Abstract
We analyzed the outcomes of patients who survived disease-free for 1-year or more following second allogeneic hematopoietic cell transplantation (HCT) for relapsed acute leukemia or myelodysplastic syndromes between 1980 and 2009. A total of 1285 patients received a second allogeneic transplant following disease relapse; among these 325 survived relapse-free at 1-year after the second HCT. The median time from first to second HCT was 17 and 24 months for children and adults, respectively. A myeloablative preparative regimen was used in the second transplant in 62% of children and 45% of adult patients. The overall 10-year conditional survival rates after second transplantation in this cohort of patients who had survived disease-free for at least one year were 55% in children and 39% in adults. Relapse was the leading cause of mortality (77% and 54% of deaths in children and adults, respectively). In multivariate analyses, only disease status prior to second HCT was significantly associated with higher risk for overall mortality (HR 1.71 for patients with disease not in complete remission prior to second HCT, P<0.01). Chronic graft-versus-host disease (GVHD) developed in 43% and 75% of children and adults following second transplant. Chronic GVHD was the leading cause of non-relapse mortality followed by organ failure and infection. The cumulative incidence of developing at least one of the studied late effects at 10-years after second HCT was 63% in children and 55% in adults. The most frequent late effects in children were growth disturbance (10-year cumulative incidence 22%) and cataracts (20%), and in adults were cataracts (20%) and avascular necrosis (13%). Among patients with acute leukemia and myelodysplastic syndromes who receive a second allogeneic HCT for relapse and survive disease-free for at least 1-year, many can be expected to survive long term. However, they continue to be at risk for relapse and non-relapse morbidity and mortality. Novel approaches are needed to minimize relapse risk and long-term transplant morbidity in this population.
Keywords: Hematopoietic cell transplantation, Allogeneic transplant, Second transplant, Long-term survival, Late Effects
Introduction
Disease relapse is the leading cause of treatment failure following allogeneic hematopoietic cell transplantation (HCT) for hematologic malignancy and occurs in approximately 20–60% of patients.(1–5) The outcome following disease relapse after first transplant is poor with survival rates less than 10% in some populations and treatment options for these patients are limited.(5–8) Second HCT is a potentially curative option for selected patients and disease relapse is the most common indication for second allogeneic transplant.(9) The decision to undergo a second transplant is complex given the heightened risks of disease recurrence, acute toxicity, post-transplant late effects, and transplant related mortality (TRM).
Rates of overall survival following second allogeneic HCT range between 28% and 60% with disease-free survival rates of 25–56%.(1, 2, 9–15) Studies of second transplant in children have demonstrated more favorable survival, but are limited by small patient numbers.(11, 16) Previous studies of second transplant have been limited in sample size and hence, have been inconsistent in identifying favorable factors for longer survival following second allogeneic HCT. Notwithstanding the limitation of small sample size, factors associated with superior survival include younger recipient age, longer duration of remission between transplants, complete remission (CR) at second transplant, bone marrow as the stem cell source, the use of a fully HLA-matched donor, the presence of acute and chronic graft-versus-host disease (GVHD), and transplantation from a female donor.(1, 9–12, 17, 18) An area of controversy has been the impact of the intensity of conditioning regimens on survival since some studies have identified reduced intensity conditioning (RIC) regimens to favorably impact survival, while others found survival to benefit from high-dose myeloablative regimens containing total body irradiation (TBI).(2, 12, 15) An additional area of discussion is the impact of using the same or alternate donor with the second transplant.
Much attention has been paid to analyzing late effects following single allogeneic HCT. The Bone Marrow Transplant Survivor Study (BMTSS) reported that 66%-79% of long-term survivors of HCT suffered from at least one chronic health condition.(19–21) The rates of long-term survival and the incidence of late effects following second allogeneic transplantation has not been well described. Given the cumulative exposure to chemotherapy and radiation, recipients of two or more transplants may be at substantial risk for late complications.
In this study, we selected a cohort of patients who were alive and in remission for at least 1 year or more following a second allogeneic HCT for relapsed acute leukemia or myelodysplastic syndrome (MDS) in order to describe: (1) long-term survival and predictive factors for survival outcomes, and (2) cumulative incidence of late effects in this population.
Materials and Methods
Data Source and Patients
Data for this study were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on hematopoietic cell transplantations to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Data are collected before transplant, 100 days and six months after transplant, and annually thereafter, or until death. Observational studies conducted by the CIBMTR are performed under guidance of the Institutional Review Board of the NMDP and are in compliance with all applicable federal regulations pertaining to the protection of human research participants.
Transplant essential data is collected for all patients participating in CIBMTR data collection. This includes demographic, disease type and stage, survival, relapse, graft type, the presence of GVHD, and cause of death data. A subset of CIBMTR participants are selected for comprehensive research level data collection by weighted randomization. Late effects data are collected from this group of patients. Transplant centers report the presence of clinically significant organ impairment or disorder at 6-months and one year following transplant, and annually thereafter. Centers are specifically asked to report the presence of the following late effects: stroke/seizure, myocardial infarction, cirrhosis, gonadal dysfunction requiring hormone replacement, renal failure severe enough to warrant dialysis, avascular necrosis, cataracts, growth hormone deficiency/growth disturbance, hypothyroidism, and bronchiolitis obliterans.
Study Population
The study population included children (age ≤18 years) and adults (age >18 years) who had survived disease-free for at least one year following second allogeneic HCT for acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), juvenile myelomonocytic leukemia (JMML), and MDS between January 1, 1980 and December 31, 2009. There was no exclusion based on type of conditioning regimen. The intensity of the conditioning regimen was based on the definitions published by Bacigalupo, et al. (22) All types of donor grafts were included with the exception of syngeneic twins. Patients who had an allogeneic HCT following autologous transplant were not included in the analysis.
Among the 2129 second allogeneic HCT reported to the CIBMTR for patients with ALL, AML, JMML and MDS during the study time period, 1285 (63%) transplants were performed for disease relapse. Other indications for second HCT were non-engraftment or graft failure (31%) and new malignancy (1%). The reason for second transplant was unknown for 5% of the population. Among those transplanted for disease relapse, 952 (74%) patients died or experienced disease relapse in the first year following second transplant. From the 333 remaining patients, 8 were further excluded because of unknown graft type. The final study cohort consisted of 325 second transplant recipients who had survived in remission for at least 1 year after the second transplant.
Statistical Analysis
Descriptive statistics were used to describe patient demographic, disease and HCT-related variables. All outcomes were evaluated, unless clarified, by calculating the probability of that outcome after second transplant conditioned upon the fact that the patient had survived and remained disease free for at least one year after the second transplant. The Kaplan-Meier method was used to estimate the probability of survival. The cumulative incidence function was used to estimate relapse-related death, non-relapse mortality, and late effects. Rates of individual late effects occurring between the first and second HCT were calculated. Rates of individual late effects were calculated after first HCT (censored at second transplant). Cumulative incidences of late effects and probabilities of other outcomes were estimated for 2- and 10-years following the second HCT and are reported separately for children and adult survivors. Proportional hazards model was developed to assess risk factors for the conditional risk of overall mortality among study population. Potential risk factors considered include age separately among children and adults as well as comparing children versus adults, diagnosis category, disease status at first and second transplant (CR vs. relapse/progressive disease/partial remission), first-second transplant donor pair (related-related vs. related-unrelated vs. unrelated-unrelated, same donor vs. unrelated-unrelated, different donor vs. other), development of GVHD before second transplant (none vs. acute GVHD alone vs. acute GVHD and chronic GVHD), interval between first and second transplants, and conditioning intensity at second transplant (myeloablative vs. non-myeloablative/reduced intensity). Patients with JMML were excluded from the proportional hazard model due to small patient numbers. Proportional hazards models were not developed to assess risk factors for late effects due to sample size limitations. Analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC).
Results
Patient and Transplant Characteristics
Patient and transplant characteristics are presented in Table 1. Of the 325 patients eligible for analysis, 146 were children and 179 were adults. The median interval between the first and second transplant was 17 months (range, 2–149 months) and 24 months (range, 2–158 months) for children and adults, respectively. The majority of children (74%) were in CR prior to second HCT compared with 49% of adults. Children received a myeloablative conditioning regimen in 89% of first and 62% of second transplants. The corresponding values for adults were 79% of first and 45% of second transplants. Of those who received a myeloablative regimen in the first HCT 61% of children and 44% of adults had a myeloablative regimen with second transplant. Total body irradiation (TBI) was used in the conditioning regimen in 45% of first and 40% of second pediatric and 55% and 25% of adult transplants. No conditioning agent or radiation was used with the second transplant in 3% of children and 7% of adult patients. The median follow-up after second transplant among both children and adult survivors was 72 months (range 17–219 and 12–288 months, respectively).
Table 1.
Patient and transplant characteristics
| Characteristics at second HCT | Age at second transplant |
|
|---|---|---|
| Children (≤ 18 years) N (%) |
Adults (> 18 years) N (%) |
|
| Number of patients | 146 | 179 |
| Number of centers | 71 | 92 |
| Male/Female | 92 (63) / 54 (37) | 99 (55) / 80 (45) |
| Disease | ||
| Acute myeloid leukemia | 64 (44) | 111 (62) |
| Acute lymphoblastic leukemia | 66 (45) | 54 (30) |
| Myelodysplastic syndromes | 12 (8) | 14 (8) |
| Juvenile myelomonocytic leukemia | 4 (3) | 0 |
| Patient age at 1st transplant (years), median (range) | 7 (<1–16) | 35 (14–66) |
| Patient age at 2nd transplant (years), median (range) | 9 (1–17) | 38 (19–66) |
| Lansky/Karnofsky score ≥90 | 101 (69) | 96 (54) |
| Donor type | ||
| HLA-matched sibling | 78 (53) | 99 (55) |
| Other related | 8 (5) | 8 (4) |
| Unrelated | 60 (41) | 72 (40) |
| Graft type | ||
| Bone marrow | 94 (64) | 63 (35) |
| Peripheral blood stem cells | 34 (23) | 113 (63) |
| Umbilical cord blood | 18 (12) | 3 (2) |
| Disease status prior to 1st HCT* | ||
| Early | 67 (46) | 90 (50) |
| Intermediate | 49 (34) | 35 (20) |
| Advanced | 26 (18) | 54 (30) |
| Unknown | 4 (3) | 0 |
| Disease remission status prior to 2nd HCT | ||
| Complete remission | 108 (74) | 88 (49) |
| Relapse/progression | 29 (20) | 77 (43) |
| Unknown | 9 (6) | 14 (8) |
| Interval from 1st HCT to relapse (months), median (range) | 14 (<1–145) | 18 (<1–157) |
| < 12 months | 67 (46) | 64 (36) |
| 12–23 months | 42 (29) | 44 (25) |
| ≥ 24 months | 37 (25) | 71 (40) |
| Interval from 1st HCT to 2nd HCT, months, median (range) | 17 (2–149) | 24 (2–158) |
| <12 months | 46 (32) | 41 (23) |
| 12–23 months | 48 (33) | 49 (27) |
| ≥ 24 months | 52 (36) | 89 (50) |
| 1st HCT – 2nd HCT donor pair | ||
| Same related donor | 72 (50) | 80 (45) |
| Different related donor | 8 (6) | 21 (12) |
| Related donor – unrelated donor | 9 (6) | 7 (4) |
| Related donor – donor relationship unknown | 4 (2) | 3 (2) |
| Same unrelated donor | 15 (10) | 26 (15) |
| Different unrelated donor | 27 (18) | 33 (18) |
| Unrelated donor – related donor | 3 (2) | 3 (2) |
| Unrelated, donor – donor relationship unknown | 9 (6) | 6 (3) |
| Conditioning regimen intensity for 1st HCT | ||
| Myeloablative | 130 (89) | 141 (79) |
| Reduced intensity | 5 (3) | 33 (18) |
| Conditioning regimen intensity for 2nd HCT | ||
| Myeloablative | 90 (62) | 81 (45) |
| Reduced intensity | 38 (26) | 73 (41) |
| None | 5 (3) | 12 (7) |
| Unknown | 5 (3) | 12 (7) |
| Year of 2nd HCT | ||
| 1980–1989 | 14 (10) | 20 (11) |
| 1990–1999 | 67 (45) | 53 (30) |
| 2000–2009 | 65 (45) | 106 (59) |
Disease status classification: Early risk disease included acute leukemia in first complete remission, myelodysplastic syndrome refractory anemia or refractory anemia with ringed sideroblasts, or unspecified myelodysplastic syndrome with pre-transplant marrow blasts <5%; intermediate risk disease included acute leukemia in second or greater complete remission; advanced risk disease included acute leukemia in relapse or primary induction failure, myelodysplastic syndrome refractory anemia with excess blasts or excess blasts in transformation or marrow blasts >5% and juvenile myelomonocytic leukemia
Survival, Non-relapse Mortality and Relapse Outcomes
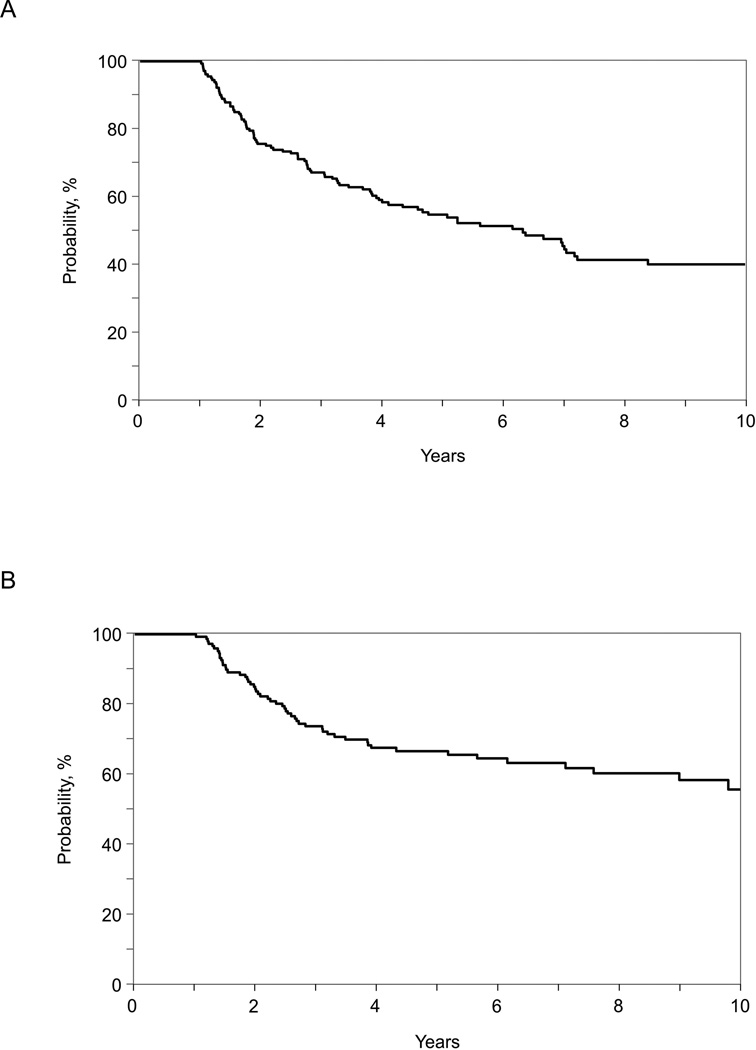
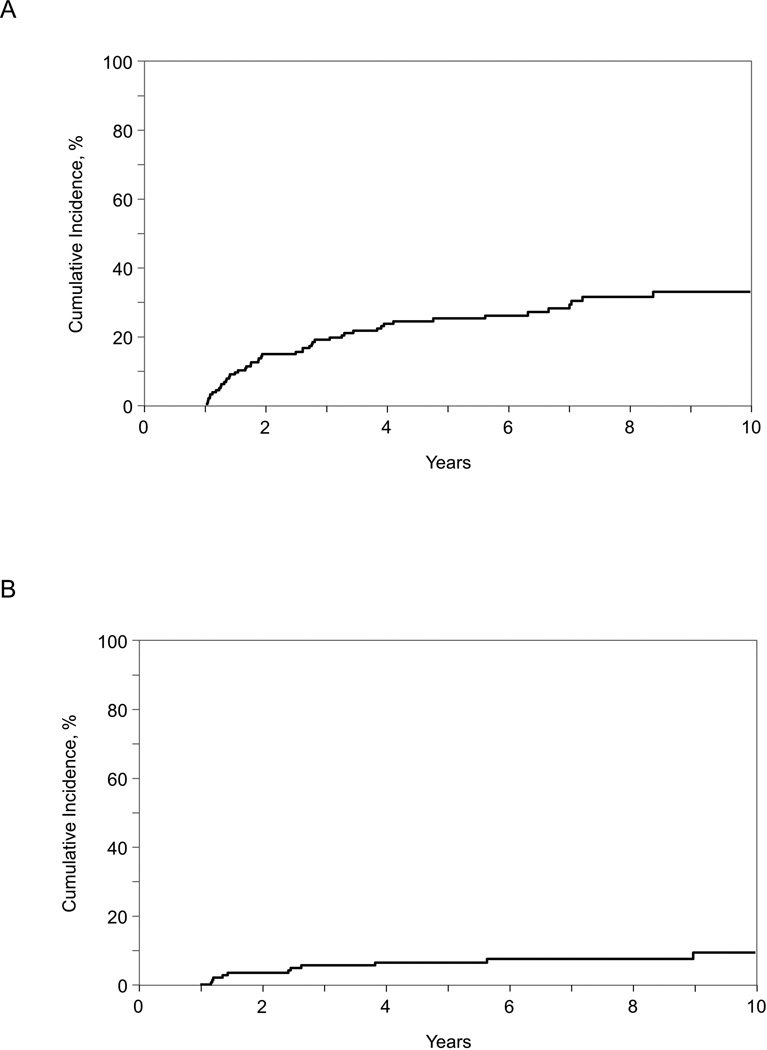
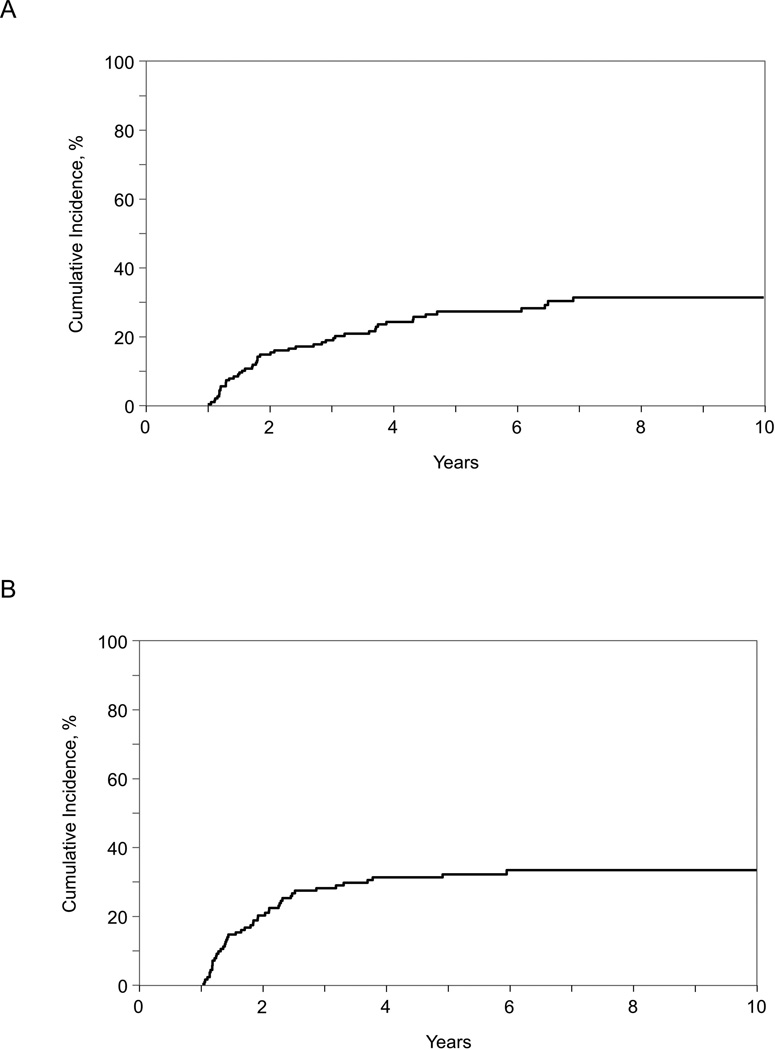
Among the 1-year survivors included in our study, 2- and 10-year conditional survival rates were 83% (95% CI, 77–89%) and 55% (95% CI, 44–65%) among children and 75% (95% CI, 69–81%) and 39% (95% CI, 31–48%) among adults (Table 2 and Figure 1). Cumulative incidences of non-relapse mortality among children were 4% and 10% at 2-years at 10-years, respectively (Table 2 and Figure 2). The corresponding figures among adults were 15% and 34%, respectively. The cumulative incidence of relapse at 2-years and 10-years was 21% and 34% in children and 15% and 32% in adults, respectively (Table 2 and Figure 3). Disease progression or relapse was the major cause of death (43/56 [77%] for children and 52/96 [54%] for adults). Overall causes of non-relapse mortality included GVHD (32%), organ failure (25%), infection (16%), secondary malignancy (5%), and other/unknown cause (23%). The leading causes of non-relapse mortality in children were GVHD (5%), organ failure (5%), and secondary malignancy (4%). The leading causes of non-relapse mortality in adult patients were GVHD (16%), organ failure (11%), and infection (9%).
Table 2.
Conditional probability of overall survival and cumulative incidence of non-relapse mortality and relapse among 1-year disease free survivors after second allogeneic hematopoietic cell transplantation
| Outcomes | Children | Adults | ||
|---|---|---|---|---|
| Patients at risk |
% (95% CI) | Patients at risk |
% (95% CI) |
|
| Relapse | ||||
| 2 years | 105 | 21 (15–28) | 118 | 15 (10–20) |
| 6 years | 47 | 34 (26–43) | 49 | 27 (21–35) |
| 10 years | 19 | 34 (26–43) | 18 | 32 (24–40) |
| Non-relapse mortality | ||||
| 2 years | 105 | 4 (2–8) | 118 | 15 (10–21) |
| 6 years | 47 | 8 (4–14) | 49 | 27 (20–34) |
| 10 years | 19 | 10 (5–17) | 18 | 34 (26–42) |
| Overall survival | ||||
| 2 years | 118 | 83 (77–89) | 133 | 75 (69–81) |
| 6 years | 52 | 64 (55–72) | 55 | 51 (43–58) |
| 10 years | 18 | 55 (44–65) | 22 | 39 (31–48) |
Figure 1.
Overall survival among 1 year disease-free survivors of second allogeneic transplantation for AML, ALL, JMML and MDS: (A) adult patients (age ≥18 years at second transplant), (B) children (age <18 years at second transplant)
Figure 2.
Non-relapse mortality among 1 year disease-free survivors of second allogeneic transplantation for AML, ALL, JMML and MDS: (A) adult patients (age >18 years at second transplant), (B) children (age <18 years at second transplant)
Figure 3.
Relapse among 1 year disease-free survivors of second allogeneic transplantation for AML, ALL, JMML and MDS: (A) adult patients (age >18 years at second transplant), (B) children (age <18 years at second transplant)
In proportional hazard models, disease status at the time of second HCT was the only independent predictor for overall mortality. The overall survival at 5 years for patients who were in complete remission at the time of the second transplant was 66% (95% CI, 59–73%) compared to 48% (95% CI, 38–58%) for those not in remission at the time of transplant. Compared to patients who were in CR, patients with active disease had significantly higher risks for overall mortality (hazard ratio 1.71 [95% CI, 1.22–2.38], P=0.0017).
Late Effects and GVHD
Between first and second allogeneic HCT, at least one late effect was reported in 12% of patients. The most common reported late effects in children were stroke/seizures (3%) and growth disturbance (3%). Gonadal dysfunction (5%) and cataracts (5%) were the most frequently reported late effects among for adults. Grade 2–4 acute GVHD was reported following the first allogeneic transplant in 30% of children and 26% of adults. Grade 2–4 acute GVHD was diagnosed following the second transplant in 47% of children and 46% of adults. Chronic GVHD requiring treatment was reported prior to the second transplant in 16% and 32% of children and adults, respectively. New onset chronic GVHD requiring treatment was diagnosed following the second transplant among 43% of children and 75% of adults. Data regarding the status or severity of chronic GVHD are not uniformly reported by centers to the CIBMTR database and these data are not presented.
The cumulative incidence of developing any late effect at 2-years and 10-years following second HCT was 42% (95% CI, 32–52%) and 63% (95% CI, 53–73%) among children and 45% (95% CI, 36–54%) and 55% (95% CI, 46–64%) among adults, respectively. The cumulative incidences of specific late effects are reported in Table 3. At 10-years following second transplant, the incidence was >10% for gonadal dysfunction, cataracts, growth hormone deficiency/growth disturbance, and hypothyroidism in pediatric survivors. Only avascular necrosis and cataracts were reported with an incidence greater than 10% in adult survivors. The 10-year cumulative incidence of second cancers after second transplant was 1% (95% CI, 0–4%) in pediatric and 8% (95% CI, 4–13%) in adult survivors. The primary sites for second cancers included non-melanoma skin cancer (N=9), oral cavity (N=2), sarcoma (N=2), skin melanoma (N=1) and gastrointestinal tract (N=1). In addition, 1 patient had post-transplant lymphoproliferative disorder and 2 patients were reported to have squamous cell cancers of unknown site (the primary site could not be confirmed by the transplant center).
Table 3.
Late effects among 1-year disease-free survivors following second allogeneic hematopoietic cell transplantation
| Patient and transplant characteristics |
Age group at second transplant | |||||
|---|---|---|---|---|---|---|
| Children (≤ 18 years) | Adults (>18 years) | |||||
| Rate prior to 2nd HCT* N (%) |
CI at 2- years† % (95% CI) |
CI at 10- years† % (95% CI) |
Rate prior to 2nd HCT* N (%) |
CI at 2- years† % (95% CI) |
CI at 10- years† % (95% CI) |
|
| Seizure/stroke | 4 (3) | 4 (1–7) | 4 (2–8) | 2 (1) | 0 | 1 (0–2) |
| Myocardial infarction | 0 | 0 | 0 | 0 | 1 (0–2) | 1 (0–2) |
| Gonadal dysfunction | 3 (2) | 6 (3–11) | 16 (10–23) | 9 (5) | 4 (2–7) | 5 (2–8) |
| Renal failure | 1 (1) | 1 (0–4) | 4 (1–8) | 2 (1) | 3 (1–6) | 4 (1–7) |
| Avascular necrosis | 2 (1) | 4 (2–8) | 5 (2–9) | 4 (2) | 10 (6–15) | 13 (9–19) |
| Cataracts | 1 (1) | 9 (4–14) | 20 (13–28) | 9 (5) | 14 (9–20) | 20 (14–26) |
| Growth hormone deficiency/growth failure | 4 (3) | 8 (4–14) | 22 (15–30) | 0 | 0 | 0 |
| Hypothyroidism | 1 (1) | 7 (3–12) | 13 (7–20) | 2 (1) | 2 (1–5) | 4 (1–7) |
| Cirrhosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Bronchiolitis obliterans | 0 | 3 (1–7) | 4 (1–8) | 1 (1) | 4 (2–8) | 4 (2–8) |
| Second cancers | 0 | 1 (0–3) | 1 (0–4) | 2 (1) | 1 (0–3) | 8 (4–13) |
CI = cumulative incidence
Rate of late effects after first allogeneic transplant, but before second transplant (censored at time of second transplantation)
Cumulative incidence estimates
Discussion
In the present era with several advances in post-transplantation supportive care practices, it is not unusual for a second allogeneic transplant to be considered as treatment for patients whose disease relapses or progresses after a first allogeneic transplant. We present the outcomes of a relatively large cohort of patients reported to the CIBMTR who received a second allogeneic transplant for disease relapse and had survived in remission for at least 1 year. By focusing on long-term survival and late effects in this population, our study addresses important gaps in the literature. Our findings will inform the medical decision making of transplant providers as they consider a second allogeneic transplantation in patients with relapsed acute leukemia and MDS.
There were several key findings in our analysis. First, we show that the majority of recipients of second allogeneic transplants relapsed or died within the first year (74% in our study) confirming earlier results from smaller studies.(1, 2, 15, 23, 24) Second, we have shown that disease status at the time of second transplant was the most-important predictor of subsequent long-term survival. Patients with CR at the time of second transplant had higher survival chances than those who were not in CR. Also, while considering the very high risk nature of their disease, a substantial number of patients who were alive and disease-free at 1 year after second transplant continued to survive on long-term (up to 10 years) follow-up. However, relapse continued to be the major cause of treatment failure even among the disease-free survivors beyond the first year after second transplantation. Finally, a relatively large proportion of second transplant survivors suffered from long-term toxicities.
The overall survival of patients who survived the first year after their second transplant was favorable at 2-years (83% in children and 75% in adults). The overall survival gradually declined before stabilizing between years 7 and 8 with overall survival at 10-years of 55% in children and 39% in adults. Disease relapse or progression was the leading cause of mortality following second transplant and accounted for 77% and 52% of deaths in children and adults, respectively. This is consistent with the published literature of all patients following second HCT.(1, 9, 15, 17, 25) As expected, non-relapse mortality rates were generally lower children compared to adults.(1, 9, 15, 25)
Disease status in remission and longer duration between first transplant and relapse and second HCT are the most consistent factors influencing survival following second HCT across multiple studies.(3, 5, 11, 15, 17, 26) We evaluated these factors as well as other published potential prognostic factors including diagnosis, patient age, disease status at first HCT, donor pairing at first and second HCT, GVHD following first transplant, and conditioning intensity at second HCT.(1–3, 9, 24, 27, 28) Stem cell source was not evaluated as there were insufficient patients in each group to draw conclusions. Remission status was the only factor predictive of survival following second transplant in our study. Similar to other studies, patients in a CR had a survival advantage compared to those transplanted with active disease.(15, 17, 24, 26, 29) This finding supports the use of additional therapy, if feasible, to achieve a CR prior to second HCT for patients with relapsed acute leukemia and MDS in order to maximize the potential for long-term survival. Patients with disease relapse following a first allogeneic HCT should be encouraged to participate in clinical trials of novel treatment approaches.(30)
We did not find an association of donor switching between first and second transplant and overall survival. This may be explained by the relatively small number of donor-recipient pairs in each of the groups analyzed (related-related vs. related-unrelated vs. unrelated-unrelated [same donor] vs. unrelated-unrelated [different donor] vs. other). Some prior investigations were similarly restricted in the ability to detect a survival difference attributable to change in donor pairing due to limited sample size. However, the results of this and prior studies do not support the strategy of changing donors between first and second transplant in an effort to improve overall survival.(1, 2, 11, 15, 25) When comparing the results of our study to published literature, it is worth noting that survival and risk factor analysis in prior studies included all patients irrespective of survival since transplantation while we focused only on those who survived disease-free the initial post-transplant year. This may explain why the time between first and second HCT was not predictive of survival as patients with shorter duration between transplants may have relapsed or died during the first year.
We report the cumulative incidences of developing at least a single late effect at 10-years following second HCT of 63% in children and 55% in adult patients. The cumulative incidence of late effects between 2 and 10 years did not increase substantially in adults. The increase in the CI of late effects in children between 2 and 10 years was primarily due to gonadal failure. The median age of 9 years indicates that many of the patients were pre-pubertal at the time of second transplant. The cumulative incidences of late effects presented are similar to what is described in the literature in long-term survivors of single transplant. Patients in the BMTSS reported at least one chronic health condition in 32%–38.2% of two year survivors and in 74% of ten-year survivors of first transplant for leukemia and aplastic anemia.(20, 21, 31) This is also comparable to the 79% of 145 pediatric survivors of single transplant followed for a median of 11 years and lower than the 91% found in 99 Australian patients followed for a median of 74 months following first HCT.(19, 32) The specific late-effects evaluated differ in each of the studies referenced. However, the categories of conditions investigated are similar and reasonably allow for comparison between studies. CIBMTR data forms capture a limited number of late complications, and hence, we may have underestimated the true incidence of late effects in second allogeneic transplant survivors. Irrespective, our study emphasizes the need for continued long-term surveillance for late effects in this population.(33, 34)
We acknowledge several limitations to our retrospective cohort study. First, there is the potential for selection bias at the level of the individual centers regarding which patients are offered a second transplant (e.g., patients may be more likely to receive a second transplant if they have less comorbidities, better performance status, absence of severe GVHD and longer time between first transplant and relapse). As noted above, data were collected on selected late effects. Capture of long-term follow-up information by transplant centers for their transplant recipients can be challenging. However, our follow-up information was robust with the completeness index of follow-up (ratio of observed versus expected follow-up for the cohort) of 94% at 5-years and 83% at 10-years after second transplantation. Screening practices can differ between institutions resulting in potential under-reporting of specific effects. The median follow-up of our cohort was approximately 6 years. While this is an acceptable period of time, it is possible that a longer duration of follow-up may result in an increase in the number of reported late effects. Finally, even though our study is the largest to date, the number of patients included and the number of post-transplant events was still relatively small and future studies will have to readdress this issue in larger patient cohorts. On the same note, the number of umbilical cord blood recipients was small and outcomes with second transplants using this graft source will have to be characterized in larger studies.
In summary, the 1-year overall survival following second allogeneic transplant for relapsed acute leukemia and MDS is suboptimal. However, many patients who live disease-free for at least 1-year post-second transplant can be expected to survive long term and their survival is best optimized when patients receive a second transplant in CR. The majority of late failures are due to relapse and late effects are frequently encountered. Future trials focusing on reducing risks of relapse, novel treatments for treating post-transplant relapse and implementing systematic surveillance for long-term toxicities in this population are warranted.
Highlights.
At one-year following second HCT for relapsed leukemia 74% of patients either died or relapsed.
Being in CR at the time of second HCT was the most-important predictor of long-term survival.
A relatively large proportion of second HCT survivors suffer from long-term toxicities.
Acknowledgments
CIBMTR Funding Support:
The Center for International Blood and Marrow Transplant Research (CIBMTR) is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: None of the authors has a financial conflict of interest to disclose.
References
- 1.Bosi A, Laszlo D, Labopin M, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. Journal of clinical oncology. 2001;19:3675–3684. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 2.Shaw BE, Mufti GJ, Mackinnon S, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone marrow transplantation. 2008;42:783–789. doi: 10.1038/bmt.2008.255. [DOI] [PubMed] [Google Scholar]
- 3.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biology of blood and marrow transplantatin. 2007;13:116–123. doi: 10.1016/j.bbmt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Poon LM, Bassett R, Jr., Rondon G, et al. Outcomes of second allogeneic hematopoietic stem cell transplantation for patients with acute lymphoblastic leukemia. Bone marrow transplantation. 2013;48:666–670. doi: 10.1038/bmt.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spyridonidis A, Labopin M, Schmid C, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 7.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 8.Fathi AT, Chen YB. Treatment of Relapse of Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation. Current hematologic malignancy reports. 2014 doi: 10.1007/s11899-014-0209-2. [DOI] [PubMed] [Google Scholar]
- 9.Michallet M, Tanguy ML, Socie G, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Societe Francaise de Greffe de moelle (SFGM) British journal of haematology. 2000;108:400–407. doi: 10.1046/j.1365-2141.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 10.Arfons LM, Tomblyn M, Rocha V, Lazarus HM. Second hematopoietic stem cell transplantation in myeloid malignancies. Current opinion in hematology. 2009;16:112–123. doi: 10.1097/MOH.0b013e3283257a87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meshinchi S, Leisenring WM, Carpenter PA, et al. Survival after second hematopoietic stem cell transplantation for recurrent pediatric acute myeloid leukemia. Biology of blood and marrow transplantation. 2003;9:706–713. doi: 10.1016/j.bbmt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Pawson R, Potter MN, Theocharous P, et al. Treatment of relapse after allogeneic bone marrow transplantation with reduced intensity conditioning (FLAG +/− Ida) and second allogeneic stem cell transplant. British journal of haematology. 2001;115:622–629. doi: 10.1046/j.1365-2141.2001.03150.x. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Horikoshi Y, Okamoto Y, et al. Second allogeneic hematopoietic SCT for relapsed ALL in children. Bone marrow transplantation. 2012;47:1307–1311. doi: 10.1038/bmt.2012.29. [DOI] [PubMed] [Google Scholar]
- 14.Blau IW, Basara N, Bischoff M, et al. Second allogeneic hematopoietic stem cell transplantation as treatment for leukemia relapsing following a first transplant. Bone marrow transplantation. 2000;25:41–45. doi: 10.1038/sj.bmt.1702101. [DOI] [PubMed] [Google Scholar]
- 15.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone marrow transplantation. 2004;34:721–727. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 16.Patel SA, Coulter DW, Grovas AC, et al. Cytosine Arabinoside and Mitoxantrone Followed by Second Allogeneic Transplant for the Treatment of Children With Refractory Juvenile Myelomonocytic Leukemia. Journal of pediatric hematology/oncology. 2014;36:491–494. doi: 10.1097/MPH.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosing C, Saliba RM, Shahjahan M, et al. Disease burden may identify patients more likely to benefit from second allogeneic hematopoietic stem cell transplantation to treat relapsed acute myelogenous leukemia. Bone marrow transplantation. 2005;36:157–162. doi: 10.1038/sj.bmt.1705011. [DOI] [PubMed] [Google Scholar]
- 18.Kedmi M, Resnick IB, Dray L, et al. A retrospective review of the outcome after second or subsequent allogeneic transplantation. Biology of blood and marrow transplantation. 2009;15:483–489. doi: 10.1016/j.bbmt.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118:1413–1420. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139. doi: 10.1182/blood-2009-06-229369. quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biology of blood and marrow transplantation. 2013;19:1073–1080. doi: 10.1016/j.bbmt.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishi K, Takahashi S, Gondo H, et al. Second allogeneic bone marrow transplantation for post-transplant leukemia relapse: results of a survey of 66 cases in 24 Japanese institutes. Bone marrow transplantation. 1997;19:461–466. doi: 10.1038/sj.bmt.1700680. [DOI] [PubMed] [Google Scholar]
- 24.Bosi A, Bacci S, Miniero R, et al. Second allogeneic bone marrow transplantation in acute leukemia: a multicenter study from the Gruppo Italiano Trapianto Di Midollo Osseo (GITMO) Leukemia. 1997;11:420–424. doi: 10.1038/sj.leu.2400585. [DOI] [PubMed] [Google Scholar]
- 25.Christopeit M, Kuss O, Finke J, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. Journal of clinical oncology. 2013;31:3259–3271. doi: 10.1200/JCO.2012.44.7961. [DOI] [PubMed] [Google Scholar]
- 26.Kishi K, Hiasa Y, Tanaka H, et al. [Identification of predictive factors associated with recurrent restenosis after second percutaneous transluminal coronary angioplasty] Journal of cardiology. 1997;29:7–12. [PubMed] [Google Scholar]
- 27.Radich JP, Sanders JE, Buckner CD, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. Journal of clinical oncology. 1993;11:304–313. doi: 10.1200/JCO.1993.11.2.304. [DOI] [PubMed] [Google Scholar]
- 28.Nagler A, Or R, Naparstek E, Varadi G, Slavin S. Second allogeneic stem cell transplantation using nonmyeloablative conditioning for patients who relapsed or developed secondary malignancies following autologous transplantation. Experimental hematology. 2000;28:1096–1104. doi: 10.1016/s0301-472x(00)00511-7. [DOI] [PubMed] [Google Scholar]
- 29.Mrsic M, Horowitz MM, Atkinson K, et al. Second HLA-identical sibling transplants for leukemia recurrence. Bone marrow transplantation. 1992;9:269–275. [PubMed] [Google Scholar]
- 30.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker KS, Ness KK, Weisdorf D, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Leukemia. 2010;24:2039–2047. doi: 10.1038/leu.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gifford G, Sim J, Horne A, Ma D. Health Status, Late Effects and Long Term Survivorship of Allogeneic Bone Marrow Transplantation: A Retrospective Study. Internal medicine journal. 2014;44:139–147. doi: 10.1111/imj.12336. [DOI] [PubMed] [Google Scholar]
- 33.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone marrow transplantation. 2012;47:337–341. doi: 10.1038/bmt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biology of blood and marrow transplantation. 2012;18:348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]