Abstract
In 2011, a new histologic classification of lung adenocarcinomas was proposed from a joint working group of the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS), based on the recommendation of an international and multidisciplinary panel. This classification proposed a method of comprehensive histologic subtyping (lepidic, acinar, papillary, micropapillary, and solid pattern) based on semi-quantitative assessment of histologic patterns (in 5% increments) with the ultimate goal of choosing a single, predominant pattern. Prognostic subsets could then be described for the classification. Patients with completely resected adenocarcinomas in situ (AIS) and minimally invasive adenocarcinomas (MIA) experienced low risk of recurrence. Patients with micropapillary or solid predominant tumors have a high risk for recurrence or cancer-related death. Patients with acinar and papillary predominant tumors comprise an intermediate-risk group. Herein, we review the outline of the proposed IASLC/ATS/ERS classification, a summary of published validation studies of this new classification and then discuss surgical key issues; we mainly focused on limited resection as an adequate treatment for early-stage lung adenocarcinomas as well as pre- and intraoperative diagnoses. We also review the published studies that identified the importance of histological subtypes in predicting recurrence, both rates and patterns, in early-stage lung adenocarcinomas. This new classification for the most common type of lung cancer is useful for surgeons, as its implementation would require only hematoxylin and eosin (H&E) histology slides, which is the common type of stain used in hospitals. It can be implemented with routine pathology evaluation and with no additional costs.
Keywords: lung adenocarcinoma, histologic classification, limited resection, micropapillary, small lung nodules
INTRODUCTION
Lung adenocarcinoma, which is the most frequent histologic subtype of non-small cell lung cancer (NSCLC) encountered by surgeons, is heterogeneous in clinical and radiological presentation, histological appearance, surgical outcome, and molecular biological profile.1 Over the past decade, the single most important factor that has helped determine clinical management and prognosis for patients with lung cancer has been tumor-nodal-metastases (TNM) staging.2 Although the histological classification of lung cancer has been periodically revised by the World Health Organization (WHO), the last classification, which was published in 2004, had approximately 90% of lung adenocarcinomas classified as a single category – mixed subtype.1
While there have been several advances over the past decade in defining the molecular alterations within lung adenocarcinomas, some of which have aided in the development of targeted therapies such as EGFR mutation in patients with advanced disease, none of the molecular patterns have proven useful for a uniform classification system of lung adenocarcinoma in surgically resected tumors. There is mounting evidence that suggests that histological patterns in lung adenocarcinomas can be used to define prognostically variable subsets. With the pressing need for an update a new classification for lung adenocarcinomas was developed by a joint working group of the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society (IASLC/ATS/ERS) in 2011.1
Development of the IASLC/ATS/ERS lung adenocarcinoma classification
To provide an internationally acceptable histological classification system that could be applied to define prognostically variable subsets, an international, multidisciplinary panel was developed by the IASLC/ATS/ERS including pathologists, thoracic surgeons, thoracic medial oncologists, pulmonologists, radiologists, and molecular biologists. The panel members performed a systematic review of published literature and initially selected 11,368 relevant articles. Of these, 312 articles met the specified eligibility criteria for a full-text review. Following review of the selected publications for strength and qualify of the scientific evidence, and in conjunction with each specialty group, a writing committee developed the recommendations for histological classification.
Discussions took place during a series of meetings of the multidisciplinary panel in 2008 and 2009 leading to a revised classification system that led to initiation of individual projects by panel members to validate the proposed system in their patient cohorts. Based on the data generated, the panel proposed 10 significant changes to the histological classification of lung adenocarcinomas in an effort to help improve predictive ability of clinical outcomes and therapeutic benefits. These recommendations were detailed in the 2011 joint publication by the IASLC/ATS/ERS1; this publication forms the foundation for the current lung adenocarcinoma histological classification.
In this review, we will first present the outline of the proposed IASLC/ATS/ERS classification and a summary of published validation studies of this new classification. Then, we will discuss the surgical key issues, with a focus on the pre- and intraoperative diagnosis of histological subtypes, and the role of limited resection and systematic lymph node sampling or dissection in the management of early-stage lung adenocarcinoma patients. We will also review the published studies that have identified the importance of histological subtypes in predicting recurrence, both rates and patterns, in early-stage lung adenocarcinoma.
The proposed IASLC/ATS/ERS classification of lung adenocarcinoma
The proposed IASLC/ATS/ERS classification (Table 1)1 addressed resected tumors and cytology of small biopsies. As a part of the proposed classification, the panel recommended that clinicians stop using the confusing term “bronchioloalveolar carcinoma” (BAC); this term has been used for a at least five different tumors defined in the new classification including non-invasive tumors with atypical pneumocyte proliferation along the pre-existing alveolar wall and a variety of invasive adenocarcinomas including lepidic predominant adenocarcinoma, invasive adenocarcinomas with non-predominant lepidic pattern and invasive mucinous adenocarcinomas.
Table 1.
The IASLC/ATS/ERS Classification of Lung Adenocarcinoma in Resection Specimens1
| Preinvasive lesions |
| Atypical adenomatous hyperplasia |
| Adenocarcinoma in situ (≤3 cm formerly BAC*) |
| Nonmucinous |
| Mucinous |
| Mixed mucinous/nonmucinous |
| Minimally invasive adenocarcinoma (≤3 cm lepidic predominant tumor with ≤5 mm invasion) |
| Nonmucinous |
| Mucinous |
| Mixed mucinous/nonmucinous |
| Invasive adenocarcinoma |
| Lepidic predominant (formerly nonmucinous BAC pattern, with >5 mm invasion) |
| Acinar predominant |
| Papillary predominant |
| Micropapillary predominant |
| Solid predominant with mucin production |
| Variants of invasive adenocarcinoma |
| Invasive mucinous adenocarcinoma (formerly mucinous BAC) |
| Colloid |
| Fetal (low and high grade) |
| Enteric |
BAC, bronchioloalveolar adenocarcinoma
In the new IASLC/ATS/ERS classification, 4 major concepts were recommended for resected specimens: 1) The new categories developed were adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), both of which were defined as small (≤ 3 cm), and solitary adenocarcinomas that consisted of lepidic growth pattern without invasion (AIS) or with ≤ 5 mm invasion (MIA). Tumor invasion was defined as having a histologic pattern other than lepidic (acinar, papillary, micropapillary, or solid), an active myofibroblastic stroma correlated with invasive tumor cells, and presence of lymphatic, vascular, or pleural invasion; 2) For invasive adenocarcinoma histologic proliferative patterns are recorded, semiquantitatively, in 5% increments (Figure 1). In the lepidic predominant setting, lepidic predominant adenocarcinoma is defined as a tumor with > 5 mm invasion or > 3 cm in total size. Then, the predominant histologic pattern is determined according to the percentages of the subtypes in the individual tumors. The major histologic patterns are lepidic, acinar, papillary, micropapillary, and solid (Figure 2). This comprehensive histological subtyping is thought to provide better stratification of the formerly defined “mixed subtype” adenocarcinomas in outcomes;3 3) “Micropapillary pattern” was added as one of major histologic subtypes due to its association with poor prognosis. Micropapillary adenocarcinomas consist of tumor cells that grow in papillary tufts that lack fibrovascular cores and frequently show vascular and stromal invasion; and 4) Adenocarcinomas, which were formerly known as “mucinous BAC,” were classified, depending on the extent of invasive growth, as mucinous AIS, mucinous MIA, or, for invasive tumors, as invasive mucinous adenocarcinoma. The relevance of cytology and small biopsies is discussed in another section of this manuscript.
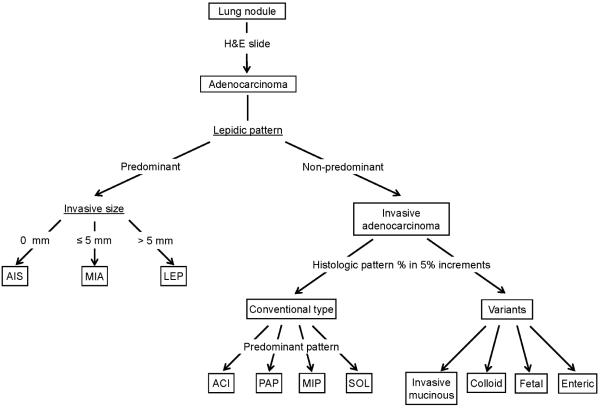
Figure 1. Histologic subtyping for surgeon.
Resected lung nodules that are diagnosed as adenocarcinoma are first classified based on the degree of lepidic growth pattern. Tumors with lepidic predominant growth are composed of 3 groups: adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and lepidic predominant invasive adenocarcinoma (LEP). AIS is defined as a ≤3 cm tumor with a pure lepidic pattern (no invasion). MIA is defined as a ≤3 cm tumor with ≤5 mm stromal invasion. LEP is defined as a tumor that is >3 cm in total size and/or >5 mm in invasive size with a non-mucinous lepidic predominant pattern. Each histologic pattern % is recorded in 5% increments. When showing conventional lung adenocarcinoma morphology, non-lepidic predominant invasive adenocarcinomas are classified into acinar (ACI), papillary (PAP), micropapillary (MIP), or solid subtype (SOL) on the basis of predominant histologic pattern. Variant histologies included invasive mucinous, colloid, fetal and enteric subtype.
AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; LEP, lepidic; MIP, micropapillary; SOL, solid; ACI, acinar; PAP, papillary
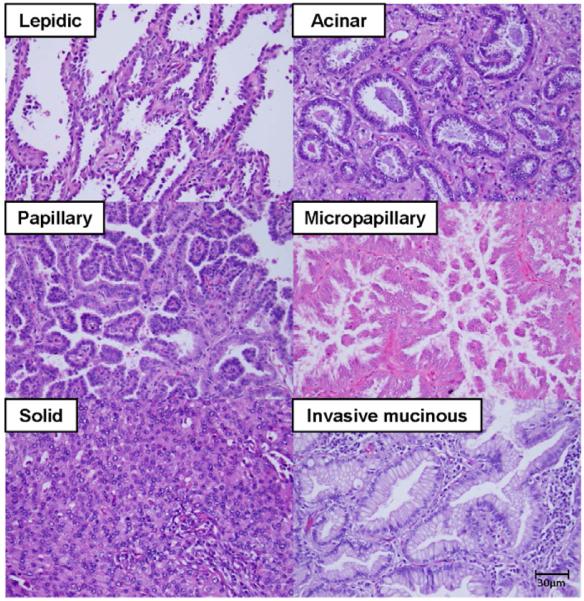
Figure 2. Major histologic subtypes of lung adenocarcinoma.
All pictures are intermediate magnification images (x200, hematoxylin and eosin staining). LEP growth pattern shows that tumor cells appeared to replace normal pneumocytes on alveolar walls. ACI adenocarcinoma shows malignant glands invading a fibrous stroma. PAP adenocarcinoma consists of cuboidal tumor cells growing along fibrovascular cores in a papillary configuration. MIP growth pattern shows small papillary clusters in airspace without fibrovascular cores. SOL adenocarcinoma consists of sheets of tumor cells with abundant cytoplasm. Invasive mucinous adenocarcinoma shows columnar cells filled with abundant mucin invading with an ACI pattern.
LEP, lepidic; ACI, acinar; PAP, papillary; MIP, micropapillary; SOL, solid
Validation studies of the 2011 IASLC/ATS/ERS lung adenocarcinoma classification
Correlation between histological subtypes and prognosis
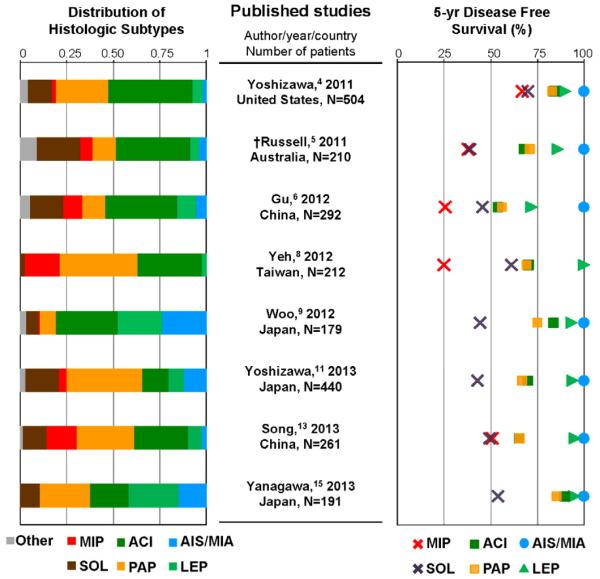
We reviewed the published literature that validated the correlation between the newly proposed histological classification and patient outcomes in at least 150 surgically resected series; we identified 19 published studies that met the criteria (see Table 2).3-21 Seventeen studies demonstrated significant differences in patient outcomes among the histologic subtypes3-12, 14, 15, 17-21 while only 2 studies did not.13, 16 The survival rate for predominant histological subtypes and the outcome and the distribution of each histological subtypes is outlined in Figure 3.3-8, 10, 12, 14, 20 It is important to also note that all studies reported that AIS and MIA had a 100% disease-free survival rate.
Table 2.
Published studies validating the IASLC/ATS/ERS lung adenocarcinoma classification and prognosis
| Author/year | Country | Number of patients |
% of pathological stage I patients |
Surgical procedures (*standard/limited) (%) |
Survival significant |
|---|---|---|---|---|---|
| Yoshizawa,3
2011 |
United States |
514 | 100% | 83%/16% | DFS |
| Russell,4
2011 |
Australia | 210 | 62% | 88%/12% | OS |
| Gu,5 2012 | China | 292 | 65% | 100%/- | DFS and OS |
| Warth,6 2012 | Germany | 487 | 42% | 97%/3% | DFS and OS |
| Yeh,7 2012 | Taiwan | 212 | 100% | 95%/5% | DFS |
| Woo,8 2012 | Japan | 179 | 100% | 100%/- | DFS |
| Tsuta,9 2013 | Japan | 904 | 64% | N/A | OS |
| Yoshizawa,10
2013 |
Japan | 440 | 76% | 79%/21% | DFS and OS |
| Hung,11 2013 | Taiwan | 283 | 100% | 84%/16% | DFS |
| Song,12 2013 | China | 261 | 100% | 100%/- | DFS and OS |
| Urer,13 2013 | Turkey | 226 | 38% | 100%/- | Not significant for OS |
| Yanagawa,14
2013 |
Japan | 191 | 100% | 92%/8% | DFS |
| Zhang,15 2013 | China | 176 | 100% | 100%/- | DFS and OS |
| Westaway,16
2013 |
Australia | 152 | 52% | 95%/5% | Not significant for OS |
| Hung,17 2014 | Taiwan | 573 | 65% | NA | DFS and OS |
| Cha,18 2014 | Korea | 511 | 63% | 93%/7% | DFS and OS |
| Yanagawa,19
2014 |
Japan | 486 | 77% | 78%/22% | OS |
| Mansuet- Lupo,20 2014 |
France | 407 | 46% | 100%/- | OS |
| Ito,21 2014 | Japan | 188 | 100% | 54%/46% | DFS and OS |
Only studies that included >150 patients were included in this table.
standard = pneumonectomy, bilobectomy, or lobectomy limited = segmentectomy or wedge resection
DFS, disease free survival; OS, overall survival; NA, not applicable
Figure 3. Prognosis and histologic pattern distribusion according to the new IASLC/ATS/ERS classification.
AIS and MIA had 100% disease free survival rate in all studies except for the study from Yeh et al. Among invasive adenocarcinoma subtypes, LEP-predominant invasive adenocarcinoma has better prognosis as compared with other subtypes. In contrast, MIP and SOL-predominant subtypes generally have poorer prognosis than the others. In all studies, ACI and PAP subtypes account for the majority of histologic subtype distribution. In most studies their survival rates are similar or lower than those of AIS/MIA or LEP subtypes and higher than those of SOL or MIP subtypes.
AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; DFS, disease-free survival; LEP, lepidic; MIP, micropapillary; SOL, solid; ACI, acinar; PAP, papillary
†Russell et al was the only study included that used a study endpoint of overall survival (OS).
*5-yr DFS rate could not be applied because of a small number of patients in MIP group.
Among the invasive adenocarcinoma subtypes, lepidic predominant invasive adenocarcinoma (LEP) has a better prognosis as compared with other subtypes. In contrast, micropapillary (MIP) and solid (SOL) predominant subtypes have poorer prognosis than other subtypes. In most studies, acinar (ACI) and papillary (PAP) subtypes account for the majority of histologic subtype distribution and their survival rates are similar. In most studies, their survival rates are lower than AIS/MIA or LEP subtype and higher than the SOL and MIP subtypes. As previously mentioned, significant differences in prognosis among histological subtypes were not recognized in only 2 studies.13, 16 One explanation for this could be that the discrepancy could be due to poor reproducibility in judging invasion or MIP component,22 and remarkably no MIP predominant tumors were identified in one of these studies.13 An alternative explanation could be that the difference in stage distribution might affect the results because in higher stages post-recurrent treatment might influence a patient’s overall survival. Furthermore these studies used overall survival for their survival analysis rather than disease or recurrence free survival; since most early stage lung adenocarcinoma patients die from causes other than their cancer, DFS or RFS is a better survival analysis method as it reflects the biologic behavior of the cancer rather than death due to unrelated factors.
Correlation between histologic subtypes and genetic mutations
Recent advance in molecular biology, such as the discovery that gene mutations could guide a patient’s management and/or treatment decisions for adjuvant therapy or therapy for recurrent disease, are of great interest to surgeons. Following publication of the IASLC/ATS/ERS classification, several groups have correlated histological subtypes with mutational status of the tumors in an effort to learn about the underlying biology. We selected and reviewed 9 studies that each analyzed ≥100 patient tumors for mutational status (EGFR, KRAS, or ALK mutations) and investigated if there is a correlation between presence of a mutation and the histologic subtype in the new IASLC/ATS/ERS classification (Table 3).9, 10, 20, 23-28 The major finding from these studies is that there is no specific histologic molecular correlation between histologic patterns and driver mutation status.
Table 3.
Published studies validating the IASLC/ATS/ERS lung adenocarcinoma classification and gene mutation
| Author/ year |
Country | Study cohort stage/ Number of patients |
Positive rate (%) |
Association between gene mutation and histologic subtype |
||||
|---|---|---|---|---|---|---|---|---|
| EGFR | KRAS | ALK | EGFR | KRAS | ALK | |||
| Zhang,23
2012 |
China | I-IV (N = 349) |
76% | 2% | 4% | Acinar | IMA | - |
| Tsuta,9
2013 |
Japan | I-IV (N = 904) |
41% | 11% | 5% | Papillary | IMA | MIP, acinar |
| Villa,24
2013 |
United States |
I-IV (N = 200) |
21% | - | - | Lepidic | - | - |
| Rekhtman,25
2013 |
United States |
I-IV (N = 180) |
19% | 35% | - | Lepidic, acinar, papillary |
Solid | - |
| Yoshizawa,10
2013 |
Japan | I-IIIA (N = 167) |
54% | 13% | - | Lepidic, papillary |
Mucinous component |
- |
| Song,26
2013 |
China | I-IIIA (N = 161) |
42% | - | - | Lepidic, MIP | - | - |
| Kadota,27
2014 |
United States |
I-III (N = 864) |
15% | 26% | 4%* | Lepidic | Extracellular mucin |
Signet ring cell |
| Mansuet- Lupo,20 2014 |
France | I-IV (N=407) |
10% | 34% | - | - | IMA | - |
| Sun,28
2014 |
China | IB (N = 136) |
38% | - | - | MIP | - | - |
ALK expression was detected using immunohistochemistry.
Only studies that included >100 patients and a performed statistical analysis were included.
IMA, invasive mucinous adenocarcinoma; MIP, micropapillary
Regarding EGFR gene mutations, in many studies it had significant positive correlations with lepidic predominant and papillary subtypes.9, 10, 24-27 Some studies, especially those from the East Asian cohort, showed a positive association between EGFR mutations and the micropapillary subtype.26, 28 The KRAS mutation showed a significant positive correlation with mucinous subtypes in some studies.9, 10, 23
As the distribution of these mutually exclusive gene mutations differs among different ethnicities (EGFR gene mutations are more frequent in Asian cohorts than in Western cohorts and vice versa for KRAS mutations), it remains to be seen whether or not individual histological patterns associated with mutational statuses differ among races. There were no reported differences among multiple ethnicities in ALK mutation positive population in 3 studies.9, 23, 27
Recently, the CD74-NRG1 fusion gene was discovered and it was reported that 4 fusion-positive tumors were found in 102 lung adenocarcinomas that were negative for known oncogenic alterations; all positive cases were of were classified as an invasive mucinous subtype.29 It is believed that this mutation may represent a therapeutic target for invasive mucinous adenocarcinomas.
Implication of the IASLC/ATS/ERS Classification for Surgical Management of Early Stage Lung Adenocarcinoma
One of the goals of developing the new IASLC/ATS/ERS classification of lung adenocarcinoma was to define prognostically variable groups of patients based on histological subtypes. Differentiating between low risk and high risk lung adenocarcinomas is of high clinical importance in the management of early-stage lung cancer, the majority of which are adenocarcinomas. In this section, we will review the published studies that provide the guidelines for assessment and surgical treatment for early stage lung cancer and the potential implications of the EASLC/ATS/ERS classification.
I. Preoperative characterization
Can AIS/MIA be predicted radiologically prior to a planned resection?
One of the main opportunities in defining the concepts of AIS and MIA in the proposed IASLC/AIS/ERS classification is to facilitate pretreatment radiological identification that will allow further study of the role of limited resection given the indolent nature of these lesions.1 Multiple retrospective studies have tried to establish precise radiologic parameters to identify pathologically non-invasive tumors on computer tomography (CT) and/or positron emission tomography (PET) scans.30-33 Shimada et al (n = 363) suggested that a tumor disappearance ratio (TDR) ≥ 50% without spiculation could be highly predictive of noninvasive or minimally invasive tumors.31 In a recent study at our institution, we found that C/T (consolidation to tumor) ratio < 0.25 and SUVmax < 2.2 (median of the cohort, n = 181) could stratify the risk of recurrence following limited resection in a step-wise fashion.32 Takahashi et al reported the usefulness of a ground-glass opacity ratio ≤ 50%, TDR ≤ 75%, and consolidation diameter > 1 cm as a means of predicting invasive adenocarcinomas33. While the data obtained from these retrospective studies is informative, it cannot be conclusive due to the inherent limitations in the selection criteria that could possibly influence the outcomes.
In a prospective study (JCOG 0201), tumor size ≤ 2 cm and C/T ratio ≤ 0.25 was regarded as the cutoff for radiologically noninvasive tumors.30 Two prospective trials (JCOG 0802 and JCOG 0804) have begun in Japan to compare the role of limited resection and lobectomy. JCOG 0802 is focused on radiologically invasive adenocarcinomas (C/T ratio > 0.25) and JCOG 0804 is a single-arm study that intends to investigate the efficacy of limited resection for radiologically non-invasive adenocarcinomas (C/T ratio ≤ 0.25).
Can cytology or small biopsy provide accurate preoperative diagnosis?
The utility of cytology for tumor subtyping of NSCLCs and molecular testing has been previously reported and is quite reliable for not only making a specific diagnosis of adenocarcinoma versus squamous cell carcinoma or small cell carcinoma, but also to provide material for molecular testing.34 However, the histological subtyping of lung adenocarcinomas in cytologic specimens is, at best, challenging due to the histologic heterogeneity in the tumor. Adenocarcinomas in situ and minimally invasive adenocarcinomas cannot be diagnosed in small biopsies or cytology specimens.1 Also, identifying the histologic pattern of cytology specimens might be difficult and unreliable.35 On the other hand, Ferretti et al retrospectively investigated the adequacy of CT-guided trans-thoracic needle biopsy specimens for histological and immunohistochemical diagnosis, as well as mutation analysis, and reported that their CT-guided core needle biopsy would be useful for a histological subtyping in accordance with the new IASLC/ATS/ERS classification system.36 Further multi-institutional data are needed before valid conclusions can be reached.
II. Intraoperative diagnosis
Are frozen sections useful in intraoperative decision-making?
Although some investigators have reported the usefulness of a frozen section during limited resection for intraoperative diagnosis of lung nodules,37 the utility and accuracy of intraoperative frozen section analysis in distinguishing AIS/MIA from other invasive adenocarcinoma subtypes remains unclear. We investigated the utility of using frozen sections for predicting histologic subtype and found that it has high specificity (94% and 96%, respectively) but low sensitivity (37% and 69%, respectively) for detecting MIP and SOL patterns.38 The degree of invasion was frequently overestimated and the frozen section diagnosis was discovered to be inaccurate in distinguishing MIA and LEP-predominant invasive adenocarcinomas. In addition to the histological pattern and invasiveness, other evaluations such as resected margin status, pleural invasion, and lymphovascular invasion were needed for the intraoperative surgical diagnosis.
III. Surgical indication for limited resection
Is limited resection adequate oncologic treatment for early stage lung cancer?
With the expected increase in the detection of small lung nodules on CT screenings, the role of limited resection as a treatment for lung adenocarcinoma is of great interest to the surgeons.39
Limited resection for AIS/MIA
Pathologically non-invasive adenocarcinomas or tumors showing a pure ground glass nature in preoperative CT scans are reported to have 100% disease-free survival after limited resection (see Figure 3).37 However, most of the lesions in the reported studies underwent anatomical resection rather than limited resection (see Table 2). In addition, the data for the prognosis of MIA resected by limited resection is lacking. Therefore, the true prognosis of AIS/MIA after limited resection still remains unclear. Although rare, delayed surgical margin recurrences following limited resection for ground glass lesions has been reported.40 The results of these ongoing prospective studies (JCOG 0802, CALGB 140503 and JCOG 0804) may shed light on whether only considering limited resection for ground glass lesions is the correct course of action.
Limited resection for invasive adenocarcinoma subtypes
There is insufficient evidence supporting limited resection as a treatment option for invasive adenocarcinoma subtypes; lobectomy is still regarded as the standard-of-care treatment for invasive adenocarcinoma subtypes. Recently, Landrneau et al retrospectively compared 312 matched patients who underwent segmentectomy or lobectomy for stage I NSCLC. They reported that, in a propensity-matched comparison, lobectomy was associated with modestly increased freedom from recurrence and overall survival; however, the differences were not statistically significant.41 The results of 2 randomized trials (JCOG 0802 and CALGB 140503) are pending. In both studies, lesions that were radiologically indicative of pathologically noninvasive tumors were excluded (former, excluded C/T ratio ≤ 0.25 tumors; latter, excluded pure ground-glass nodules). It is believed that in these trials a great deal of data about patients who underwent limited resection for invasive adenocarcinoma subtypes will be collected to compare the outcomes between limited resection and lobectomy for invasive histological subtypes.
Can we predict lymph node metastasis from histological characteristics?
The role of lymph node sampling versus lymph node dissection or no formal evaluation of lymph nodes is a controversial topic with respect to small lung adenocarcinomas. Sica et al demonstrated that the SOL and MIP subtypes were overrepresented in brain and lymph node metastases.42 Hung et al retrospectively reviewed 573 lung adenocarcinoma patients who underwent resection, 160 (27.9%) of whom had lymph node metastasis. They reported that MIP and SOL predominant subgroups were significantly associated with lymph node metastases at the resection site and that they were a significant association with aggressive postoperative behavior that favors early distant metastasis.17 Zhang et al concluded in their retrospective study of 243 patients with resected NSCLC that tumor size was not a reliable predictor of the N0 status and that histologic subtyping might be useful in avoiding systematic lymph node dissection in more than one-third of small (≤ 2 cm) NSCLC.43 In our retrospective study, we identified that the presence of MIP pattern and absence of LEP pattern was significantly associated with occult mediastinal lymph node metastasis in patients with clinically diagnosed N0/N1 lung adenocarcinoma.44 In order to utilize these findings for intraoperative decision-making, an improvement in the diagnostic accuracy of the aforementioned frozen sections would be key issue. Tsutani et al suggested that solid size < 0.8 cm or SUVmax < 1.5 could be useful lymph node negative criteria and they also validated the notion that patients who met the node-negative criteria had a better prognosis than those did not meet the criteria.45
IV. Postresection follow-up
While the surgical resection criteria for limited resection versus lobectomy for small lung nodules is still being investigated in prospective studies, postresection recurrences and postrecurrence survival in relation to histological subtypes has already been reported11, 46. This knowledge can help inform surgical decision-making.
How to predict the possibility of recurrence from the final pathological results that include histological subtyping?
The IASLC/ATS/ERS lung adenocarcinoma classification recommends that pathologists report the predominant histological subtypes and the percentage of each patterns, as well as the invasive tumor size. This information can inform the surgeon regarding the potential for recurrences. In our retrospective analysis of stage I lung adenocarcinoma patients (n = 1,038), we found that tumors with > 50% LEP component have 100% recurrence-free survival and patients with LEP-predominant adenocarcinoma (but with ≤ 50%) who experienced recurrence had potential risk factors, including limited resection with close margins (≤ 0.5 cm), presence of MIP component, and lymphatic or vascular invasion.47 We also found that invasive tumor size had a more significant prognostic value than total tumor size. Our results were corroborated by other investigators reports.48, 3, 14
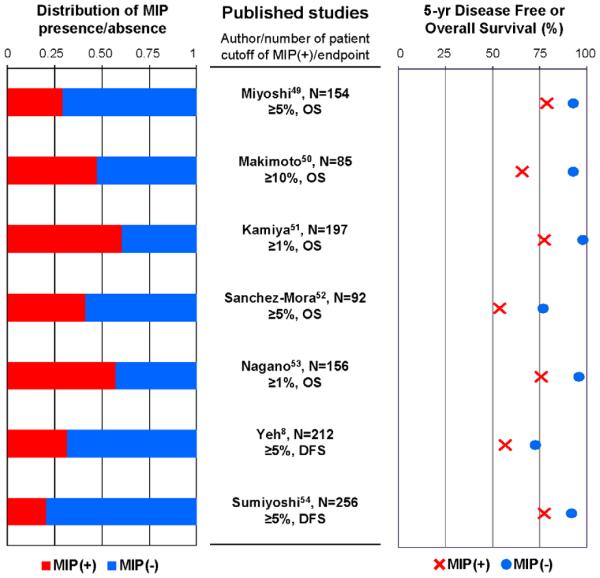
Regarding surgical margins and MIP component, in our retrospective study we identified that MIP component ≥ 5% is significantly associated with an increased risk of recurrence in patients with lung adenocarcinoma treated by limited resection.46 It and also revealed that MIP component ≥ 5% is significantly associated with an increased risk of local recurrence when the surgical margin is < 1 cm.46 Other investigators have reported that MIP pattern frequently involves lymphovascular invasion, even when using a very low threshold (e.g., 5%) could be a significantly worse prognostic factor than those without MIP component7, 49-54. A summary of the published studies that investigated the prognostic value of the presence of MIP component is shown in Figure 4. It is noteworthy that the distribution of patients with MIP component is not small, although the percentage of MIP-predominant adenocarcinomas is low (see Figure 3). These results raise awareness of the presence of MIP component in small lung adenocarcinomas that underwent limited resection.
Figure 4. Prognosis based on presence or absence of MIP component.
In all studies, the survival rate of patients with MIP component is lower than those without MIP component. The distribution of patients with MIP component is not small, although the percentage of MIP-predominant adenocarcinoma is very low.
MIP, micropapillary; OS, overall survival; DFS, disease free survival
Recent reports have also exposed the poor prognostic outcome of lung adenocarcinomas with SOL component. In their retrospective study of stage I lung adenocarcinoma patients, Hung et al reported that MIP and SOL histological subtypes were significantly associated with a poor disease-free survival and that SOL subtype tended to exhibit a worse postrecurrence survival rate than other subtypes (p = 0.0074).11 Our recent study of 151 patients with recurrence after stage I adenocarcinoma resection also showed that the postrecurrence survival rate of the high-grade subtypes (i.e., MIP, SOL, colloid, and invasive mucinous) was significantly poorer than that of other subtypes.55
While the majority of stage I lung adenocarcinomas are intermediate-grade (ACI and PAP), we reported that C/T ratio > 25%,32 high SUVmax on PET scan,32 presence of MIP or SOL component, close margins in the presence of MIP component,46 high mitotic counts,56 presence of cribriform pattern,57 TTF-1 negativity,58 high Ki-67 labeling index,56 , and the immunoinhibitory tumor microenvironment59 are indicative of a poor prognosis and higher recurrence rate, and that it may help differentiate higher risk patients. Our ongoing investigations identified tumor cells within an alveolar space that is separate from the main tumor (spread through alveolar spaces [STAS]) that may explain the reason for recurrence in patients with close margins. We found that STAS was significantly associated with MIP and SOL histological subtypes and lymphatic invasion. We also discovered that, in patients who underwent limited resection rather than lobectomy, those with STAS had a poorer prognosis than those without it.60 The available evidence for the management of small lung nodules is detailed in Figure 5.
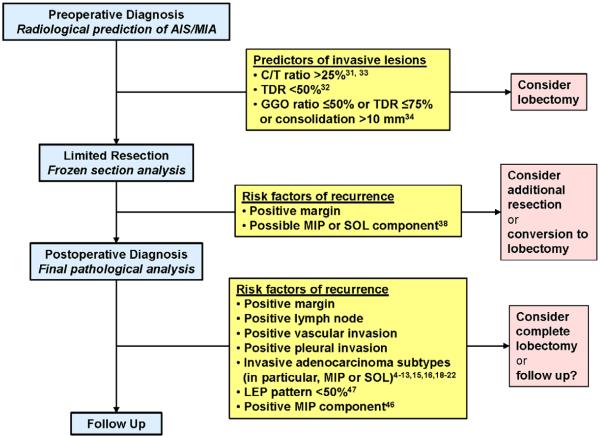
Figure 5. Published evidence for selecting the extent of surgical resection for clinical stage IA lung adenocarcinoma.
AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; C/T ratio, consolidation to tumor size ratio; TDR, tumor disappearance ratio; LEP, lepidic; MIP, micropapillary; SOL, solid
In conclusion, we reviewed the implications of the new IASLC/ATS/ERS classification of lung adenocarcinoma on surgical management of early stage lung adenocarcinoma and discussed the surgical key issues, mainly focusing on limited resection for AIS/MIA as well as pre- and intraoperative diagnosis. The application of the new classification system in small biopsies, including cytology, is still challenging and requires further investigation. The ongoing investigations facilitated by this new classification system are expected to provide guidelines for better management of patients with invasive, early-stage lung adenocarcinoma.
Acknowledgements
We would like to thank Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance.
Funding Support: This work was supported, in part, by the International Association for the Study of Lung Cancer Young Investigator Award; National Lung Cancer Partnership/LUNGevity Foundation Research Grant; American Association for Thoracic Surgery Third Edward D. Churchill Research Scholarship; William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; the National Cancer Institute (grants R21 CA164568-01A1, R21 CA164585-01A1, U54 CA137788, P30 CA008748, and U54 CA132378); and the U.S. Department of Defense (grants PR101053 and LC110202).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 3.Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 4.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 5.Gu J, Lu C, Guo J, Chen L, Chu Y, Ji Y, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol. 2013;107:474–80. doi: 10.1002/jso.23259. [DOI] [PubMed] [Google Scholar]
- 6.Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–46. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 7.Yeh YC, Wu YC, Chen CY, Wang LS, Hsu WH, Chou TY. Stromal invasion and micropapillary pattern in 212 consecutive surgically resected stage I lung adenocarcinomas: histopathological categories for prognosis prediction. J Clin Pathol. 2012;65:910–8. doi: 10.1136/jclinpath-2012-200882. [DOI] [PubMed] [Google Scholar]
- 8.Woo T, Okudela K, Mitsui H, Tajiri M, Yamamoto T, Rino Y, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int. 2012;62:785–91. doi: 10.1111/pin.12016. [DOI] [PubMed] [Google Scholar]
- 9.Tsuta K, Kawago M, Inoue E, Yoshida A, Takahashi F, Sakurai H, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81:371–6. doi: 10.1016/j.lungcan.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 11.Hung JJ, Jeng WJ, Chou TY, Hsu WH, Wu KJ, Huang BS, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg. 2013;258:1079–86. doi: 10.1097/SLA.0b013e31828920c0. [DOI] [PubMed] [Google Scholar]
- 12.Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Prognostic value of the IASLC/ATS/ERS classification in stage I lung adenocarcinoma patients--based on a hospital study in China. Eur J Surg Oncol. 2013;39:1262–8. doi: 10.1016/j.ejso.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Urer HN, Kocaturk CI, Gunluoglu MZ, Arda N, Bedirhan MA, Fener N, et al. Relationship between lung adenocarcinoma histological subtype and patient prognosis. Ann Thorac Cardiovasc Surg. 2014;20:12–8. doi: 10.5761/atcs.oa.12.02073. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol. 2013;8:612–8. doi: 10.1097/JTO.0b013e318287c3eb. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Wu J, Tan Q, Zhu L, Gao W. Why do pathological stage IA lung adenocarcinomas vary from prognosis?: a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol. 2013;8:1196–202. doi: 10.1097/JTO.0b013e31829f09a7. [DOI] [PubMed] [Google Scholar]
- 16.Westaway DD, Toon CW, Farzin M, Sioson L, Watson N, Brady PW, et al. The International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society grading system has limited prognostic significance in advanced resected pulmonary adenocarcinoma. Pathology. 2013;45:553–8. doi: 10.1097/PAT.0b013e32836532ae. [DOI] [PubMed] [Google Scholar]
- 17.Hung JJ, Yeh YC, Jeng WJ, Wu KJ, Huang BS, Wu YC, et al. Predictive Value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma in Tumor Recurrence and Patient Survival. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.50.1049. [DOI] [PubMed] [Google Scholar]
- 18.Cha MJ, Lee HY, Lee KS, Jeong JY, Han J, Shim YM, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg. 2014;147:921–8. doi: 10.1016/j.jtcvs.2013.09.045. e2. [DOI] [PubMed] [Google Scholar]
- 19.Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. The Correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) Classification With Prognosis and EGFR Mutation in Lung Adenocarcinoma. Ann Thorac Surg. doi: 10.1016/j.athoracsur.2014.04.108. 2014. [DOI] [PubMed] [Google Scholar]
- 20.Mansuet-Lupo A, Bobbio A, Blons H, Becht E, Ouakrim H, Didelot A, et al. The new histological classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: the experience of a French cohort. Chest. 2014 doi: 10.1378/chest.13-2499. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Miyata Y, Kushitani K, Yoshiya T, Mimae T, Ibuki Y, et al. Prediction for prognosis of resected pT1a-1bN0M0 adenocarcinoma based on tumor size and histological status: Relationship of TNM and IASLC/ATS/ERS classifications. Lung Cancer. 2014;85:270–5. doi: 10.1016/j.lungcan.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Thunnissen E, Beasley MB, Borczuk AC, Brambilla E, Chirieac LR, Dacic S, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol. 2012;25:1574–83. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. 2012;18:1947–53. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villa C, Cagle PT, Johnson M, Patel JD, Yeldandi AV, Raj R, et al. Correlation of EGFR Mutation Status With Predominant Histologic Subtype of Adenocarcinoma According to the New Lung Adenocarcinoma Classification of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society. Arch Pathol Lab Med. 2014 doi: 10.5858/arpa.2013-0376-OA. [DOI] [PubMed] [Google Scholar]
- 25.Rekhtman N, Ang DC, Riely GJ, Ladanyi M, Moreira AL. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol. 2013;26:1307–19. doi: 10.1038/modpathol.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol. 2013;30:645. doi: 10.1007/s12032-013-0645-1. [DOI] [PubMed] [Google Scholar]
- 27.Kadota K, Yeh YC, D'Angelo SP, Moreira AL, Kuk D, Sima CS, et al. Associations Between Mutations and Histologic Patterns of Mucin in Lung Adenocarcinoma: Invasive Mucinous Pattern and Extracellular Mucin Are Associated With KRAS Mutation. Am J Surg Pathol. 2014;38:1118–27. doi: 10.1097/PAS.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Yu X, Shi X, Hong W, Zhao J, Shi L. Correlation of survival and EGFR mutation with predominant histologic subtype according to the new lung adenocarcinoma classification in stage IB patients. World J Surg Oncol. 2014;12:148. doi: 10.1186/1477-7819-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Cuesta L, Plenker D, Osada H, Sun R, Menon R, Leenders F, et al. CD74-NRG1 Fusions in Lung Adenocarcinoma. Cancer discovery. 2014;4:415–22. doi: 10.1158/2159-8290.CD-13-0633. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, Koike T, Asakawa T, Kusumoto M, Asamura H, Nagai K, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201) J Thorac Oncol. 2011;6:751–6. doi: 10.1097/JTO.0b013e31821038ab. [DOI] [PubMed] [Google Scholar]
- 31.Shimada Y, Yoshida J, Hishida T, Nishimura M, Ishii G, Nagai K. Predictive factors of pathologically proven noninvasive tumor characteristics in T1aN0M0 peripheral non-small cell lung cancer. Chest. 2012;141:1003–9. doi: 10.1378/chest.11-0017. [DOI] [PubMed] [Google Scholar]
- 32.Nitadori J, Bograd AJ, Morales EA, Rizk NP, Dunphy MP, Sima CS, et al. Preoperative consolidation-to-tumor ratio and SUVmax stratify the risk of recurrence in patients undergoing limited resection for lung adenocarcinoma </=2 cm. Ann Surg Oncol. 2013;20:4282–8. doi: 10.1245/s10434-013-3212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi M, Shigematsu Y, Ohta M, Tokumasu H, Matsukura T, Hirai T. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg. 2014;147:54–9. doi: 10.1016/j.jtcvs.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 34.Rekhtman N, Brandt SM, Sigel CS, Friedlander MA, Riely GJ, Travis WD, et al. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol. 2011;6:451–8. doi: 10.1097/JTO.0b013e31820517a3. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez EF, Monaco SE, Dacic S. Cytologic subtyping of lung adenocarcinoma by using the proposed International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) adenocarcinoma classification. Cancer Cytopathol. 2013;121:629–37. doi: 10.1002/cncy.21314. [DOI] [PubMed] [Google Scholar]
- 36.Ferretti GR, Busser B, de Fraipont F, Reymond E, McLeer-Florin A, Mescam-Mancini L, et al. Adequacy of CT-guided biopsies with histomolecular subtyping of pulmonary adenocarcinomas: influence of ATS/ERS/IASLC guidelines. Lung Cancer. 2013;82:69–75. doi: 10.1016/j.lungcan.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida J, Nagai K, Yokose T, Nishimura M, Kakinuma R, Ohmatsu H, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg. 2005;129:991–6. doi: 10.1016/j.jtcvs.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 38.Yeh YC, Nitadori JI, Kadota K, Yoshizawa A, Rekhtman N, Moreira AL, et al. Using Frozen Section to Identify Histologic Patterns in Stage I Lung Adenocarcinoma </= 3 cm: Accuracy and Interobserver Agreement. Histopathology. 2014 doi: 10.1111/his.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol. 2010;5:1583–93. doi: 10.1097/jto.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida J, Ishii G, Yokose T, Aokage K, Hishida T, Nishimura M, et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol. 2010;5:546–50. doi: 10.1097/JTO.0b013e3181d0a480. [DOI] [PubMed] [Google Scholar]
- 41.Landreneau RJ, Normolle DP, Christie NA, Awais O, Wizorek JJ, Abbas G, et al. Recurrence and Survival Outcomes After Anatomic Segmentectomy Versus Lobectomy for Clinical Stage I Non-Small-Cell Lung Cancer: A Propensity-Matched Analysis. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.50.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sica G, Yoshizawa A, Sima CS, Azzoli CG, Downey RJ, Rusch VW, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. The American journal of surgical pathology. 2010;34:1155–62. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Sun Y, Shen L, Li Y, Xiang J, Zhang Y, et al. Predictive factors of lymph node status in small peripheral non-small cell lung cancers: tumor histology is more reliable. Ann Surg Oncol. 2013;20:1949–54. doi: 10.1245/s10434-012-2829-x. [DOI] [PubMed] [Google Scholar]
- 44.Yeh YC, Nitadori J, Kadota K, Sima CS, Rizk NP, Rusch VW, et al. Micropapillary histology is associated with occult lymph node metastasis (pN2) in patients with clinically N2-negative (cN0/N1) lung adenocarcinoma. J Thorac Oncol. 2013;8:S671. [Google Scholar]
- 45.Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Sublobar resection for lung adenocarcinoma meeting node-negative criteria on preoperative imaging. Ann Thorac Surg. 2014;97:1701–7. doi: 10.1016/j.athoracsur.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–20. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadota K, Villena-Vargas J, Yoshizawa A, Motoi N, Sima CS, Riely GJ, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol. 2014;38:448–60. doi: 10.1097/PAS.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokose T, Suzuki K, Nagai K, Nishiwaki Y, Sasaki S, Ochiai A. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung cancer (Amsterdam, Netherlands) 2000;29:179–88. doi: 10.1016/s0169-5002(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 49.Miyoshi T, Satoh Y, Okumura S, Nakagawa K, Shirakusa T, Tsuchiya E, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–9. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Makimoto Y, Nabeshima K, Iwasaki H, Miyoshi T, Enatsu S, Shiraishi T, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi's type C tumours) Histopathology. 2005;46:677–84. doi: 10.1111/j.1365-2559.2005.02126.x. [DOI] [PubMed] [Google Scholar]
- 51.Kamiya K, Hayashi Y, Douguchi J, Hashiguchi A, Yamada T, Izumi Y, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21:992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Mora N, Presmanes MC, Monroy V, Moreno N, Lara-Martinez JM, Aladro MH, et al. Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance. Case series. Hum Pathol. 2008;39:324–30. doi: 10.1016/j.humpath.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 53.Nagano T, Ishii G, Nagai K, Ito T, Kawase A, Takahashi K, et al. Structural and biological properties of a papillary component generating a micropapillary component in lung adenocarcinoma. Lung Cancer. 2010;67:282–9. doi: 10.1016/j.lungcan.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Sumiyoshi S, Yoshizawa A, Sonobe M, Kobayashi M, Fujimoto M, Tsuruyama T, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer. 2013;81:53–9. doi: 10.1016/j.lungcan.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Ujiie H, Buitrago D, Kadota K, Huang J, Travis WD, Rusch VW, et al. Sites, symptoms, CT scan findings and survival in patients with recurrence after curative-intent surgical resection for stage I lung adenocarcinoma. J Thorac Oncol. 2013;8:S164. [Google Scholar]
- 56.Kadota K, Suzuki K, Kachala SS, Zabor EC, Sima CS, Moreira AL, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25:1117–27. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadota K, Yeh YC, Sima CS, Rusch VW, Moreira AL, Adusumilli PS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2014;27:690–700. doi: 10.1038/modpathol.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kadota K, Nitadori J, Sarkaria IS, Sima CS, Jia X, Yoshizawa A, et al. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer. 2013;119:931–8. doi: 10.1002/cncr.27863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–8. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nitadori J, Kadota K, Sima CS, Ujiie H, Rizk NP, Rusch VW, et al. Spread through alveolar spaces (STAS): a newly recognized pattern of invasion in lung adenocarcinoma associated with increased recurrence in patients undergoing limited resection for ≤2cm tumors. J Thorac Oncol. 2013;8:S955. [Google Scholar]