Abstract
To refine inhibitory circuitry models for ON and OFF pathways in zebrafish retina, GABAergic properties of zebrafish bipolar cells were studied with two techniques: whole cell patch responses to GABA puffs in retinal slice, and voltage probe responses in isolated cells. Retinal slices documented predominantly axon terminal responses; isolated cells revealed mainly soma-dendritic responses. In the slice, GABA elicited a conductance increase, GABA responses were more robust at axon terminals than dendrites, and Erev varied with [Cl−]in. Axon terminals of ON- and OFF-type cells were similarly sensitive to GABA (30–40pA peak current); axotomized cells were unresponsive. Bicuculline-sensitive, picrotoxin-sensitive, and picrotoxin-insensitive components were identified. Muscimol was as effective as GABA; baclofen was ineffective. Isolated bipolar cells were either intact or axotomized. Even in cells without an axon, GABA or muscimol (but not baclofen) hyperpolarized dendritic and somatic regions, suggesting significant distal expression. Median fluorescence change for GABA was −0.22 log units (~−16mV); median half-amplitude dose was 0.4μM. Reduced [Cl−]out blocked GABA responses. GABA hyperpolarized isolated ON-bipolar cells, OFF-cells were either unresponsive or depolarized. Hyperpolarizing GABA responses in isolated cells were bicuculline and TPMPA insensitive, but blocked or partially blocked by picrotoxin or zinc. In summary, axon terminals contain bicuculline-sensitive GABAA receptors and both picrotoxin-sensitive and insensitive GABAC receptors. Dendritic processes express zinc- and picrotoxin-sensitive GABAC receptors.
Keywords: retina, bipolar cells, GABA, zebrafish
Introduction
In retina, immunocytochemical studies of neural circuitry show that the dendritic arbors and axon terminals of bipolar cells are surrounded by GABAergic processes. In the outer plexiform layer, processes of GABAergic horizontal cells surround bipolar cell dendrites; while in the inner plexiform layer, processes of GABAergic amacrine cells surround bipolar axon terminals. Physiological studies report localization of GABA sensitivity. GABA-evoked currents are greatest at bipolar terminals (i.e., Tachibana & Kaneko, 1987; Karschin & Wassle, 1990; Lukasiewicz et al., 1994; Tachibana & Kaneko, 1998) with smaller amplitude currents typically elicited from the soma and/or dendritic arbor. In some species large dendritic currents also occur (Qian & Dowling, 1995). Overall GABAergic inhibitory feedback from neighboring amacrine cells is dominant (Tachibana & Kaneko, 1987, 1998), and occurs through direct GABAergic input onto bipolar cell terminals.
In many species, signals from GABAergic horizontal cells may arrive both directly and indirectly onto bipolar cell dendrites. The indirect route is through a feedback synapse involving photoreceptors (Burkhardt, 1977; Kondo & Toyoda, 1983; Wu, 1986; Wu & Maple, 1998). Direct morphological contacts between GABAergic horizontal cells and bipolar cells have also been seen, albeit infrequently (Dowling & Werblin, 1969; Lasansky, 1973; Fisher & Boycott, 1974; Kolb & Jones, 1984; Linberg & Fisher, 1988). In zebrafish at least one class of horizontal cell is GABAergic and is a candidate presynaptic element (Connaughton et al., 1999; Marc & Cameron, 2001; Yazulla & Studholme, 2001; Nelson et al., 2006). The horizontal cell layer of zebrafish stains richly for VGAT, vesicular transporters for GABA, and the outer plexiform layer stains for GABA receptors (Yazulla & Studholme, 2001). This suggests distal GABAergic synaptic activity. Although the postsynaptic elements have not been identified, bipolar cell dendrites are a likely target for horizontal cell GABA. GABAergic interplexiform cells also appear to synapse onto bipolar cell dendrites in some species (Kolb & West, 1977; Crooks & Kolb, 1992), though GABAergic interplexiform cells have not been reported in zebrafish (Connaughton et al., 1999; Marc & Cameron, 2001; Yazulla & Studholme, 2001).
GABA application typically elicits a chloride current that hyperpolarizes bipolar cells. GABA-elicited currents have both transient and sustained components. The transient response is mediated by GABAA receptors; while the sustained component results from the activation of GABAC receptors (Tachibana & Kaneko, 1987; Qian & Dowling, 1993; Feigenspan & Bormann, 1994; Qian & Dowling, 1995; Lukasiewicz & Wong, 1997; Lukasiewicz & Shields, 1998; McGillem et al., 2000). Baclofen-sensitive GABAB receptors have only been identified on salamander bipolar cells (Maguire et al., 1989), though a different GABAB-like receptor modulating Ca+2 currents has been identified in goldfish Mb bipolar terminals (Heidelberger & Matthews, 1991). Interestingly, though most bipolar cells appear to express both GABAA and GABAC receptors, it is the GABAC receptor that underlies the majority of GABA-elicited responses in these cells (Lukasiewicz & Wong, 1997; Lukasiewicz & Shields, 1998; Nelson et al., 1999; Kaneda et al., 2000; McGillem et al., 2000). The combined expression of GABAA and GABAC receptors on bipolar cells (Zhang & Yang, 1997; Euler & Wassle, 1998; Du & Yang, 2000) allows these cells to respond to a range of GABA concentrations and time courses within the synaptic cleft, as these different receptor types display different sensitivities and kinetics to GABA and/or GABA agonists (Woodward et al., 1993; Lukasiewicz & Wong, 1997; Lukasiewicz & Shields, 1998; Lukasiewicz et al., 2004).
Microelectrode studies show GABAA and GABAC receptors may perform functionally distinct roles. GABAC receptors preferentially suppress ON responses of third order neurons (Zhang & Slaughter, 1995); while GABAA activation suppresses the OFF pathway (Zhang & Yang, 1997). Further, GABAergic ligands affect the amplitude and time course of the ERG b-wave (Kapousta-Bruneau, 2000; Dong & Hare, 2002; Hanitzsch et al., 2004; Lukasiewicz et al., 2004). Pharmacological studies examining GABAergic inputs to mammalian rod bipolar cells have identified responses mediated predominantly by GABAC receptors (Feigenspan & Bormann, 1994; Vaquero & de la Villa, 1999; McGillem et al., 2000; Frech & Backus, 2004). However, recent findings indicate recurrent inhibition of rod bipolar terminals by A17 amacrine cells is mediated only through GABAA type receptors (Singer & Diamond, 2003). The net effect of GABAergic inputs is to modulate calcium current activity in bipolar cell terminals (Maguire et al., 1989; Matthews et al., 1994; Wu & Zhu, 2000; Singer & Diamond, 2003; Frech & Backus, 2004) altering neurotransmitter release (Mack et al., 2000; Singer & Diamond, 2003; Hull & von Gersdorff, 2004) and/or suppressing spontaneous EPSCs in postsynaptic ganglion cells (Freed et al., 2003).
Zebrafish is a current model for vertebrate vision. The voltage-gated (Connaughton & Maguire, 1998) and glutamate-gated (Connaughton & Nelson, 2000) responses of bipolar cells have been documented and their morphology described (Connaughton et al., 2004). However, the inhibitory circuitry modifying bipolar cell responses has not been determined in this species. This information is important, given the continued identification of zebrafish with visual system mutations localized to the retina. The present work is a study of inhibitory mechanisms on zebrafish bipolar cells. We have employed parallel techniques: the retinal slice with cell attached patch electrodes, and dissociated cell preparations using the oxonol fluorescent probe. Recordings in the slice best revealed GABAergic inputs to bipolar cell terminals; GABA responses at the dendrites were typically small and/or absent in this preparation. In contrast, recordings of inhibitory responses from dissociated cells appeared to originate predominantly in soma and dendritic regions. In both preparations, multiple GABA receptor types were identified. Parts of this work has appeared previously in abstract form (Connaughton et al., 2000).
Methods
Care and maintenance of animals
Zebrafish (Danio rerio) were obtained from a local supplier (Petsmart, Inc.) and maintained in the laboratory in 40L aquaria at 28–29°C on a 14hr light:10hr dark photoperiod until needed for experiments. Each day, temperature and water level in the aquaria was checked and the fish were fed (Tetramin flakes). Animal care and use protocols were approved by the appropriate committees at both American University and the National Institutes of Health.
Preparation of retinal slices and patch clamp recordings
Retinal slices were prepared as described previously (Connaughton & Nelson, 2000; Connaughton, 2003). In brief, fish were removed from aquaria and decapitated with a dorsal-ventral incision. Following enucleation, the anterior segment was removed. The retina (with attached sclera) was placed vitreal-side down on a piece of Millipore filter paper (0.45μm pore diameter). The filter paper and retina were transferred into the recording chamber and anchored into place on Vaseline strips. Retinal slices (~100μm thick) were cut with a fresh razor and each slice rotated 90° within the recording chamber to view the retina in cross section. Tissue was submerged in the standard Ringer’s solution containing 120mM NaCl (Na-isethionate in low Cl− solution), 2mM KCl, 1mM MgCl2, 3mM CaCl2, 4mM HEPES, and 3mM D-glucose brought to pH 7.4–7.5 with NaOH. In some experiments 5mM CoCl2 was added to the bath solution, to block synaptic transmission.
Bipolar cells were initially identified by a soma position in the distal to mid inner nuclear layer (INL). Lucifer Yellow (1% solution), present in the patch pipette, stained recorded neurons and confirmed bipolar cell morphology. Ligand-gated currents were recorded from zebrafish bipolar cells using whole-cell voltage clamp techniques. A cesium-based intracellular solution blocked K+ currents. This solution (Low Cl−) contained 12mM CsCl, 104mM Cs-gluconate (116 mM CsCl in high Cl−solution), 1mM EGTA, 4mM HEPES, and 0.1mM CaCl2 brought to pH 7.4–7.5 with KOH. Calculated liquid junction potentials (bath pipette) for 3 combinations of pipette and bath solutions are as follows: High Cl− internal solution and standard external solution (lacking cobalt) = 5.3mV; Low Cl− internal solution and standard external solution (lacking cobalt) = 15.8mV; Low Cl− solution and cobalt-containing external solution = 16.0mV (Kenyon, 2002). Text and figures are uncorrected. GABA agonists were applied using a second puffer pipette, positioned to stimulate processes in either the inner or the outer plexiform layer. Each GABA agonist was applied in a separate experiment. The GABA antagonists bicuculline (≤200μM) and picrotoxin (≤200μM) were applied in the bath solution.
Currents were elicited by focal puffs of GABA (100μM or 1mM), muscimol (200μM), or ± baclofen (200μM) onto bipolar cell dendrites or axonal boutons (50ms puff, 15psi). To determine reversal potentials of these currents, holding potentials (Vhold) were increased stepwise (20 mV increments) from either − 60mV or − 70mV to reach a maximum of +10mV or +20mV. Raw current traces were boxcar filtered in pCLAMP (9 data points on each side) prior to data analysis. Peak current amplitude at each holding potential was measured and plotted as a current-voltage relationship (Microcal Origin version 7.0). The reversal potential (Erev) for each current was determined by a linear regression through the I-V plot (only fits with r2 ≥ 0.7 included).
Patch and puff pipettes of ~1μm tip diameter were pulled from thin-walled, filamented borosilicate glass (World Precision Instruments) using a Flaming-Brown P-80 pipette puller (Sutter Instruments, Novato, CA). Cells were visualized using an Olympus fixed-stage compound epifluorescence microscope fitted with a 40× water immersion lens and Hoffman modulation contrast optics. Data were collected using an Axopatch 1-C patch clamp amplifier and pCLAMP software (ver. 6.0 and 8.0).
Cell isolation and fluorescent probe measurements
Following established protocols (Connaughton & Dowling, 1998; Nelson et al., 2003), zebrafish were dark adapted overnight and decapitated. The retinas were removed and cell adhesions were enzymatically broken in a solution consisting 33U/ml papain (Worthington), 70% Leibovitz (L-15) medium, 0.19U/ml dispase (Sigma Chemical Co.), and 0.05% DNAase (Sigma Chemical Co.). The pH was adjusted to neutral with 0.07M NaOH. The cells were physically separated by gentle trituration, and the resultant suspension plated on a rectangular stripe of poly-D-lysine, coated across the center of plastic culture dishes. Cells were allowed to settle and adhere for at least 1.5 hours before the start of experiments.
Dissociated bipolar cells were identified morphologically, with a round cell body connected to a thin axon, or by a flask-shaped cell body with dendritic tufts. Plated cells were superfused under a raised cover glass (mean fluid velocity = 2600μm/s) with the above standard extracellular solution (lacking cobalt) and containing the fluorescent, voltage-sensitive dye oxonol (DiBaC4(5), 80nM). Cells were perfused for at least 10min for initial probe equilibration. Agonists and antagonists were applied as a stock solution diluted in the extracellular solution. Voltage-probe responses to GABA, GABA agonists, glycine, or glutamate were obtained in parallel for each cell in a microscope field using intensified fluorescence video microscopy, with images captured at 30s intervals. The integrated fluorescence of each cell (FL) was summed and the fluorescence of equivalent background areas subtracted before logarithmic conversion. Histograms of log(FL) responses to treatments revealed skewed distributions, so that (for positive going responses) means were greater than histogram peaks. Medians and histogram peaks agreed well, however, and so median responses are adopted here to characterize typical responses from within distributions. The significance of differences among treatments was evaluated using 2-tailed Mann-Whitney U tests.
Oxonol entry causes retinal cells to fluoresce with a brightness corresponding to internal concentration. A depolarization was indicated by an increase in fluorescence, while a hyperpolarization was indicated by a decrease in fluorescence as the negatively charged oxonol redistributed in Nernstian fashion across the cell membrane (Nelson et al., 1999; Nelson et al., 2003). Following treatments with inhibitory or excitatory ligands, 1μM gramicidin was administered to permeabilize the cells to monovalent cations. This caused the membrane potential to increase to 0mV (Nelson et al., 2003). Only cells or cell regions depolarized by gramicidin are included in this study. The median fluorescence increase induced in bipolar cells by gramicidin was 0.52 (P25/75; 0.36/0.67) log units (n = 269). Nelson et al. (1999) found 1 log unit fluorescence increase from resting fluorescence corresponded to 129mV of Goldman potential, making the corresponding range in median membrane potential about −65 to −79 mV. The median gramicidin time constant at the 2600μm/min flow rate was 1.65 min for cell bodies (n = 197), 1.75min for dendrites (n = 60), and 1.95min for terminals (n = 5). These values were not significantly different (p ≥ 0.05; Mann Whitney U test). Oxonol was purchased from Molecular Probes (Eugene, OR). All other chemicals were purchased from Sigma (St. Louis, Mo).
GABA responses observed using the oxonol probe were recorded in both axon bearing and axotomized cells (soma and dendrites only). Most dissociated bipolar cells lacked axons and terminals. The morphology of axonless bipolar neurons, however, was characteristic: a flask-shaped/elongate cell body with dendritic tufts. While round somata without processes were far more common in these dissociations (and a large fraction of these are likely to be bipolar neurons) the physiological patterns of such cells are not included here. Comparison of responses of either intact cells vs. axotomized cells, or between different cell regions (dendrites vs. soma) were found to be similar (p > 0.05; Mann-Whitney U test). Further, the likelihood of GABA-induced hyperpolarization did not differ between isolated axon-bearing or axotomized cells (axon-bearing, n = 38 of 61 responders or 62%; axotomized, n = 94 of 150 responders or 63%). As a result, data collected all isolated bipolar cells were pooled for analysis, and they are referred to simply as isolated cells or regions within the text.
Results
In addition to documenting GABA responses in zebrafish bipolar cells, this study also compares dissociated and non-dissociated responses of these cells as almost all experiments were performed both within the retinal slice and using cultured cells. Exceptions to this include analysis of the effects of zinc and TPMPA, which were performed using isolated cells. In both preparations, GABA responses were correlated with Bon (ON-) and Boff (OFF-) type bipolar cells, either based on cell morphology (slice) or glutamate responses (isolated cells). Results from both preparations are discussed together, with examples presented from each.
Localization of GABA responses
The location of bipolar cell GABA receptors was determined by focally applying GABA (100μM or 1mM) onto either the dendritic arbor in the outer plexiform layer (OPL; Figure 1) or the axon terminals in different zones of the inner plexiform layer (IPL; Figure 1). High concentrations of GABA (i.e., 1mM) were more effective, as they were probably better able to overcome uptake and removal by in vivo transporter systems (Wu, 1986).
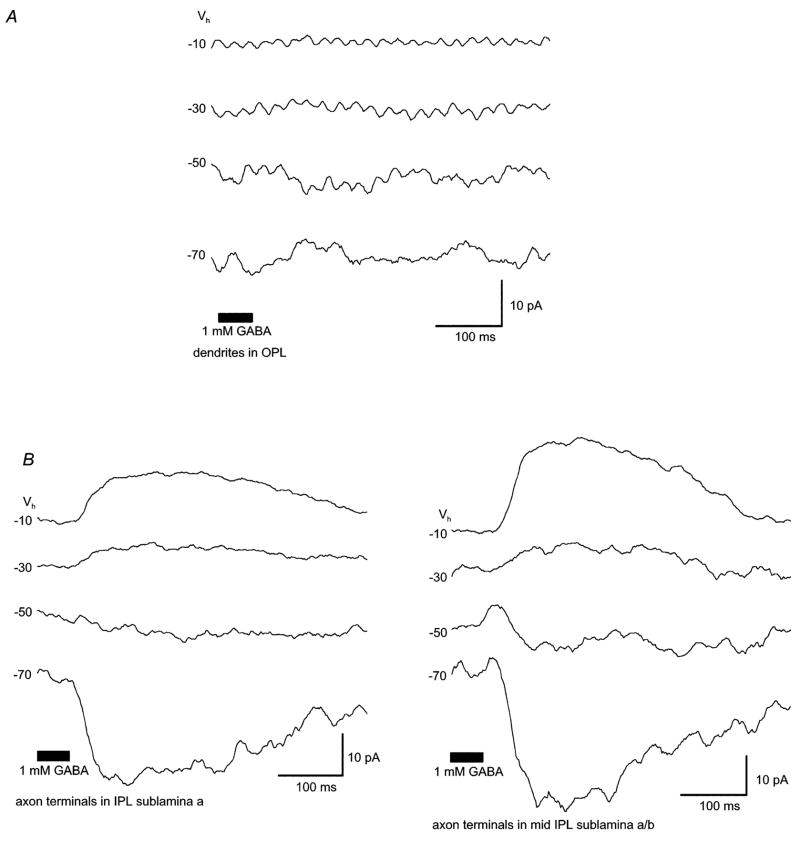
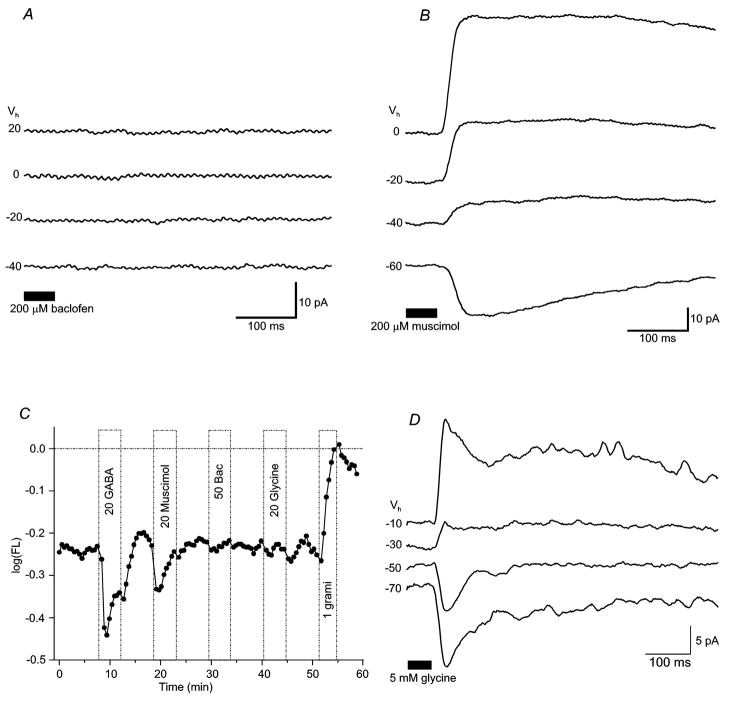
Figure 1. In the retinal slice preparation, GABA-evoked currents were more robust in the axon terminal than in the dendritic arbor.
In the retinal slice preparation, focal application of GABA onto (A) the dendritic arbor (OPL) or (B) axonal bouton(s) of bipolar cells (IPL) evoked different amplitudes of whole-cell currents. GABA responses elicited from axon terminals (~30 to 40pA) could be recorded from all levels of the IPL (IPL sublamina a, mid IPL sublamina a/b) and were typically more robust than those elicited from the dendritic arbor (≤5pA). Only a minority of cells responded to GABA application in the OPL (n = 3 of 12 cells; 1mM GABA puff). All traces shown were recorded from the same cell. Values to the left of each trace are the holding potentials, the bar at the bottom denotes the duration of GABA application. Pipette solution is low Cl− (ECl = −61mV).
GABA currents in the slice were recorded using two different intracellular solutions and the cobalt-containing Ringer’s solution. Initially, recordings were made with the Low Cl− intracellular solution (ECl = −61mV). Using this solution, focal application of the lower GABA dose (100μM) to the dendritic arbor failed to elicit a response in all cells examined (n = 6 of 6). Similarly, application of 100μM GABA onto axonal boutons in the IPL elicited a response in only some (3 of 6) cells. These GABA-elicited currents were characterized by a conductance increase and an average reversal potential of −32.7 ± 8.47mV. Four of the six cells tested (two responding to GABA and two not responding) had identifiable axon terminals.
Experiments performed using 1mM GABA and the Low Cl− pipette solution gave similar results, as the application of GABA to the IPL elicited robust responses in 10 of 20 cells. All 10 responders had identifiable axon terminals. Eight of the ten cells not responding to 1mM GABA lacked terminals. The reversal potential of these GABA-elicited responses averaged − 38.02 ± 5.6mV (n = 10; ECl = − 61mV). The fact that, overall, cells without axon terminal boutons typically lacked GABA responses suggests IPL GABA receptors are localized to this region, and are not found on the remaining axonal process. GABA responses were elicited from bipolar cells with terminals located throughout the IPL (Figure 1), including monostratified Boff cells with terminals in sublamina a (n = 3), monostratified Bon cells with terminals in sublamina b (n = 4), and multistratified Boff and Bon cells with terminals in both sublaminae (n = 2). Application of 1mM GABA onto the dendritic arbor elicited a response in a minority of cells (3 of 12 cells), in one case with and in two cases without cobalt in the extracellular solution.
GABA-elicited currents recorded following stimulation of axon terminals were typically 30–40pA. Peak current amplitude, recorded at a holding potential of − 60mV (High Cl− intracellular solution) averaged 36 ± 16.5pA (n = 7) for 1mM GABA and 21.6 ± 14.7pA (n = 21 cells) for 100μM GABA. These currents were consistently more robust than currents elicited at the dendritic arbor (typically ~5pA) suggesting a greater density of GABA receptors on axonal boutons. Given the more robust and consistent responses recorded from GABA application onto bipolar terminals, the remaining experiments in the slice focused on these responses only.
GABA responses were accompanied by a conductance increase. The reversal potentials of GABA responses were sensitive to changes in ECl (Figures 2A and 2B) resulting from altering internal [Cl−]. Using 1mM GABA, Erev increased from − 38.02 ± 5.6mV (n = 10; Low Cl− solution) to 0.39 ± 4.29mV (n = 6; High Cl− solution). Similarly, with the lower GABA dose (100μM) Erev increased from −32.7 ± 8.47mV (n = 3; Low Cl-) to − 0.25 ± 5.21mV (n = 20; High Cl−). This data indicates GABA elicited a chloride-sensitive current. This change in reversal potential with altered internal chloride was observed in both Boff type cells (n = 12) and Bon type cells (n = 11) using either GABA concentration.
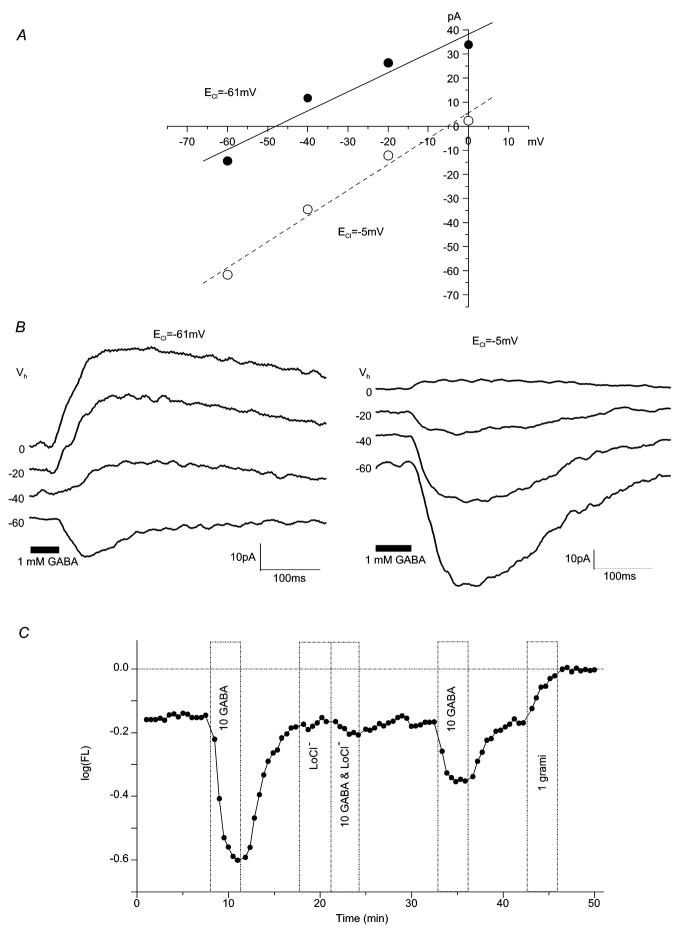
Figure 2. GABA elicits a chloride current in zebrafish bipolar cells.
(A) GABA-evoked current-voltage relationships and (B) the accompanying whole-cell current traces change with high and low intracellular chloride concentrations. For [Cl−]i of 12mM (ECl = − 61mV) the mean Erev was − 38.02 ± 5.6mV (n = 10; 1mM GABA); for [Cl−]i of 116mM (ECl = −5mV) the mean Erev was 0.39 ± 4.29mV (n = 6; 1mM GABA). Vhold values (Vh) are given to the left of each trace (B). (C) Hyperpolarizing GABA responses recorded with a voltage probe in an isolated bipolar cell are blocked by removal of [Cl−]o “LoCl” (Na isethionate substituted for NaCl in the extracellular media, [Cl−]o = 10mM). In this and following voltage-probe figures, the voltage probe is 80nm oxonol, added to all solutions and log(FL) refers to background-subtracted oxonol fluorescence. Increases in log(FL) correspond to depolarization, and decreases to hyperpolarization. The ‘0’ level of log(FL) is set by the final 1μM gramicidin treatment (“1 grami”), that forces cell membrane potential to zero. 1 log of FL decrease corresponds to − 72mV, 1 log of FL increase corresponds to 129mV (Nelson et al., 1999). Treatments are shown by the bars, with concentrations given in μM.
Chloride-sensitivity was also observed in isolated cells as low extracellular Cl− eliminated GABA responses in these conditions (Figure 2C). In normal external chloride, GABA reduced voltage probe fluorescence, signaling membrane hyperpolarization. Reducing chloride in the extracellular medium greatly attenuated this response (Figure 2C), confirming that bipolar cell GABA responses are chloride mediated. In these experiments, 100% of bipolar cell hyperpolarizations (n = 16) were decreased by 80% or more following the reduction of [Cl−]out to ~10μM. Quantitatively, the median control hyperpolarization of − 0.24 log units decreased to a median response of 0.0 log units in low chloride. The median recovery response was − 0.10 log units. As compared to combined control and recovery responses (median − 0.13 log units), the median GABA response in low chloride was significantly reduced (p < 0.001 Mann-Whitney U test).
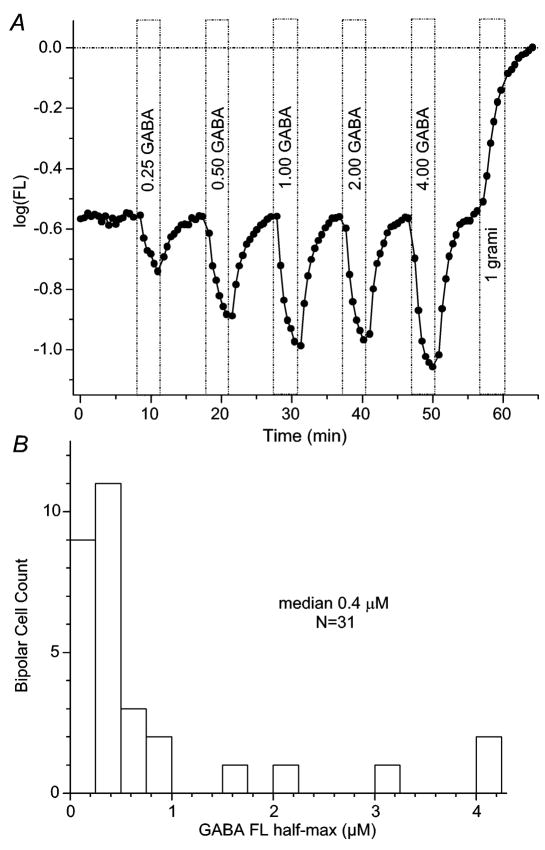
Isolated zebrafish bipolar cells were highly sensitive to GABA, in contrast to the high GABA concentrations (1mM) required in the slice, but in agreement with published findings on isolated cells (i.e, Feigenspan & Bormann, 1994; Matthews et al., 1994; Vaquero & de la Villa, 1999; Qian et al., 2001). Sub-saturating GABA-induced hyperpolarizations were typically observed only for doses less than 1μM (Figure 3A). Examination of the concentration dependence of voltage probe GABA responses (Figure 3B) revealed a median half-amplitude point at 0.4μM (P25/75; 0.2/0.8μM). Combining all experiments, the median peak hyperpolarization induced by GABA was −0.22 log units (P25/75; − 0.37/− 0.15 log units; n = 156; GABA concentration of 10 or 20μM only), corresponding to a hyperpolarization of ~ −16mV (Nelson et al., 1999). Few regions were either depolarized by GABA or responded with after hyperpolarizations (Nelson et al., 2006).
Figure 3. Isolated zebrafish bipolar cells are sensitive to a range of GABA concentrations.
(A) Responses to different GABA concentrations in an isolated bipolar cell. GABA concentrations ranging from 0.25–4μM (bars) elicited hyperpolarizing responses from isolated bipolar neurons. In this example half-maximum response (half-max) occurred at ~0.5μM. (B) Histogram of half-maximum GABA concentrations for responses from 31 isolated bipolar cells. The median is 0.4μM. Figure conventions for voltage probe recordings are the same as described in Figure 2.
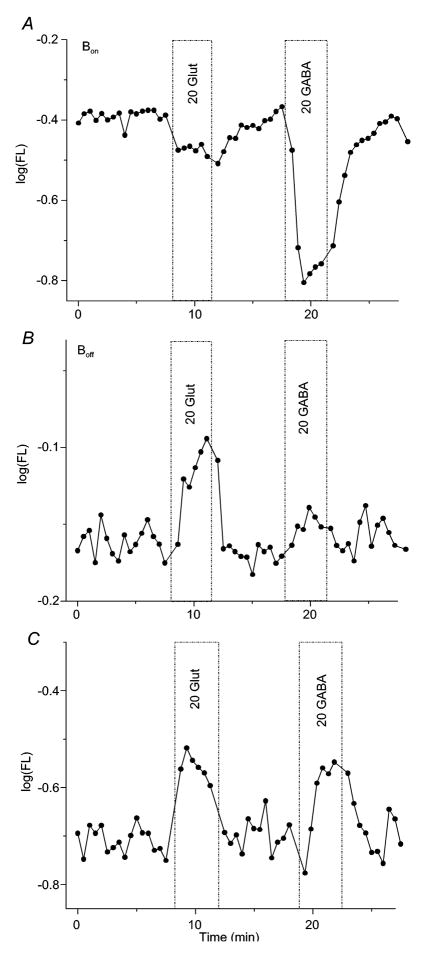
As documented in the slice, GABA responses of both ON- and OFF-bipolar cells were identified in isolated cells. ON-type responses, identified by glutamate hyperpolarizations, were highly associated with GABA hyperpolarizations (Figure 4A), as 83% of isolated cells responded to both compounds. In contrast, OFF-type responses, identified by glutamate depolarizations, were not associated with GABA hyperpolarizations. Rather, 38% of OFF-cells were depolarized by both glutamate and GABA application, and GABA did not evoke a change in membrane fluorescence in the remaining 62%. GABA insensitive and GABA depolarized OFF-type bipolar cells are illustrated in Figures 4B and 4C. Cells that were insensitive to glutamate could not be classified as ON- or OFF-types. GABA hyperpolarizations were characteristic of this group, and they were encountered at a frequency intermediate between identified ON- and OFF-types. In this glutamate-insensitive group, GABA hyperpolarizations were found in 7 of 12 regions. Overall, the positive association of GABA hyperpolarizations with ON-type bipolars, and the negative association with OFF-type bipolars were significant (p < 0.001; Chi Square test).
Figure 4. GABA responses of isolated soma-dendritic regions of Bon and Boff bipolar cells.
(A) Bon type cell, characterized by glutamate hyperpolarization (“20 glut”), also hyperpolarizes robustly to GABA (“20 GABA”). (B) Boff type unit, characterized by glutamate depolarization, does not respond to GABA. (C) Boff type unit, characterized by glutamate depolarization, is depolarized by GABA. Figure conventions for voltage probe recordings are the same as described in Figure 2.
Though GABA application in the slice evoked only small responses at the dendritic arbor, isolated axotomized cells displayed robust decreases in membrane fluorescence following GABA application. This latter finding confirms GABA receptors are located on the dendritic arbor and soma of zebrafish bipolar cells and suggests that dendrites are capable of large GABAergic responses even in the absence of an axon terminal.
Multiple GABA receptor types are present on zebrafish bipolar cells
To examine whether GABAA, GABAB, or GABAC receptors were active, specific agonists and antagonists were applied. Within the slice, these experiments were performed using the cesium-based (High Cl−) intracellular solution and 5mM cobalt in the bath. Agonists were puffed onto bipolar terminals and antagonists were dissolved in the bath solution. For fluorescent probe measurements, the different compounds were dissolved directly into the standard extracellular perfusate (lacking cobalt).
Application of baclofen (200μM), a GABAB receptor agonist, failed to elicit a response from bipolar cells examined in the retinal slice (Figure 5A, n = 6 of 6 cells). Similarly, in isolated cells, perfusion of baclofen (100μM) had little effect on membrane potential (Figure 5C). In 75% of isolated cells (n = 21 of 28), no changes were observed. In the remainder, small miscellaneous effects occurred including low amplitude hyperpolarizations (n = 3), a small depolarization (n = 1), and small after hyperpolarizations (n = 3). The distribution of responses, including depolarizing, hyperpolarizing and non-responses following baclofen application was significantly different from the strongly hyperpolarized distribution evoked by GABA (p < 0.001 Mann-Whitney U test).
Figure 5. Muscimol, not baclofen, is an effective agonist of GABA receptors on bipolar cell axon terminals.
Whole-cell current traces show responses to (A) 200μM ± baclofen or (B) 200μM muscimol applied onto axon terminals. Erev of muscimol-elicited currents averaged −45.4 ± 21.6mV (n = 4). Baclofen failed to elicit responses. Each drug was tested in separate experiments. (C) Voltage-probe records from a dissociated bipolar cell reveal both GABA- (“20 GABA”) and muscimol-(“20 Muscimol”) induced hyperpolarizations. Baclofen (“50 Bac”) and glycine (“20 gly”) were not effective in this cell, though glycine induced hyperpolarizations in others. (D) Whole-cell current traces show responses to 5 mM glycine applied onto bipolar cell dendrites. Erev of dendritic glycine-elicited currents averaged − 49 ± 9mV (n = 8). Bipolar cells were also sensitive to glycine applied to axonal boutons (not shown). Figure conventions for voltage-probe records are the same as described in Figure 2.
In contrast, the GABAA/C receptor agonist muscimol elicited consistent responses (Figure 5B) both within the slice (200μM; n = 6 of 7 cells) and in voltage-probe recordings (Figure 5C). The currents evoked by puffing muscimol on bipolar cells recorded in retinal slices were similar in time course and amplitude to those elicited by GABA. The average peak current elicited by muscimol (lowCl− pipette solution) at Vhold = − 60mV was 6.2 ± 11.6pA (n = 4); while the average GABA current recorded under these conditions was 10.4 ± 3.29pA (n = 4). The calculated reversal potential of muscimol-elicited currents was − 45.4 ± 21.6mV (n = 4), similar to GABA-evoked reversal potentials recorded with the same solutions.
Muscimol was equally as effective as GABA in hyperpolarizing all regions of isolated bipolar cells, with 62% of isolated cells hyperpolarized by 10 or 20uM muscimol. Of 84 cells treated with both GABA and muscimol, 47 (or 56%) were hyperpolarized by each treatment, far more than the 33 (or 39%) predicted by independent association (p < 0.001, Chi-square test). GABA and muscimol responses were highly correlated.
Some (12 of 49) isolated bipolar cells were hyperpolarized by glycine, with a median amplitude of −0.16 log units. The bipolar cell of Figure 5C, however, does not have a significant response to 20μM glycine. Seventy-three percent of bipolar cells recorded with voltage-probe did not respond to glycine (n = 36 of 49). When found, the amplitude distribution of glycine hyperpolarizations was not significantly different than that found for GABA hyperpolarizations (p > 0.05, 2-tail Mann-Whitney U test). In 47 cells treated with both GABA and glycine, hyperpolarization by both inhibitory transmitters was seen in 6 cases, about the same number (7) expected based on independent association. GABA and glycine hyperpolarizations were not linked (p > 0.05, Chi-square), and probably reflect different receptor systems. Eight of the 12 glycine-induced hyperpolarizations occurred in isolated axotomized cells, suggestive of soma-dendritic glycine receptors. Within the retinal slice, puffs of glycine (1mM or 5mM) also elicited responses from ~30% of bipolar cells (Figure 5D). Following application in the OPL, cells responded with a conductance increase and a mean reversal potential of −49 ± 9mV (n = 8). Bipolar cell terminals were also sensitive to glycine. When puffed into the IPL, glycine again evoked a conductance increase, with an average reversal potential of −38 ± 10.8mV (n = 7). Though current amplitude recorded from individual cells was sometimes greater at the dendritic arbor than the axon terminal, the average amplitude of glycine-evoked currents from both regions was similar (6 ± 5.3pA at the dendrites; 5 ± 1.9pA at the terminals, Vhold= −70mV, low Cl− intracellular solution). Thus, direct glycinergic inputs are found on both zebrafish bipolar dendrites and axon terminals, similar to findings for GABA. However, a much lower fraction of cells, less than half, are glycine sensitive.
The above results with selective GABAergic agonists reveal that ionotropic GABA receptors on zebrafish bipolar cells are directly activated by exogenous GABA, and that metabotropic receptors evoke little standing current. In order to discriminate between the different ionotropic GABA receptor types, selective antagonists were applied. In retinal slice, 100–200μM bicuculline, a GABAA antagonist, partially blocked responses elicited by 100μM GABA, with all cells tested displaying bicuculline sensitivity. On average, bicuculline blocked 39% (range = 21% − 49%; n = 4) of the GABA-elicited current in cell-attached patch recordings (Figure 6B). In isolated bipolar cells, bicuculline was also found to be an incomplete blocking agent (Figure 6C). Bicuculline partially blocked GABA-induced hyperpolarizations in only 32% (7 of 22 regions). (A complete block was identified in one cell.) Complete block was defined as < 20% of control response amplitude remaining; partial block was defined as >20% but < 80% of control response amplitude remaining. In the presence of bicuculline, the median amplitude of response for isolated cells was − 0.195 log units, the identical value obtained for both control and recovery responses. These response amplitudes in both treated and treatment-free groups were not significantly different (p > 0.05, Mann-Whitney-U test). Thus, in both slice and isolated cells partial or no bicuculline sensitivity was the rule; with most isolated cell responses identified as bicuculline-insensitive.
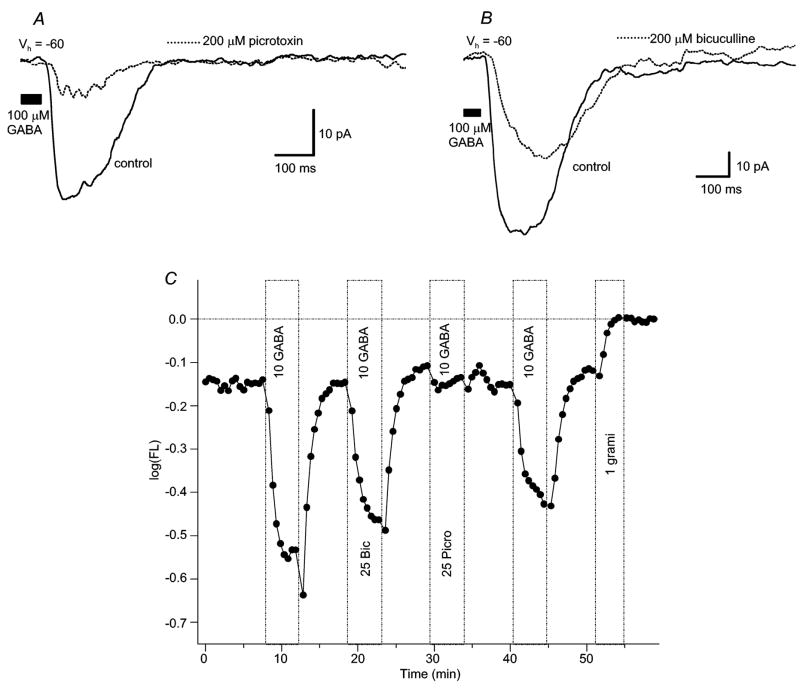
Figure 6. Picrotoxin is more effective than bicuculline at blocking GABA-evoked currents elicited at bipolar cell axon terminals or isolated soma-dendritic regions.
(A) Whole-cell current traces show a decrease in GABA-gated currents observed after bath application of 200μM picrotoxin. (B) Whole cell current traces show lesser decrease in current after bath application of 200μM bicuculline. Vhold= −60mV for (A) and (B). (C) Dissociated bipolar cells displayed similar antagonist sensitivity: picrotoxin decreased GABA-evoked fluorescence responses by ~80%, while bicuculline only partially antagonized the response. Figure conventions for voltage-probe records are the same as described in Figure 2.
In isolated cells, bicuculline partially blocked muscimol-induced hyperpolarizations in 20% of cases (Fig. 7A). No complete blocks were observed. In the regions studied, the median control and recovery muscimol response (−0.195 log units) was again not significantly different (p > 0.05, Mann-Whitney U-test) from responses recorded in the presence of bicuculline (−0.31 log units). Overall, in both the slice and isolated cell recordings, bicuculline application typically blocked < 50% of the GABA-elicited (or muscimol-elicited) response.
Figure 7. In isolated bipolar cells, zinc, but not TPMPA, antagonizes GABA-responses, muscimol responses are not blocked by bicuculline.
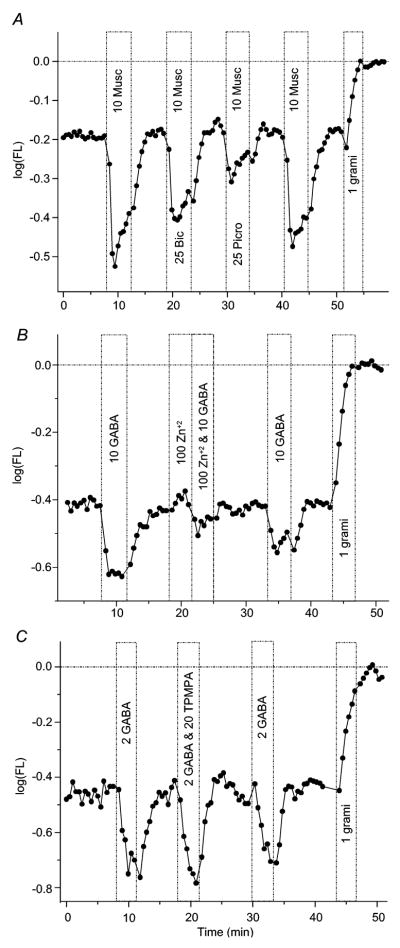
(A) In an isolated bipolar cell, muscimol (“10 Musc”), like GABA, evokes a transient hyperpolarizing response. This muscimol response is only partially blocked by bicuculline, suggesting muscimol does not selectively activate GABAA receptors. The picrotoxin block is more complete, suggesting GABAC receptors are activated by muscimol and at least partially blocked by picrotoxin. (B) The co-application of zinc (100μM) together with GABA (10μM) reversibly abolished GABA-elicited hyperpolarization in an isolated bipolar cell. These findings, together with bicuculline insensitivity and high GABA sensitivity, suggest GABAC receptors are expressed on zebrafish bipolar cells. (C) TPMPA (20μM), a GABAC selective antagonist, fails to abolish hyperpolarizations evoked by GABA (2μM) in an isolated bipolar cell, even in 10x excess. Figure conventions for voltage-probe records are the same as described in Figure 2.
Picrotoxin is a mixed GABAA and GABAC receptor antagonist. Application of 100–200μM picrotoxin (with puffs of 100μM GABA) also resulted in a partial block of GABA-elicited currents recorded in the retinal slice, with all cells tested sensitive to picrotoxin. GABA was typically applied 2–4x after picrotoxin application, with changes in amplitude measured after the final application. In these conditions, a maximum 74% decrease (Figure 6A) in GABA-elicited current amplitude was observed (range = 10% to 74%; n = 7). In isolated cells, picrotoxin application (25 and 50μM) decreased GABA-induced hyperpolarizations, with 100% of cells tested displaying partial or complete picrotoxin sensitivity. Picrotoxin decreased GABA-evoked changes in membrane fluorescence an average of 81%, with a complete block observed in 65% of cells. In isolated cells, the median of control and recovery responses was − 0.175 log units. In the presence of picrotoxin the median response was significantly decreased to 0.0 log units (Figure 6C) (p < 0.001, Mann-Whitney U-test).
Further, in isolated cells, picrotoxin also completely or partially blocked muscimol-induced hyperpolarizations in 100% of regions tested (Figure 7A). In these regions, the median control and recovery amplitude of muscimol hyperpolarizations was − 0.195 log units. Picrotoxin significantly decreased this to − 0.05 log units (p ≤ 0.01, Mann-Whitney U test). Overall, picrotoxin was a far more effective antagonist than bicuculline for the GABA- (and/or muscimol-) induced hyperpolarizations in isolated bipolar cells.
Co-application of bicuculline (200μM) and picrotoxin (100μM) resulted in the greatest block of currents elicited by 100μM GABA (65% average decrease; n = 3) in the retinal slice, and gave the most consistent reduction in current amplitude. Though there was no complete block of GABA-evoked responses in the slice, all cells tested were sensitive to the co-application of blockers. The effect of the combination of blockers on isolated cells was similar to that observed with picrotoxin alone, with 100% of cells tested sensitive to the co-application of antagonists and most showing a complete block of GABA-elicited responses. In this latter group the median hyperpolarizing response amplitude in control and recovery treatments was − 0.14 log units. With the antagonist combination, this was reduced to 0.0 log units, a significant effect (p < 0.001, Mann-Whitney U test).
GABA responses of isolated cells were sensitive to exogenous zinc application (Figure 7B). At low doses (20μM), 72% of GABA hyperpolarizations were significantly antagonized, with 3 being completely blocked, and 2 partially blocked. At higher doses (100μM), all regions displayed zinc sensitivity (n = 10 of 10, with 4 completely blocked, and 6 partially blocked). The fraction blocked or partially blocked at the two doses was not significantly different (p > 0.05; Chi Square test). Overall, 88% of GABA hyperpolarizations in isolated cells were blocked or partly blocked by zinc. The median, GABA-induced hyperpolarization in control and recovery treatments was − 0.21 log units, whereas the median hyperpolarization with zinc treatment was significantly reduced to − 0.04 (p < 0.001, Mann-Whitney U test). Zinc-sensitive GABA receptors are present on zebrafish bipolar cells.
Surprisingly, TPMPA, a competitive antagonist at GABAC receptors, failed to block GABA hyperpolarizations in most (79%) isolated bipolar cells. Two agonist/antagonist ratios were used: 10μM GABA with 20μM TPMPA, and 2μM GABA with 20μM TPMPA. In experiments using the former concentrations (1-to-2 ratio), 1 of 11 hyperpolarizations blocked. With the latter concentrations (1-to-10 ratio), 2 of 13 hyperpolarizations were blocked or partially blocked. The results obtained using the different agonist/antagonist ratios were not significantly different (p > 0.05; Chi Square test). Combining all data (n = 24) shows the median control and recovery response was − 0.205 log units. This diminished to − 0.188 log units during TPMPA application. This difference is not significant (p > 0.05, Mann-Whitney U test). An example of TPMPA failing to block a GABA hyperpolarization, even at a 10 to 1 antagonist/agonist ratio, appears in figure 7C.
Discussion
GABA responses on retinal bipolar cells have been identified in many species. Invariably bipolar cells express a combination of GABA receptors, with the GABAA and/or GABAC types predominating. Our results showing GABA responses with the pharmacological properties of A- and C-type receptors on the processes of zebrafish bipolar cells are consistent with these previous immunocytochemical (Greferath et al., 1993; Yazulla & Studholme, 2001; Zhang et al., 2003; Klooster et al., 2004) and physiological (i.e., Feigenspan et al., 1993; Qian & Dowling, 1993, 1995; Qian et al., 1997; Du & Yang, 2000; Qian et al., 2001) findings. GABA responses on isolated zebrafish bipolar cell dendrites were largely insensitive to bicuculline, but very sensitive to picrotoxin, suggesting these processes express GABAC-type receptors. On axon terminals, however, GABA-elicited currents were partially blocked by either bicuculline and/or picrotoxin, indicating both GABAA and GABAC receptors are expressed on synaptic boutons. The direct action of GABA on GABAB type receptors was not identified in either isolated cells or retinal slices.
GABA receptors on zebrafish bipolar cells have unique antagonist sensitivity
An interesting finding in our results is that antagonist pharmacology of the GABA receptors on zebrafish bipolar cells appears to be somewhat unique. In particular, our results suggest that there are multiple subtypes of GABAC receptors expressed on these cells. GABA currents recorded from axon terminal stimulation in the retinal slice were only partially blocked by picrotoxin, revealing an insensitive component. Even co-application of bicuculline and picrotoxin did not produce a complete block, suggesting a mixture of picrotoxin-sensitive and picrotoxin-insensitive GABAC receptors on axon terminals of zebrafish bipolar cells. Though picrotoxin was an effective antagonist of GABA responses in isolated cells, a GABA response component sometimes remained (see Figure 6C), indicating an insensitive component could also be present on the dendrites. Though picrotoxin-insensitive GABAC receptors have been identified in rat bipolar cells (Feigenspan et al., 1993; Feigenspan & Bormann, 1994), this finding is in contrast to reports from other teleost retinas (Tachibana & Kaneko, 1987; Qian & Dowling, 1993; Matthews et al., 1994; Qian & Dowling, 1994, 1995; Han et al., 1997).
In isolated cell voltage probe recordings, GABA responses sensitive to picrotoxin were also partially blocked by zinc, but not TPMPA. TPMPA completely blocks focally applied GABAC-mediated bipolar cell responses in salamander (Gao et al., 2000), rabbit (McGillem et al., 2000) mouse (Frech & Backus, 2004; Lukasiewicz et al., 2004) and goldfish (Hull et al., 2006). TPMPA was also an effective antagonist of GABAC-mediated light-evoked IPSCs in mouse rod bipolar cells (Eggers & Lukasiewicz, 2006). GABA responses of zebrafish bipolar cells appear to be TPMPA insensitive, even at high antagonist/agonist ratios. Zinc is also a reported antagonist of GABAC receptors (Qian & Dowling, 1995; Qian et al., 1997; Qian et al., 1998; Han & Yang, 1999; Kaneda et al., 2000), while its effects on GABAA receptors vary and appear only at high concentrations. GABAA receptors on mouse and carp bipolar cells are inhibited by zinc (Han & Yang, 1999; Kaneda et al., 2000) but only at higher doses or to a much lesser extent than GABAC receptors. GABAA responses on skate bipolar cells are enhanced (Qian et al., 1997), and GABAA receptors on hybrid bass bipolar cells are insensitive to zinc (Qian & Dowling, 1995). In the present study, low zinc concentrations (20μM) blocked most GABA-elicited changes in membrane voltage in isolated bipolar cells. These findings are consistent with the effects of a similar concentration (10μM) on hybrid bass bipolar cells (Qian & Dowling, 1995) and suggest the zinc-sensitive receptors are GABAC types. We found neither further blockade, nor enhancement at the high (100μM) zinc dose. As zinc has been shown to either antagonize or enhance GABAA responses at high doses (Qian et al., 1997; Kaneda et al., 2000); this suggests a lack of zinc sensitive GABAA receptors in zebrafish bipolar cells.
The effects of zinc and TPMPA were examined at ratios in which the antagonist was either 2x or 10x more concentrated than GABA. In other preparations, much lower TPMPA/GABA (McGillem et al., 2000; Zhou & Dacheux, 2005; Ivanova et al., 2006) and zinc/GABA (Qian & Dowling, 1995; Qian et al., 1996; Han & Yang, 1999; Kaneda et al., 2000) ratios successfully blocked GABAC responses. Similarly, in the slice, bicuculline and picrotoxin were applied at concentrations of 100 or 200μM, with 100μM GABA. This GABA concentration reflects the concentration within the puffer pipette and is most likely an overestimate of the concentration actually seen by the receptors due to diffusion after puffing, uptake, and the proximity of the puffer pipette to the receptors (i.e., (Lukasiewicz & Wong, 1997; Lukasiewicz & Shields, 1998). Comparable antagonist/GABA ratios were effective in blocking GABA responses in other preparations (Qian & Dowling, 1993, 1995; Blanco et al., 1996; Han et al., 1997; Lukasiewicz & Shields, 1998; McGillem et al., 2000; Pan, 2001; Zhou & Dacheux, 2005). This data suggests that the antagonist/GABA ratios used here should have been sufficient to block responses elicited through GABAC type receptors. Thus, the TPMPA- and picrotoxin-insensitivity observed in zebrafish bipolar cells is most likely due to the GABAC receptor subtypes expressed on these cells.
Overall, recordings made in retinal slices or using isolated cells indicate bicuculline-sensitive GABAA receptors are present. In addition, a diversity of GABAC receptor subtypes is expressed. Some GABAC receptors display “classic” pharmacology and are bicuculline-insensitive and picrotoxin-sensitive. These are found on both the dendrites and axon terminals. The dendritic GABAC receptors are also sensitive to zinc and insensitive to TPMPA. On axon terminals (and dendrites), some presumed GABAC receptors are picrotoxin-insensitive. Typically both picrotoxin-sensitive and insensitive subtypes are found on the same cell. This pharmacological pattern, while somewhat distinctive to zebrafish, displays characteristics similar to those found in other fish (Tachibana & Kaneko, 1987; Qian & Dowling, 1993; Matthews et al., 1994; Qian & Dowling, 1994, 1995; Han et al., 1997), though picrotoxin-insensitivity has been identified only in rat (Feigenspan et al., 1993; Feigenspan & Bormann, 1994). TPMPA-insensitive GABAC receptors have not been previously reported, though a small GABA-evoked current remained in rabbit OFF-bipolar cells following co-application of bicuculline and TPMPA (Zhou & Dacheux, 2005).
Variation in the distribution of GABAA and GABAC receptors
The distribution of GABAA and GABAC responses on zebrafish bipolar cells appears to vary in two ways: (1) between the dendritic and terminal processes of individual cells and (2) among the different types of bipolar cells. Overall, GABAC responses are more abundant than GABAA responses on all cells. GABAC responses are found on both dendrites and terminals; while GABAA responses are more prominent on the terminals. Similar to our findings, immunocytochemical data (Yazulla & Studholme, 2001) shows positive label for GABAA and GABAC receptor subunits in both plexiform layers, but with the GABACρ subunit labeling particularly intense in the OPL. GABAA subunits were also selectively distributed. Only OFF-bipolar cells and the large Bon-s6 bipolar cells expressed the α3 subunit of the GABAA receptor. Dendritic label of α3 OFF bipolar cells was particularly intense. This may be related to the difference in GABA physiology seen between OFF- and ON-type dendritic regions in the present study. The GABA α1 subunit was more broadly distributed.
Variability in GABA receptor expression may also occur among the different classes of bipolar cells, as found in rat (Euler & Wassle, 1998), bullfrog (Du & Yang, 2000), and skate (Qian et al., 2001). Physiological studies indicate bullfrog OFF-bipolars have a greater density of GABAA receptors at their dendrites and more GABAC receptors at their terminals. ON-bipolar cells have more GABAA receptors than GABAC receptors on both sets of processes (Du & Yang, 2000). Similarly, salamander OFF-bipolar cells express α1, β1, β2/3, and γ2 subunits of GABAA receptors (Zhang et al., 2003) and ON- and OFF-bipolar cells in rat are labeled with an antibody to the ρ1 subunit of GABAC receptor (Klooster et al., 2004). We have previously identified multiple subtypes of ON- and OFF-bipolar cells in zebrafish (Connaughton et al., 2004). Our voltage probe recordings from isolated cells suggest these ON- and OFF- types express different combinations of receptors. GABA-induced soma-dendritic hyperpolarizations were observed in ON-type cells that were also hyperpolarized by glutamate, but not in OFF-type cells that were depolarized by glutamate. In the slice, however, GABA responses were identified on dendritic and axonal processes of both ON- and OFF- cell types. While we do not know why there is a difference in the GABA response of OFF-bipolar cells recorded here, we speculate that the lack of a hyperpolarizing response of the isolated cells to externally applied GABA may reflect a difference in GABA receptor expression among OFF-bipolar subtypes.
Differential antagonist sensitivities may reflect differences in subunit composition of the different GABA receptors
Zinc, TPMPA, and picrotoxin sensitivities suggest bipolar cell GABA subunit compositions. Zinc sensitivity of GABAC receptors can be determined by the ρ subunits (Wang et al., 1995; Zhang et al., 2001; Qian & Pan, 2002), with the different homomeric receptors having differential sensitivities to zinc (Qian et al., 1998). White perch homomeric ρ2A receptors displayed the greatest zinc sensitivity of the four ρ subunits (Qian et al., 1998). Further, zinc sensitivity of the perch ρ1B subunit is enhanced if it is co-expressed with the γ2 subunit in Xenopus oocytes (Qian & Ripps, 1999; Qian & Pan, 2002). Given this information we hypothesize that GABAC receptors on zebrafish bipolar cells are most likely either homomers composed of one ρ subunit (such as ρ2A) or heteromers composed of different combinations subunits (such as ρ1B and γ2). If this were the case, zebrafish receptors assembled from ρ2A subunits or ρ1Bγ2 would have the greatest sensitivity to zinc. The other subunit combinations would also be sensitive to zinc, but to a lesser extent. GABAA receptors would be insensitive to zinc, due to the presence of the γ subunit. In agreement with this, both plexiform layers in the zebrafish retina were labeled with antibodies to ρ, as well as β2/3, receptor subunits (Yazulla & Studholme, 2001).
The hypothesis that ρ1B subunits form GABAC receptors on some zebrafish bipolar cells is further supported by the results of the TPMPA experiments. TPMPA-insensitivity observed here contradicts most reports within the literature. However, TPMPA-insensitivity has been observed in expression systems (Pan et al., 2005) and suggests possible GABA receptor subunit composition in zebrafish. The different ρ subunits (i.e., ρ1A, ρ1B, ρ2A, and ρ2B) display differential sensitivities to TPMPA: A-type receptors are highly sensitive to TPMPA; B-type receptors are less sensitive (Pan et al., 2005). As the GABA responses in most isolated bipolar cells were insensitive to TPMPA, these receptors are probably assembled from ρ1B and/or ρ2B subunits. These two subunits are also differentially sensitive to picrotoxin (Qian et al., 1998), with picrotoxin insensitivity in rat determined by the rρ2 subunit (Zhang et al., 1995). The varied expression of the ρ2 and ρ1 subunits among zebrafish bipolar cells could account for the range of picrotoxin sensitivities observed in the slice and in isolated cells. Focusing on the ρ2B subunits, one might speculate that picrotoxin sensitive or insensitive assemblies might be formed depending on whether the γ2 subunit happened to be incorporated (Qian & Ripps, 1999).
Glycine sensitivity
Glycine-evoked currents (in slices) or membrane hyperpolarizations (voltage probe recordings) were observed in zebrafish bipolar cells. Responses were obtained from both dendritic and axonal processes. These results are unique as glycine-evoked currents have not been previously reported in fish bipolar cells (Kaneko et al., 1991; Shen et al., 2005). However, bipolar cells in mammals (Cui et al., 2003; Frech & Backus, 2004; Zhou & Dacheux, 2005; Eggers & Lukasiewicz, 2006; Ivanova et al., 2006) and other lower vertebrates (Maple & Wu, 1998; Qian et al., 2001; Du & Yang, 2002a, 2002b) are glycine-sensitive.
Not all zebrafish bipolar cells were sensitive to glycine, with responses observed in ~30% of either isolated cells or cells within the retinal slice. This suggests that glycinergic innervation might be type specific, whereas GABAergic innervation might be universal. The fact that zebrafish bipolar cells receive direct glycinergic inputs agrees with the immunocytochemical localization of glycine-positive processes (Connaughton et al., 1999) and glycine receptor subunits (Yazulla & Studholme, 2001) in both plexiform layers of the zebrafish retina. Glycinergic inputs to bipolar cell dendrites most likely arise from the population of glycine-containing interplexiform cells present in zebrafish (Connaughton et al., 1999; Marc & Cameron, 2001); while presynaptic glycine-containing amacrine cells most likely provide inputs to bipolar terminals.
Glycine application evokes a current with a negative Erev, similar to GABA, suggesting a chloride current. Overall, currents evoked by glycine were similar in amplitude to GABA-evoked responses at the dendritic arbor (5–10 pA), as were changes in membrane fluorescence; however, at the axon terminal, glycine-evoked currents were smaller than GABA-evoked responses. This suggests that GABA receptor activation mediates most of the inhibition onto axon terminals, whereas, inhibitory inputs to the dendritic arbor occur equally through the activation of both GABA and glycine receptors. Immunocytochemistical localization of GABA-and glycine-containing processes in the zebrafish retina again supports these findings. Glycinergic amacrine cells are half as numerous as GABAergic amacrine cells (Marc & Cameron, 2001) GABA-containing processes are located throughout both plexiform layers. Glycine heavily labels the middle of the IPL, while more sparse label is seen throughout the IPL and the OPL (Connaughton et al., 1999).
Isolated cell vs. slice recordings
As two different methods were used to explore the localization of bipolar cell GABA sensitivity, it is worth discussing potential biases of these different approaches. For example, the protocols used in voltage-probe recordings probably favored GABAC-type responses. Compounds were applied over 4 minutes, a time period that might result in greater desensitization of GABAA receptors, allowing us, in effect, to examine the effects of zinc and TPMPA on relatively isolated GABAC responses. In contrast, focal puffs of GABA into retinal slices would allow both GABAA and GABAC responses to be examined. This difference may account for the insignificant change in membrane fluorescence following bicuculline application seen in isolated cells. Another issue is the availability of dendritic GABA receptors, which may differ between the two preparations. In the slice, dendritic receptors may be located deep within the slice, rather than on the surface. Some exogenously applied compounds may be quickly removed by transporter activity (i.e., Wu, 1986). Thus, within the tissue, sparse dendritic receptors may not be easily accessed by externally applied GABA. Isolating cells removes uptake/removal systems and exposes dendritic receptors. Further, the voltage-probe recording method may increase sensitivity. Semi-saturating GABA concentrations were more than a decade lower in zebrafish bipolar cells with probe recording (0.4μM) than reported in hybrid bass bipolar cells by whole cell currents (12.5μM; Qian & Ripps, 1999). Such increased sensitivities might arise if voltage saturation preceded current saturation. For example, approximating the membrane of an isolated bipolar cell as an 8μm sphere with a resistivity of 104Ω·cm2, it would have a conductance of 200pS, in which case the 16mV median hyperpolarization would require only 3pA, far less than the 30pA maximal amplitude commonly recorded from zebrafish bipolar cells in slice preparations. For both these reasons, the voltage probe studies of isolated cells may unmask the actions of a sparse population of dendritic GABA receptors.
Summary
Taken together, our results suggest a combination of GABAA and GABAC receptors are present on zebrafish bipolar cell processes, with the bulk of the response amplitudes, whether measured by whole-cell currents or voltage probe fluorescent changes, arising from GABAC-like receptors. Glycine receptors are also present on both sets of processes, but not on all cells. Smaller GABAA response components were also seen that were selectively sensitive to bicuculline. GABAC receptors displayed a somewhat unique pharmacology as they were insensitive to TPMPA, and were only partially blocked by picrotoxin. Sensitivity to zinc and insensitivity to bicuculline follows a more conventional pattern.
GABAergic inputs to zebrafish bipolar cell dendrites most likely come from horizontal cells in the outer retina. There are at least three horizontal cell types in zebrafish (Connaughton et al., 2004) and at least one of these types contains GABA (Connaughton et al., 1999; Marc & Cameron, 2001; Yazulla & Studholme, 2001). The submicromolar sensitivity of isolated bipolar cells with only dendritic tops suggests that even extrasynaptic GABA might be an effective regulator of bipolar cell function. Inputs to axon terminals (either GABAergic or glycinergic) in the inner retina likely come from inhibitory amacrine cells (i.e., Tachibana & Kaneko, 1987, 1998). GABA receptors expressed on zebrafish bipolar cell terminals are similar in activity and general pharmacology to those described in other species. As wide field GABAergic amacrines are the presynaptic sources of GABA in all species thus far studied (Marc et al., 1978; Pourcho & Goebel, 1983; Nelson & Kolb, 1985; Freed et al., 1987; Yazulla et al., 1987), it is likely that in zebrafish also GABAergic signal-processing roles in the inner retina are similar to those found in other vertebrates.
Acknowledgments
This work was supported, in part, by the Mellon Research Fund and an American University Senate Research Award (both to VPC).
References
- Blanco R, Vaquero CF, de la Villa P. The effects of GABA and glycine on horizontal cells of the rabbit retina. Vision Res. 1996;36:3987–3995. doi: 10.1016/s0042-6989(96)00145-9. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977;40:53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Connaughton VP. Zebrafish retinal slice preparation. Methods in Cell Sci. 2003;25:49–58. doi: 10.1023/B:MICS.0000006853.54435.85. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Behar TN, Liu W-LS, Massey S. Immunocytochemical localization of excitatory and inhibitory neurotransmitters in the zebrafish retina. Visual Neurosci. 1999;16:483–490. doi: 10.1017/s0952523899163090. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Bender AM, Nelson R. GABA-evoked responses of zebrafish retinal bipolar cells. Investigative Ophthalmology and Visual Science. 2000;41:S621. [Google Scholar]
- Connaughton VP, Dowling JE. Comparative morphology of distal neurons in developing and adult zebrafish retinas. Vision Res. 1998;38:13–18. doi: 10.1016/s0042-6989(97)00146-6. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Graham D, Nelson R. Identification and morphological classification of horizontal, bipolar, and amacrine cells within the zebrafish retina using the DiOlistic technique. J Comp Neurol. 2004;477:371–385. doi: 10.1002/cne.20261. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Maguire G. Differential expression of voltage-gated K+ and Ca+2 currents in bipolar cells in teh zebrafish retinal slice. Eur J Neurosci. 1998;10:1350–1362. doi: 10.1046/j.1460-9568.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Axonal stratification patterns and glutamate-gated conductance mechanisms in zebrafish retinal bipolar cells. J Physiol. 2000;524:135–146. doi: 10.1111/j.1469-7793.2000.t01-1-00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks J, Kolb H. Localization of GABA, glycine, glutamate, and tyrosine hydroxylase in the human retina. J Comp Neurol. 1992;315:287–302. doi: 10.1002/cne.903150305. [DOI] [PubMed] [Google Scholar]
- Cui J, Ma Y-P, Lipton SA, Pan Z-H. Glycine receptors and glycinergic synaptic input at the axon terminals of mammalian retinal rod bipolar cells. J Physiol. 2003;553:895–909. doi: 10.1113/jphysiol.2003.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C-J, Hare WA. GABAC feedback pathway modulates the amplitude and kinetics of ERG b-wave in a mammalian retina in vivo. Vision Res. 2002;42:1081–1087. doi: 10.1016/s0042-6989(02)00032-9. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Werblin FS. Organizatin of the retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969;32:315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Du J-L, Yang X-L. Subcellular localization and complements of GABAA and GABAC receptors on bullfrog retinal bipolar cells. J Neurophysiol. 2000;84:666–676. doi: 10.1152/jn.2000.84.2.666. [DOI] [PubMed] [Google Scholar]
- Du J-L, Yang X-L. Bullfrog retinal bipolar cells may express heterogeneous glycine receptors at dendrites and axon terminals. Neurosci Lett. 2002a;322:177–181. doi: 10.1016/s0304-3940(01)02523-x. [DOI] [PubMed] [Google Scholar]
- Du J-L, Yang X-L. Glycinergic synaptic transmission to bullfrog retinal bipolar cells is input-specific. Neurosci. 2002b;113:779–784. doi: 10.1016/s0306-4522(02)00255-5. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz P. GABAA, GABAC, and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol. 2006;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wassle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Wassle H, Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993;361:159–161. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Boycott BB. Synaptic connexions made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proc R Soc Lond B. 1974;186:317–331. doi: 10.1098/rspb.1974.0052. [DOI] [PubMed] [Google Scholar]
- Frech MJ, Backus KH. Characterization of inhibitory postsynaptic currents in rod bipolar cells of the mouse retina. Visual Neurosci. 2004;21:645–652. doi: 10.1017/S0952523804214134. [DOI] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Rod bipolar arrayin the cat retina: pattern of input from rods and GABA-accumulating amacrine cells. J Comp Neurol. 1987;266:445–455. doi: 10.1002/cne.902660310. [DOI] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Timing of quantal release from the retinal bipolar terminal is regulated by a feedback circuit. Neuron. 2003;38:89–101. doi: 10.1016/s0896-6273(03)00166-1. [DOI] [PubMed] [Google Scholar]
- Gao F, Maple BR, Wu SM. I4AA-sensitive chloride current contributes to the center light responses of bipolar cells in the tiger salamander retina. J Neurophysiol. 2000;83:3473–3482. doi: 10.1152/jn.2000.83.6.3473. [DOI] [PubMed] [Google Scholar]
- Greferath U, Muller F, Wassle H, Shivers B, Seeburg P. Localization of GABAA receptors in the rat retina. Visual Neurosci. 1993;10:551–561. doi: 10.1017/s0952523800004764. [DOI] [PubMed] [Google Scholar]
- Han M-H, Li Y, Yang X-L. Desensitizing GABAC receptors on carp retinal bipolar cells. Neuroreport. 1997;8:1331–1335. doi: 10.1097/00001756-199704140-00003. [DOI] [PubMed] [Google Scholar]
- Han M-H, Yang X-L. Zn+2 differentially modulates kinetics of GABAC vs. GABAA receptors in carp retinal bipolar cells. Neuroreport. 1999;10:2593–2597. doi: 10.1097/00001756-199908200-00028. [DOI] [PubMed] [Google Scholar]
- Hanitzsch R, Kuppers L, Flade A. The effect of GABA and the GABA-uptake-blocker NO-711 on the b-wave of the ERG and the responses of horizontal cells to light. Graefe’s Arch Clin Exp Ophthalmol. 2004;242:784–791. doi: 10.1007/s00417-004-0919-6. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Inhibition of calcium influx and calcium current by gamma-aminobutyric acid in single synaptic terminals. Proc Natl Acad Sci. 1991;88:7135–7139. doi: 10.1073/pnas.88.16.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Li G-L, von Gersdorff H. GABA transporters regulate a standing GABAC receptor-mediated current at a retinal presynaptic terminal. J Neurosci. 2006;26:6979–6984. doi: 10.1523/JNEUROSCI.1386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, von Gersdorff H. Fast endocytosis is inhibited by GABA-mediated chloride influx at a presynaptic terminal. Neuron. 2004;44:469–482. doi: 10.1016/j.neuron.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller E, Wassle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Andrasfalvy B, Kaneko A. Modulation of Zn+2 of GABA responses in bipolar cells of the mouse retina. Visual Neurosci. 2000;17:273–281. doi: 10.1017/s0952523800172098. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Suzuki S, Pinto H, Tachibana M. Membrane currents and pharmacology of retinal bipolar cells: a comparative study on goldfish and mouse. Comp Biochem Physiol C. 1991;98:115–127. doi: 10.1016/0742-8413(91)90188-y. [DOI] [PubMed] [Google Scholar]
- Kapousta-Bruneau NV. Opposite effects of GABAA and GABAC receptor antagonists on the b-wave of the ERG recorded from the isolated rat retina. Vision Res. 2000;40:1653–1665. doi: 10.1016/s0042-6989(00)00028-6. [DOI] [PubMed] [Google Scholar]
- Karschin A, Wassle H. Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J Neurophysiol. 1990;63:860–876. doi: 10.1152/jn.1990.63.4.860. [DOI] [PubMed] [Google Scholar]
- Kenyon JL. Primer on Junctional Potentials for the Patchologist. 3. University of Nevada, School of Medicine; 2002. [Google Scholar]
- Klooster J, Cardozo BN, Yazulla S, Kamermans M. Postsynaptic localization of gamma-aminobutyric acid transporters and receptors in the outer plexiform layer of the goldfish retina: an ultrastructural study. J Comp Neurol. 2004;474:58–74. doi: 10.1002/cne.20114. [DOI] [PubMed] [Google Scholar]
- Kolb H, Jones J. Synaptic organization of the outer plexiform layer of the turtle retina: an electron microscope study of serial sections. J Neurocytol. 1984;13:567–591. doi: 10.1007/BF01148080. [DOI] [PubMed] [Google Scholar]
- Kolb H, West RW. Synaptic connections of the interplexiform cell in the retina of the cat. J Neurocytol. 1977;6:155–170. doi: 10.1007/BF01261503. [DOI] [PubMed] [Google Scholar]
- Kondo H, Toyoda J-I. GABA and glycine effects on the bipolar cells of the carp retina. Vision Res. 1983;23:1259–1264. doi: 10.1016/0042-6989(83)90101-3. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina f the larval tiger salamander. Philos Trans Royal Soc Lond B. 1973;265:471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Linberg KA, Fisher SK. Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. J Comp Neurol. 1988;268:281–297. doi: 10.1002/cne.902680211. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P, Maple BR, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz P, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol. 1998;79:3157–3167. doi: 10.1152/jn.1998.79.6.3157. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P, Wong ROL. GABAC receptors on ferret retinal bipolar cells: a diversity of subtypes in mammals? Visual Neurosci. 1997;14:989–994. doi: 10.1017/s095252380001169x. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Eggers ED, Sagdullaev BT, McCall MA. GABAC receptor-mediated inhibition in the retina. Vision Res. 2004;44:3289–3296. doi: 10.1016/j.visres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Mack AF, Behrens UD, Wagner H-J. Inhibitory control of synaptic activity in goldfish Mb bipolar cell terminals visualized by FM1–43. Visual Neurosci. 2000;17:823–829. doi: 10.1017/s0952523800176011. [DOI] [PubMed] [Google Scholar]
- Maguire G, Maple B, Lukasiewicz P, Werblin FS. Gamma-aminobutyric type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc Natl Acad Sci. 1989;86:10144–10147. doi: 10.1073/pnas.86.24.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple BR, Wu SM. Glycinergic synaptic inputs to bipolar cells in the tiger salamander retina. J Physiol. 1998;506:731–744. doi: 10.1111/j.1469-7793.1998.731bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Cameron D. A molecular phenotype atlas of the zebrafish retina. J Neurocytol. 2001;30:593–654. doi: 10.1023/a:1016516818393. [DOI] [PubMed] [Google Scholar]
- Marc RE, Stell WK, Bok D, Lam DM. GABAergic pathways in the goldfish retina. J Comp Neurol. 1978;182:221–224. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Matthews G, Ayoub GS, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci. 1994;14:1079–1090. doi: 10.1523/JNEUROSCI.14-03-01079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillem GS, Rotolo TC, Dacheux RF. GABA responses of rod bipolar cells in rabbit retinal slices. Visual Neurosci. 2000;17:381–389. doi: 10.1017/s0952523800173067. [DOI] [PubMed] [Google Scholar]
- Nelson R, Bender AM, Connaughton VP. Stimulation of sodium pump restores membrane potential to neurons excited by glutamate in zebrafish distal retina. J Physiol. 2003;549:787–800. doi: 10.1113/jphysiol.2003.042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Bender AM, Connaughton VP. Transporter-like GABA excitation of horizontal and bipolar cells in zebrafish distal retina. Investigative Ophthalmology and Visual Science. 2006 E-abstract 390. [Google Scholar]
- Nelson R, Kolb H. A17: a broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol. 1985;54:592–614. doi: 10.1152/jn.1985.54.3.592. [DOI] [PubMed] [Google Scholar]
- Nelson R, Schaffner AE, Li Y-X, Walton MK. Distribution of GABAC-like responses among acutely dissociated rat retinal neurons. Visual Neurosci. 1999;16:179–190. doi: 10.1017/s0952523899161133. [DOI] [PubMed] [Google Scholar]
- Pan Y, Khalili P, Ripps H, Qian H. Pharmacology of GABAC receptors: responses to agonists and antagonists distinguish A- and B-subtypes of homomeric rho receptors expressed in Xenopus oocytes. Neurosci Lett. 2005;376:60–65. doi: 10.1016/j.neulet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Pan Z-H. Voltage-activated Ca+2 channels and ionotropic GABA receptors localized at axon terminals of mammalian retinal bipolar cells. Visual Neurosci. 2001;18:279–288. doi: 10.1017/s095252380118212x. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983;219:25–35. doi: 10.1002/cne.902190104. [DOI] [PubMed] [Google Scholar]
- Qian H, Dowling JE. GABA responses on retinal bipolar cells. Biol Bull. 1993;185:312. doi: 10.1086/BBLv185n2p312a. [DOI] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994;14:4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Dowling JE. GABAA and GABAC receptors on hybrid bass retinal bipolar cells. J Neurophysiol. 1995;74:1920–1928. doi: 10.1152/jn.1995.74.5.1920. [DOI] [PubMed] [Google Scholar]
- Qian H, Dowling JE, Ripps H. Molecular and pharmacological properties of GABA-rho subunits from white perch retina. J Neurobiol. 1998;37:305–320. doi: 10.1002/(sici)1097-4695(19981105)37:2<305::aid-neu9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Qian H, Li L, Chappell RL, Ripps H. GABA receptors of bipolar cells from the skate retina: actions of zinc on GABA-mediated membrane currents. J Neurophysiol. 1997;78:2402–2412. doi: 10.1152/jn.1997.78.5.2402. [DOI] [PubMed] [Google Scholar]
- Qian H, Malchow RP, Chappell RL, Ripps H. Zinc enhances ionic currents induced in skate Muller (glial) cells by the inhibitory neurotransmitter GABA. Proc R Soc Lond B. 1996;263:791–796. doi: 10.1098/rspb.1996.0118. [DOI] [PubMed] [Google Scholar]
- Qian H, Pan Y. Co-assembly of GABA rho subunits with GABAA receptor gamma-2 subunit cloned from white perch retina. Mol Brain Res. 2002;103:62–70. doi: 10.1016/s0169-328x(02)00140-7. [DOI] [PubMed] [Google Scholar]
- Qian H, Ripps H. Response kinetics and pharmacological properties of hetermoeric receptors formed by coassembly of GABA rho- and gamma2- subunits. Proc R Soc Lond B. 1999;266:2419–2425. doi: 10.1098/rspb.1999.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Ripps H, Schuette E, Chappell RL. Responses of small- and large-field bipolar cells to GABA and glycine. Brain Res. 2001;893:273–277. doi: 10.1016/s0006-8993(00)03282-0. [DOI] [PubMed] [Google Scholar]
- Shen Y, Chen L, Ping Y, Yang X-L. Glycine modulates the center response of ON type rod-dominant bipolar cells in carp retina. Brain Res Bull. 2005;67:492–497. doi: 10.1016/j.brainresbull.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca+2 entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. Gamma-aminobutyric acid exerts a local inhibition action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc Natl Acad Sci. 1987;84:3501–3505. doi: 10.1073/pnas.84.10.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. Retinal bipolar cells receive negative feedback input from GABAergic amacrine cells. Visual Neurosci. 1998;1:297–305. doi: 10.1017/s0952523800001954. [DOI] [PubMed] [Google Scholar]
- Vaquero CF, de la Villa P. Localisation of the GABAC receptors at the axon terminal of rod bipolar cells of the mouse retina. Neurosci Res. 1999;35:1–7. doi: 10.1016/s0168-0102(99)00050-4. [DOI] [PubMed] [Google Scholar]
- Wang T-L, Hackam A, Guggino WB, Cutting GR. A single histidine residue is essential for zinc inhibition of GABA rho1 receptors. J Neurosci. 1995;15:7684–7691. doi: 10.1523/JNEUROSCI.15-11-07684.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward RM, Polenzani L, Miledi R. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acid-A and gamma-aminobutyric acid-B receptor agonists and antagonists. Mol Pharmacol. 1993;43:609–625. [PubMed] [Google Scholar]
- Wu D, Zhu PH. Inhibition of calcium signaling in terminal and soma of carp retinal bipolar cells by GABA. Acta Pharmacol Sin. 2000;21:709–714. [PubMed] [Google Scholar]
- Wu SM. Effects of gamma-aminobutyric acid on cones and bipolar cells of the tiger salamander retina. Brain Res. 1986;365:70–77. doi: 10.1016/0006-8993(86)90723-7. [DOI] [PubMed] [Google Scholar]
- Wu SM, Maple B. Amino acid neurotransmitters in the retina: a functional overview. Vision Res. 1998;38:1371–1384. doi: 10.1016/s0042-6989(97)00296-4. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM. Neurochemical anatomy of the zebrafish retina as determined by immunocytochemistry. J Neurocytol. 2001;30:551–592. doi: 10.1023/a:1016512617484. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, Wu JY. GABAergic input to the synaptic terminals of Mb1 bipolar cells in the goldfish retina. Brain Res. 1987;411:400–405. doi: 10.1016/0006-8993(87)91095-x. [DOI] [PubMed] [Google Scholar]
- Zhang D-O, Yang X-L. OFF pathway is preferentially suppressed by the activation of GABAA receptors in carp retina. Brain Res. 1997;759:160–162. doi: 10.1016/s0006-8993(97)00340-5. [DOI] [PubMed] [Google Scholar]
- Zhang D, Pan Z-H, Awobuluyi M, Lipton SA. Structure and function of GABAC receptors: a comparison of native versus recombinant receptors. Trends in Pharmacol Sci. 2001;22:121–132. doi: 10.1016/s0165-6147(00)01625-4. [DOI] [PubMed] [Google Scholar]
- Zhang D, Pan Z-H, Zhang X, Brideau AD, Lipton SA. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with methionine residue critical for picrotoxinin channel block. Proc Natl Acad Sci. 1995;92:11756–11760. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, De Blas AL, Miralles CP, Yang C-Y. Localization of GABAA receptor subunits alpha1, alpha3, beta1, beta2/3, gamma1, and gamma2 in the salamander retina. J Comp Neurol. 2003;459:440–453. doi: 10.1002/cne.10626. [DOI] [PubMed] [Google Scholar]
- Zhang J, Slaughter MM. Preferential suppression of the ON pathway by GABAC receptors in the amphibian retina. J Neurophysiol. 1995;74:1583–1592. doi: 10.1152/jn.1995.74.4.1583. [DOI] [PubMed] [Google Scholar]
- Zhou C, Dacheux RF. Glycine- and GABA-activated inhibitory currents on axon terminals of rabbit cone bipolar cells. Visual Neurosci. 2005;22:759–767. doi: 10.1017/S095252380522607X. [DOI] [PubMed] [Google Scholar]