Abstract
Introduction
EMS treatment of status epilepticus improves outcomes, but the benzodiazepine best suited for EMS use is unclear, given potential high environmental temperature exposures.
Objective
To describe the degradation of diazepam, lorazepam, and midazolam as a function of temperature exposure and time over 120 days of storage on active EMS units.
Methods
Study boxes containing vials of diazepam, lorazepam, and midazolam were distributed to 4 active EMS units in each of 2 EMS systems in the southwestern United States during May–August 2011. The boxes logged temperature every minute and were stored in EMS units per local agency policy. Two vials of each drug were removed from each box at 30-day intervals and underwent high-performance liquid chromatography to determine drug concentration. Concentration was analyzed as mean (and 95%CI) percent of initial labeled concentration as a function of time and mean kinetic temperature (MKT).
Results
192 samples were collected (2 samples of each drug from each of 4 units per city at 4 time-points). After 120 days, the mean relative concentration (95%CI) of diazepam was 97.0% (95.7–98.2%) and of midazolam was 99.0% (97.7–100.2%). Lorazepam experienced modest degradation by 60 days (95.6% [91.6–99.5%]) and substantial degradation at 90 days (90.3% [85.2-95.4%]) and 120 days (86.5% [80.7–92.3%]). Mean MKT was 31.6°C (95%CI 27.1–36.1). Increasing MKT was associated with greater degradation of lorazepam, but not midazolam or diazepam.
Conclusions
Midazolam and diazepam experienced minimal degradation throughout 120 days of EMS deployment in high-heat environments. Lorazepam experienced significant degradation over 120 days and appeared especially sensitive to higher MKT exposure.
Keywords: emergency medical services, benzodiazepines, temperature
Introduction
Emergency medical services (EMS) treatment of status epilepticus with benzodiazepines improves outcomes.1,2 Recent evidence suggests that, among the commonly used benzodiazepines, midazolam may be the most effective in achieving seizure cessation prior to hospital arrival.2,3 EMS medications are frequently stored without temperature-control procedures, which may negatively impact the medication through degradation, and heat stability is an important factor in determining which benzodiazepine to deploy in an EMS system.4–7 Diazepam and lorazepam experience some heat-dependent degradation while midazolam is heat-stable for at least 60 days.8,9 The effect of longer storage, especially in extreme heat conditions, is unknown.
We sought to expand on our previous work by describing the degradation of diazepam, lorazepam, and midazolam as a function of temperature exposure and time over a longer, 120-day storage period on active EMS units during the summer months in the southwestern United States.
Methods
Study Design and Setting
This experimental pharmaco-stability study of medications stored in active EMS units was designed as an independent extension of our previous work that demonstrated heat-dependant degradation of lorazepam over 60 days.9 The present study was conducted during the summer of 2011 (May through August) and focused on two EMS agencies in the southwestern United States with historically high ambient temperatures. We extended the period of observation to 120 days and added diazepam as a comparator to give insight on the behavior of all benzodiazepines currently available for prehospital use.
Vials of diazepam, lorazepam, and midazolam were distributed to 4 active EMS units in each of the two EMS systems. Instrumented boxes logged temperature every minute and were stored in EMS units per local agency policy alongside other routine medications. Use of temperature-control systems beyond normal vehicle air conditioning or garaging practices were not specified in the study protocol. Mirroring routine EMS practices, some vehicles were kept in station garages unless responding to an emergency call, while others were constantly exposed to ambient temperatures during work shifts. Two samples of each benzodiazepine were removed from each box after 30, 60, 90, and 120 days of deployment.
The methods of measurement, data collection, and data processing were identical to our previous study.9 Briefly, the instrumented study boxes measured and recorded temperature every minute. Temperatures were analyzed and summarized by determination of the mean kinetic temperature (MKT), which is commonly used in the pharmaceutical industry to describe the overall effects of temperature changes on heat-sensitive materials.10 MKT expresses the cumulative heat stress to which a medication has been exposed over time and is not a simple average of ambient temperatures.
Samples were analyzed in a commercial laboratory (DynaLabs, St. Louis, MO) by high-performance liquid chromatography (HPLC) to determine the concentration of the active drug. Samples were refrigerated after removal from the field, including during shipping, to minimize further heat-related degradation.
Data were managed within Microsoft Excel (Microsoft, Redmond, WA) and analyzed using SPSS version 19 (International Business Machines, Armonk, NY).
Outcome Measures
The primary outcome was the relative reduction in medication concentration from labeled concentration after 30–120 days of exposure.
Primary Data Analysis
Concentration was analyzed as a function of time and MKT. For each benzodiazepine, the mean relative concentration at 60, 90, and 120 days was compared to the 30-day measurement using the Student t-test. The influence of MKT and time on each medication's degradation was determined with linear regression and oneway analysis of covariance (ANCOVA), respectively.
Sample Size Determination
Sample size was estimated to provide a significance of 0.05 and a power of 0.8, assuming a mean difference of 7.5% between the 120-day relative concentrations of lorazepam compared to midazolam or diazepam. The assumed within-group sample variability (standard deviation) was 5%.
Results
A total of 192 samples were collected (2 samples in each of 4 units per city at 4 timepoints for each drug) and underwent HPLC. The cumulative mean MKT over the 120-day period was 31.6°C (95%CI 27.1–36.1°C) (Table 1).
Table 1.
Average mean kinetic temperature (MKT) and relative concentration of benzodiazepines compared to label at each measured timepoint.
| Concentration, mean % (95% CI) |
||||
|---|---|---|---|---|
| 30-day | 60-day | 90-day | 120-day | |
| Diazepam | 97.0 (96.3-97.6) | 97.1 (96.6-97.6) | 97.4 (96.6-98.3) | 97.0 (95.7-98.2) |
| Lorazepam | 101.0 (99.0-102.9) | 95.6* (91.6-99.5) | 90.3** (85.2-95.4) | 86.5** (80.7-92.3) |
| Midazolam | 101.0 (99.8-101.4) | 100.6 (99.8-101.4) | 99.0 (98.0-99.9) | 99.0 (98.1-100.2) |
| MKT | 28.1 (24.9-31.2) | 30.4 (26.0-34.7) | 31.0 (26.8-35.1) | 31.6 (27.1-36.1) |
p = 0.014
p < 0.001 when compared to 30-day concentration.
Benzodiazepine concentration over time is shown in Table 1 and the impact of time on degradation was significantly different among the benzodiazepines (ANCOVA p < 0.01). Diazepam and midazolam experienced minimal degradation at each time point. At 120 days, the mean relative concentration (95%CI) of diazepam was 97.0% (95.7–98.2%) and of midazolam was 99.0% (97.7–100.2%). Lorazepam experienced significant degradation by 60 days (95.6% [91.6–99.5]) with the concentration in half of all samples being less than 95% of labeled concentration. Relative concentration of lorazepam was 90.3% (85.2–95.4) at 90 days and 86.5% (80.7–92.3) at 120 days.
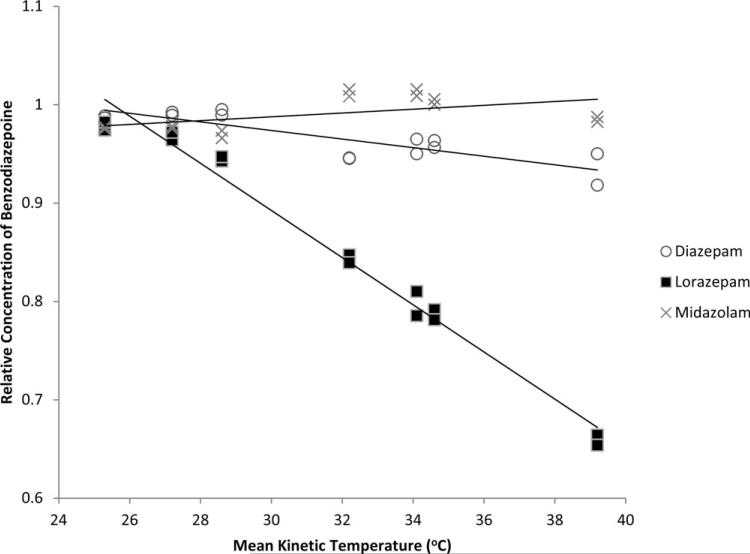
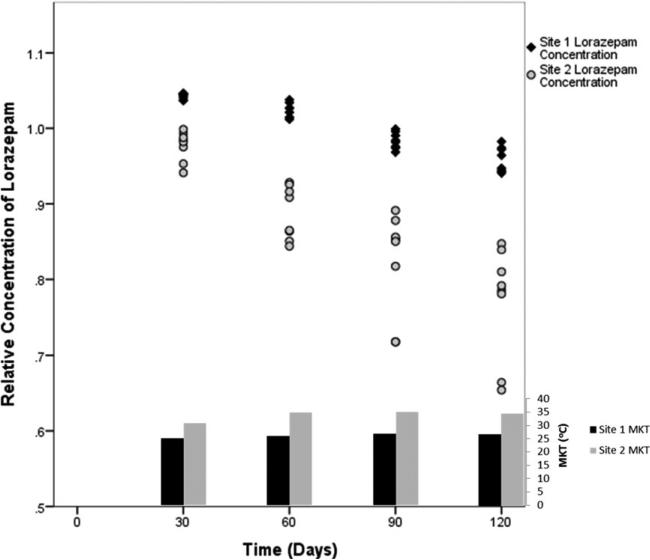
Midazolam and diazepam were stable across the range of mean kinetic temperatures, whereas increasing MKT was associated with greater degradation of lorazepam after 120 days (Figure 1; lorazepam R2 = 0.98). The mean (95%CI) daily ambient temperature for each site was not significantly different (30.6°C [23.3–37.2°C] vs. 33.3°C [26.7–40.0°C]; p = 0.83). However, there was a greater than expected observed difference of MKT between sites over the 120 days (27.0°C [22.9–31.1°C] vs. 35.0°C [30.3–39.8°C]; p = 0.009). There was no significant intersite difference in the relative concentrations of midazolam or diazepam at any timepoint. Lorazepam experienced statistically significant degradation at 60, 90, and 120 days (p = 0.009) at each site, and the magnitude of the temperature effect was different between the two sites (ANCOVA p = 0.001) (Figure 2).
Figure 1.
Relative concentrations of benzodiazepines at 120 days as a function of cumulative mean kinetic temperatures (MKT).
Figure 2.
Comparison by site of relative concentration of lorazepam and cumulative mean kinetic temperature (MKT) at 30, 60, 90, and 120 days.
DIscussion
In this study, we found that midazolam and diazepam experienced minimal degradation during 120 days of EMS deployment in high-heat environments. Lorazepam maintained acceptable concentrations of active drug for at least 30 days. However, when exposed to high heat stress, many samples experienced significant and progressive degradation by 60 days.
We have previously evaluated rates of degradation for lorazepam and midazolam over 60 days of EMS field deployment at multiple sites during the conduct of a multicenter clinical trial.9 In that study we found that midazolam remained stable at 60 days, but that lorazepam showed slight time- and temperature-dependent degradation. The current study builds upon this work by extending the period of observation to 120 days, by focusing on EMS systems with very high heat stress, and by including diazepam, the most common benzodiazepine in EMS use. The current study confirms the stability of midazolam for at least 120 days and that lorazepam is time and heat sensitive.
Gottwald et al. previously reported some experience with the degradation of diazepam and lorazepam deployed on two ambulances in San Francisco.8 Interestingly, despite higher ambient temperatures, we found that diazepam was more resilient than first reported, with no diazepam samples determined to have <90% of labeled concentration. Lorazepam's degradation was greater at 60 days and beyond, as well, reaffirming the relationship between heat stress and medication decomposition.
Ambient temperatures were similar between the two sites. However, there was an unexpected difference in the MKTs encountered at each site (27.0 vs. 35.0°C). Although unplanned, this difference allows additional insight into lorazepam's instability in the prehospital setting. Post hoc evaluation of this difference found that the agency with the lower MKT frequently keeps EMS units running with the vehicle's air conditioning system engaged, while the other agency routinely turns off the EMS units and parks them in station garages between calls. It is probable that a combination of operational and environmental factors contributed to the higher degradation rate at one site. However, it appears that altering the deployment and storage procedures for vehicles does not prevent lorazepam degradation in hot environments and further temperature-control methods, such as on-board refrigeration units, may be needed to extend useful shelf life.
It is notable that ambient temperature cannot be used as a surrogate for MKT when assessing whether, or for how long, lorazepam can be stored in an EMS unit. MKT is a dynamic variable that accounts for the potential stress caused by changing temperatures, which is one reason that the pharmacology literature supports the use of MKT, rather than simple temperature means, for evaluating heat stability of drugs.10 These data support the notion that EMS agencies should take multiple variables into consideration, including temperature exposure and length of field deployment, when determining medication storage and restocking policies.7
Based on our findings here and previously, EMS systems choosing to deploy lorazepam should employ lorazepam storage methods to limit high heat exposures and maintain controlled room temperature (MKT < 25°C) environments. Otherwise, it may be prudent to limit field deployment time of lorazepam to 30 days to minimize degradation risks. In some systems, preferential use of midazolam or diazepam may be warranted.
Limitations And Future Research
This study has limitations. First, baseline (day 0) samples were not obtained because our previous work demonstrated consistent baseline concentrations for midazolam and lorazepam.9 Furthermore, the differences in the pair of samples taken from each EMS unit for testing were insignificant. This is consistent with the quality controls and USP specifications expected in these commercial pharmaceuticals.
Second, we did not perform duplicate measures from each sample; instead, we performed redundant single measurements from independent but identically stored samples. Previous work has demonstrated the reliability of the HPLC testing,8 and our duplicate sample testing reduces the bias that an outlier may cause.
Finally, this study was not designed to evaluate the impact of different vehicle deployment or medication storage practices on drug degradation. The impact of medication refrigerators and other techniques to control heat exposure and MKT may be more important for lorazepam than the other benzodiazepines and is a topic suitable for further study.
Evaluating the impact of heat exposure on other medications commonly used by EMS should be a priority.
Conclusion
Midazolam and diazepam experienced minimal degradation throughout 120 days of EMS deployment in high-heat environments. In contrast, lorazepam degraded significantly over this time and appeared especially sensitive to higher mean kinetic temperatures.
Acknowledgments
This work was conducted as part of the RAMPART trial, which was supported by awards from the National Institute of Neurological Disorders and Stroke (NINDS) (U01NS056975 and U01NS059041); the National Institutes of Health Office of the Director CounterACT Program; and the Biomedical Advanced Research and Development Authority of the Assistant Secretary for Preparedness and Response.
The Neurological Emergencies Treatment Trials investigators
Clinical Coordinating Center
Robert Silbergleit, MD, Daniel Lowenstein, MD, William Barsan, MD, Arthur Pancioli, MD, Valerie Stevenson, BAS, RRT, CCRP, Erin Zaleski, MA, Deneil
Harney, MPH, MSW, Donna Harsh, MS, Joy Pinkerton, BSN, RN, MS, Allison Kade, BA, Nicholas Siewert, BA, Ashley Pinawin, BS, Catherin Ring, Phebe Brenne
National EMS Coordinator
Kay Vonderschmidt, MPA, MS-EM, NREMT-P
Statistical Data Management Center
Valerie Durkalski, PhD, Yuko Palesch, PhD, Catherine Dillon, Keith Pauls, Qi Wu, Wenle Zhao, PhD
National Institutes of Health
Robin Conwit, MD, Scott Janis, PhD, David Jett, PhD, Brandy Fureman, PhD
Hubs (ordered by number of subjects enrolled)
Wayne State University (178)
Hub Principal Investigator: Robert D. Welch, MD, MS Primary Study Coordinators: Lynnmarie Mango, MPH, Valerie H. Mika, MS EMS Director(s)/Coordinator: Jenny Atas, MD Other Site Investigators: Robert Dunne, MD, Douglas Wheaton, MD, Phillip Levy, MD, MPH, Marc-Anthony Velilla, MD, Robert Sherwin, MD, Brian O'Neil, MD, Angela Groves, MD, Marc Rosenthal, DO, PhD Participating EMS Service: Detroit EMS
University of Cincinnati (133)
Hub Principal Investigator: Arthur Pancioli, MD Primary Study Coordinators: Irene Ewing, RN, BSN, Peggy Waymeyer, RN EMS Director(s)/Coordinator: M. Kay Vonderschmidt, MPA, MS-EM, NREMT-P, Jason McMullan, MD Other Site Investigators: Hamilton Schwartz, MD, Brian Stettler, MD, William Knight, MD, Opeolu Adeoye, MD, Rhonda Cadena, MD, Jordan Bonomo, MD, Erin Grise, MD, Laura Heitsch, MD, George Shaw, MD, Nick Gagai, CCRP, Pamela Schmit, RN BSN, Sara Stark, Med, Traci Doellman, RN Participating EMS Services: Cincinnati Fire Department, BlueAsh Fire Department, Forest Park Fire Department, Green Township Fire Department, Florence Fire Department, Independence Fire Department
University of California San Francisco (121)
Hub Principal Investigator: J. Claude Hemphill, III, MD, MAS Primary Study Coordinators: Michele Meeker, RN, BSN, Kelley Rosborough, BA EMS Director(s)/Coordinator: Jeany Duncan EMT-P Other Site Investigators: Karl Sporer, MD, FACEP, FACP, Alan Gelb, MD; Wade Smith, MD, PhD, Prasanthi Ramanujam, MD, Kazuma Nakagawa, MD, Asma Moheet, MD, Hooman Kamel, MD, Bharath Naravetla, MD, Mary Mercer, MD, Christine Wong, MD Participating EMS Services: San Francisco Fire Department, EMS Division
University of Texas – Houston (81)
Hub Principal Investigator: Elizabeth Jones, MD Trial Principal Investigator: Truman J. Milling, MD Primary Study Coordinators: Misty Ottman, RN, BSN, Ben King, Laura LaChance EMS Directors/Coordinators: Jeff Brockman, RN, Pete Didonato, EMT-P Other Site Investigator: Paul Hinchey, MD Participating EMS Service: Austin-Travis County EMS
Emory University (75)
Hub Principal Investigator: David W. Wright, MD Trial Principal Investigators: Matthew D. Bitner, MD, Gerald W. Beltran, DO Primary Study Coordinator: Harriet Nevarez, RN, CCRC EMS Director/Coordinator: Rachel Barnhard, Andrea G. McDougal Other Site Investigators: Jeffrey F. Linzer Sr, MD, Lisa H. Merck, MD MPH, Tamara Espinoza, MD Participating EMS Service: Grady EMS
Henry Ford Health System (64)
Hub Principal Investigator: Christopher A. Lewandowski, MD Trial Principal Investigator: Taher T. Vohra, MD Primary Study Coordinators: Paula L. Crouse, RN, BSN., MA., Anna E. Baker, RN, BSN EMS Director/Coordinator: Dean R. Creech EMT-P, I/C Other Site Investigator: Andrew N. Russman, DO, Joseph B. Miller, MD, Jumana Nagarwala, MD, Daniel J. Miller, MD, Raymond Fowkes, MD, Anne Marie Lundell, RN, BSN Participating EMS Services: Detroit EMS, West Bloom-field Fire and EMS Services
Stanford University (62)
Hub Principal Investigator: James V. Quinn, MD, MS Primary Study Coordinators: Stephanie Casal. RN, CNS, Anke Hebig, Mark Liao EMS Director/Coordinator: Peter D'souza, MD Participating EMS Services: Palo Alto Fire Department, San Jose Fire Department, Redwood City Fire Department, San Mateo Fire Department
University of Arizona (58)
Hub Principal Investigator: Kurt R. Denninghoff, MD Trial Principal Investigator: Daniel W. Spaite, MD Primary Study Coordinator: Bruce Barnhart, RN, CEP EMS Director(s)/Coordinator: Willie Haro, CEP Other Site Investigator: Bentley J. Bobrow, MD Participating EMS Service: Glendale Fire Department
Virginia Commonwealth University (55)
Hub Principal Investigator: Joseph P. Ornato, MD Primary Study Coordinator: Sallie L. Noe, RN
EMS Director/Coordinator: Alan D. Payne, CCEMTP Other Site Investigators: Alan R. Towne, MD, Michael C. Kurz, MD, John T. Carmack, MD Participating EMS Service: Richmond Ambulance Authority
University of Minnesota (47)
Hub Principal Investigator: Michelle Biros, MD Trial Principal Investigator: Brian Mahoney, MD Primary Study Coordinators: Corey Sargent, Kathleen Miller, BSN, CCRC Other Site Investigators: David Hildebrandt, Chris Kummer, Doug Gesme Participating EMS Services: Hennepin County EMS
Medical College of Wisconsin (47)
Hub Principal Investigator: Tom P. Aufderheide, MD Primary Study Coordinator: Joseph T. Brandt Jr., BS, EMT-P EMS Director/Coordinator: M. Riccardo Colella, DO Other Site Investigators: Ron Pirrallo, MD, MHSA, Walter Bialkowski, MS, Benjamin Hermanson, BS, Christopher Sandoval, BS, EMT-P, Kevin Morrow, MFA, Kelly McCormick, BS, MBA, Katherine Burpee, BA, Geri Price, BS, Dawn Kawa, BA Participating EMS Services: Milwaukee County EMS, Milwaukee Fire Department, Franklin Fire Department, Greenfield Fire Department, North Shore Fire Department, Oak Creek Fire Department, South Milwaukee Fire Department, Wauwatosa Fire Department, West Allis Fire Department
University of Kentucky (31)
Hub Principal Investigator: Roger L. Humphries, MD Primary Study Coordinator: Linda Dechtenberg, RN, BSN, CCRC EMS Director/Coordinator: Christofer Sweat Other Site Investigator: L. Creed Pettigrew, MD,MPH Participating EMS Service: Lexington-Fayette Urban County Government Division of Fire & Emergency Services
University of Pennsylvania (26)
Hub Principal Investigator: Jill M. Baren, MD, MBE Trial Principal Investigator: R. Daniel Bledsoe, MD Primary Study Coordinator: Barbie Stahlman, MS, Katherine Lamond, BA, Pamela G. Nathanson, MBE Other Site Investigator: Scott E. Kasner, MD, MSCE, Peter D. Le Roux, MD Participating EMS Services: York Hospital Medic 97, White Rose Ambulance, Grantley Fire Company, Jacobus Lions Ambulance Club, West York Ambulance
Oregon Health & Science University (24)
Hub Principal Investigators: Craig R. Warden, MD, MPH, Robert A. Lowe, MD, MPH Primary Study Coordinator: Rachel N. Stone, CCRP Participating EMS Service: Clackamas Fire District #1
New York Presbyterian Hospital (10)
Hub Principal Investigator: Stephan Mayer, MD, FCCM Trial Principal Investigator: Neal Flomenbaum, MD Primary Study Coordinators: M. Cristina Falo, PhD, Lisa-Vanessa Magitbay, RN, Chirag Surti EMS Directors/Coordinators: Heidi Cordi, MD, Daniel Ribaudo Other Site Investigators: Axel Rosengart, MD, PhD, Matthew Vibbert, MD, Santiago Ortega-Gutierrez, MD, H. Alex Choi, MD, Emily Gilmore, MD, Rishi Malhotra, MD, Lawrence Berger Participating EMS Services: New York Presbyterian
Temple University(8)
Hub Principal Investigator: Nina T. Gentile, MD Trial Principal Investigators: Alvin Wang, DO, Christopher Vates, MD, Ben Usatch, MD Primary Study Coordinators: Brent B. Freeman, Stacey L. Cleary Participating EMS Services: Volunteer Medical Services Corps of Lower Merion and Narberth (Narberth Ambulance), Life Lion EMS
University of Maryland (3)
Hub Principal Investigator: Barney Stern, MD Trial Principal Investigators: Tricia Ting, MD, Gregory Krauss, MD Primary Study Coordinators: Virginia Ganley, RN, Susan Rice, RN, Jennifer Ronald EMS Director/Coordinator: Michelle Stevens, RN Other Site Investigators: Brian Browne, MD, Robert Rosenthal, MD, Peter Hill, MD Participating EMS Services: Maryland Institute for Emergency Medical Services Systems (MIEMSS), Baltimore City EMS
Footnotes
Author contributions: JM and RS conceived the study and designed the trial. All authors supervised the conduct of the trial and data collection. JM and RS analyzed the data. JM drafted the manuscript, and all authors contributed substantially to its revision. JM takes responsibility for the paper as a whole.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was presented in oral and poster format at the Society for Academic Emergency Medicine annual meeting (May 2013, Atlanta).
References
- 1.Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O’Neil N, Neuhaus JM, Segal MR, Lowenstein DH. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–7. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 2.Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, Barsan W. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMullan J, Sasson C, Pancioli A, Silbergleit R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med. 2010;17:575–82. doi: 10.1111/j.1553-2712.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown LH, Campagna JD. Medication storage in the EMS environment: understanding the science and meeting the standards. Emerg Med Serv. 2005;34(71):3–7. 90. [PubMed] [Google Scholar]
- 5.Brown LH, Krumperman K, Fullagar CJ. Out-of-hospital medication storage temperatures: a review of the literature and directions for the future. Prehosp Emerg Care. 2004;8:200–6. doi: 10.1016/j.prehos.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 6.de Guzman R, Polykratis IA, Sondeen JL, Darlington DN, Cap AP, Dubick MA. Stability of tranexamic acid after 12-week storage at temperatures from −20 degrees C to 50 degrees C. Prehosp Emerg Care. 2013;17:394–400. doi: 10.3109/10903127.2013.792891. [DOI] [PubMed] [Google Scholar]
- 7.Kupas DF, Shayhorn MA, Green P, Payton TF. Structured inspection of medications carried and stored by emergency medical services agencies identifies practices that may lead to medication errors. Prehosp Emerg Care. 2012;16:67–75. doi: 10.3109/10903127.2011.621046. [DOI] [PubMed] [Google Scholar]
- 8.Gottwald MD, Akers LC, Liu PK, Orsulak PJ, Corry MD, Bacchetti P, Fields SM, Lowenstein DH, Alldredge BK. Prehospi tal stability of diazepam and lorazepam. Am J Emerg Med. 1999;17:333–7. doi: 10.1016/s0735-6757(99)90079-7. [DOI] [PubMed] [Google Scholar]
- 9.McMullan JT, Pinnawin A, Jones E, Denninghoff K, Siewart N, Spaite DW, Zaleski E, Silbergleit R. The 60-day temperature-dependent degradation of midazolam and lorazepam in the prehospital environment. Prehosp Emerg Care. 2013;17:1–7. doi: 10.3109/10903127.2012.722177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okeke CC, Bailey L, Medwick T, Grady LT. Revised USP standards for product dating, packaging, and temperature monitoring. Am J Health Syst Pharm. 2000;57:1441–5. doi: 10.1093/ajhp/57.15.1441. [DOI] [PubMed] [Google Scholar]