., Mayer L. A. Malignant lymphomas of extrathymic origin induced in rats by murine erythroblastosis virus. J Natl Cancer Inst. 1969 Sep;43(3):735–746. [PubMed] [Google Scholar]
- Klement V., Freedman M. H., McAllister R. M., Nelson-Rees W. A., Heubner R. J. Differences in susceptibility of human cells to mouse sarcoma virus. J Natl Cancer Inst. 1971 Jul;47(1):65–73. [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H., Horst J., Vogt P. K. Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2266–2270. doi: 10.1073/pnas.70.8.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C., Kelloff G., Gilden R. V. Variations in sarcoma and leukemia virus activity in somatic cell hybrids. Int J Cancer. 1972 Sep 15;10(2):310–319. doi: 10.1002/ijc.2910100211. [DOI] [PubMed] [Google Scholar]
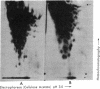
- McClain K., Kirsten W. H. Electrophoretic analysis of the RNA from a mouse leukemia virus. Cancer Res. 1972 Jul;32(7):1470–1475. [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Melderis H., Steinheider G., Kluge N., Dube S. Synthesis of mouse haemoglobin and globin mRNA in leukaemic cell cultures. Nat New Biol. 1972 Oct 25;239(95):231–234. doi: 10.1038/newbio239231a0. [DOI] [PubMed] [Google Scholar]
- Peebles P. T., Bassin R. H., Haapala D. K., Phillips L. A., Nomura S., Fischinger P. J. Rescue of murine sarcoma virus from a sarcoma-positive leukemia-negative cell line: requirement for replicating leukemia virus. J Virol. 1971 Nov;8(5):690–694. doi: 10.1128/jvi.8.5.690-694.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Murine sarcoma and leukemia viruses: genetic differences determined by RNA-DNA hybridization. Virology. 1971 Nov;46(2):480–484. doi: 10.1016/0042-6822(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Watson J. D. The structure and assembly of murine leukemia virus: intracellular viral RNA. Virology. 1971 Sep;45(3):586–597. doi: 10.1016/0042-6822(71)90174-7. [DOI] [PubMed] [Google Scholar]