Abstract
Current understanding of the thyroid disruptive properties of perfluoroalkyl substances (PFASs), particularly in aging populations, is limited. The objectives of this study were to (i) assess associations between thyroid function, as measured by serum thyrotropin (thyroid stimulating hormone, TSH), free thyroxine (fT4), total thyroxine (T4), and total triiodothyronine (T3), and serum perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in an aging population and (ii) determine if other persistent organic pollutants with thyroid disruptive properties including polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) modify such associations. We conducted a cross-sectional study of 87 men and women 55 to 74 years of age, without clinically-diagnosed thyroid disease, who resided in upper Hudson River communities in New York. Geometric means (standard deviations) of serum PFOS and PFOA were 31.6 (1.7) ng/mL and 9.17 (1.72) ng/mL, respectively. Multivariable linear regression analyses indicated that one interquartile range difference in PFOS corresponded to 4% and 9% increases in fT4 and T4 respectively. We detected statistical interactions between PFOA and age for effects on fT4 and T4; joint increases in PFOA and age were associated with increases in fT4 and T4, of 3% and 7%, respectively. We also detected statistical interactions between PFOS and total PCBs for the effect on T3 and between PFOA and total PBDEs for the effect on TSH. Our results suggest that PFASs are associated with subtle alterations in thyroid hormone levels in this population, and that these associations are likely to vary by age, and levels of PCBs and PBDEs.
Keywords: Older Adults, Perfluoroalkyl Substances, Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, Thyroid Function, Thyroid Hormones
1. Introduction
Perfluoroalkyl substances (PFASs) have been extensively used over the last five decades in a variety of consumer products and industrial applications including textiles, fire-fighting foams, and fluoropolymers mainly due to their surfactant properties (ATSDR, 2009; Prevedouros et al., 2006). Due to extensive persistence and an ability to bioaccumulate, PFASs are widespread in the environment (Giesy and Kannan, 2001; Kato et al., 2011). Their ubiquity and potential link to human health effects led to efforts to phase out U.S. production and use of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), the two most predominant PFASs, in the early 2000's (ATSDR, 2009). PFASs, however, were still detected in 99% of serum samples collected from the general U.S. population during the 2007-2008 National Health and Nutrition Examination Survey, and therefore remain a potential health concern (Kato et al., 2011).
Thyroid hormones are important for proper cardiovascular and central nervous system function, including neuropsychological function (Bauer et al., 2008; Klein and Ojamaa, 2001; Yen and Brent, 2012). Rather than directly interfering with the hypothalamus-pituitary-thyroid (HPT) axis, PFASs may compete for certain thyroid hormone binding proteins (TBPs) including albumin and transthyretin (Chang et al., 2008; Han et al., 2012; Jones et al., 2003; Weiss et al., 2009). By competing for binding sites on TBPs, PFASs may displace thyroid hormone and in doing so increase the circulating levels of biologically available hormone. However, both increases and decreases in serum thyroid hormones have been demonstrated in groups occupationally exposed to high-dose PFASs (Olsen et al., 2003; Olsen and Zobel, 2007), and in adults and children exposed to high/background levels of PFOA and PFOS (Dallaire et al., 2009; Knox et al., 2011; Lopez-Espinosa et al., 2012a; Wen et al., 2013). Inconsistent findings across studies limit current understandings of PFASs' thyroid disruptive properties and the associated public health impact. Additionally, aging populations are often under-represented in the literature, with only one study illustrating associations in an older population (Knox et al., 2011). Aging individuals may experience increased health risks due to compromised biological capacities and higher body burdens of the chemicals (Geller and Zenick, 2005). Therefore, characterization of the associated risks in such populations is important but understudied areas.
Besides PFASs, U.S. populations are also exposed to other persistent organic pollutants (POPs) with thyroid disruptive properties, including polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) (Boas et al., 2012; Centers for Disease Control Prevention, 2009). In our previous research, we reported that such POPs may interact to affect thyroid and neuropsychological function (Bloom et al., 2014; Fitzgerald et al., 2012). Similarly, there exists a likelihood for shared biological mechanisms, including interference with serum TBPs (Boas et al., 2012), and so these POPs may modify the effects of PFASs on thyroid function. However, the interaction between PFASs and other POPs has not been well addressed in other studies of human populations.
The current study is intended to further increase knowledge of the associations of PFASs with thyroid function in aging populations and the potential modifying effects of the other POPs on such associations. We assessed associations between serum PFOS and PFOA and thyroid function among older men and women residing in upper Hudson River communities. We hypothesized that PFOS and PFOA would be positively associated with free thyroxine (fT4), total thyroxine (T4), and total triiodothyronine (T3). Additionally, we examined if serum total PCBs (Σ PCBs), total PBDEs (Σ PBDEs), and dichlorodiphenyl trichlorethane (DDT) and its metabolite p,p-dichlorodiphenyl dichloroethene (DDE) modified such associations.
2. Materials and Methods
2.1. Sample Selection
The study population consisted of men and women, aged 55 to 74 years, who lived in three demographically similar communities adjacent to the Hudson River in New York State (NYS) for 25 years or more: Fort Edward, Hudson Falls, and Glens Falls (Figure 1). The study participants were originally recruited to investigate associations between environmental PCB exposure and neuropsychological function, given the proximity to General Electric plants located in Fort Edward and Hudson Falls. These plants used PCBs to manufacture electric capacitors from 1947 until 1977, and discharged more than 450,000 kg of PCBs into the upper Hudson River (U.S. EPA, 2011).
Figure 1.
Study Areas: Glens Falls, Hudson Falls, and Fort Edward, New York.
The procedures for participant recruitment have been described in detail elsewhere (Fitzgerald et al., 2007; Fitzgerald et al., 2008). Briefly, the source population was identified using an online telephone directory search engine and a digital database (InfoUSA). A total of 2,704 men and women aged 55 to 74 years residing in one of the three target communities were contacted by telephone to determine eligibility for the study. Those who had not lived in their respective areas for at least 25 years, or who had been involved in a PCB-related job for ≥ 1 year, or who had certain medical conditions, including a history of stroke, severe head injury, Parkinson's disease, Alzheimer's disease, or severe cognitive impairment, were ineligible for the study. Among those eligible and invited to participate, 40% agreed.
The final cohort consisted of 253 participants from all three communities. During the years 2000-2002, structured in-person interviews were conducted to obtain information on sociodemographics, and histories on residence, fish consumption, occupation and medication use.
A flowchart outlining the sample selection process is shown in Figure 2. Of 253 participants from the parent study, 181 had sufficient serum archived (≥ 1.0 mL), and 144 agreed to thyroid hormone and PBDE determinations in 2005. In 2010, 157 of 166 participants with adequate serum archived (≥ 0.2 mL) consented to the additional analysis of serum PFASs. A total of 109 participants had information on both PFASs and thyroid biomarkers. Participants with clinical thyroid disease or who were taking any thyroid-related medications (n=9), or sex hormone therapy (n=13) were excluded (Tahboub and Arafah, 2009). The current analysis was therefore restricted to 87 participants. The project was approved by the Institutional Review Boards of the University at Albany and the NYS Department of Health (NYSDOH).
Figure 2.
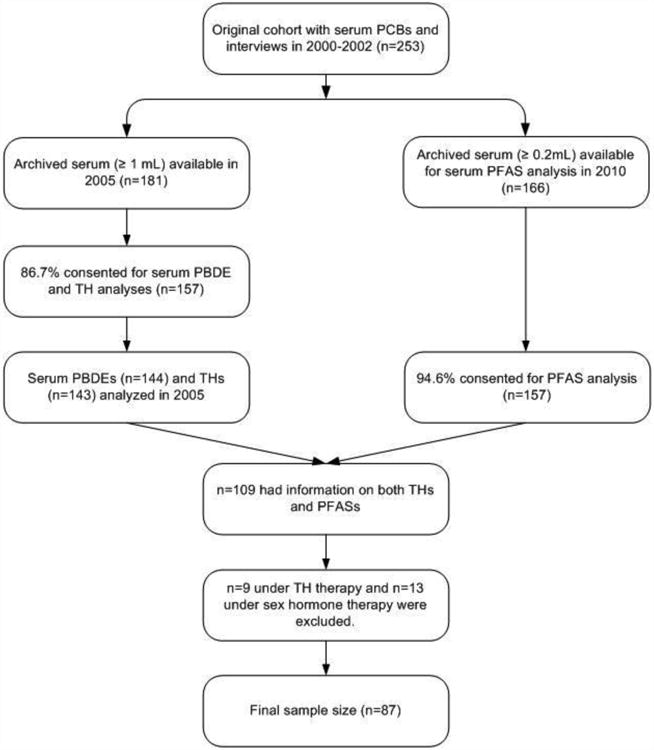
Sample Selection Process.
Abbreviations: PBDEs, Polybrominated Diphenyl Ethers; PCBs, Polychlorinated Biphenyls; PFASs, Perfluoroalkyl Substances; THs, Thyroid Hormones.
2.2. Serum Chemical Analysis
Samples of 25 mL of venous blood were drawn from fasting subjects and centrifuged to obtain serum which was pipetted into a glass container. Blood serum PCBs, organochlorine pesticides, including DDE and DDT, cholesterol, and triglycerides were analyzed at the Wadsworth Center of the NYSDOH during 2000-2002 (Fitzgerald et al., 2007; Fitzgerald et al., 2008). Remaining serum samples were archived at -20°C and later used for PBDE and PFAS analysis in 2005 and 2010, respectively. The analytical procedures for serum PBDE analysis can be found elsewhere (Fitzgerald et al., 2010). The 30 PCB congeners were summed to obtain serum Σ PCBs, and the 9 commonly detected PBDE congeners were summed to obtain serum Σ PBDEs. Concentrations of serum DDE and DDT were also summed.
The procedure for the analysis of PFOS and PFOA is described in detail elsewhere (Kannan et al., 2004). The methods utilized initial extraction of the chemicals from serum using an ion-pairing method with subsequent analysis by high performance liquid chromatograph-tandem mass spectrometer. Isotopically labeled internal standards were spiked into each sample and quantification was done by isotope dilution method. The laboratory has validated the method by participating in several inter-laboratory comparison studies and proficiency testing programs. The limit of quantitation (LOQ) ranged from 0.5 to 1 ng/mL, which was determined based on the linear range of the calibration curve prepared at a concentration range of 0.5 ng/mL to 100 ng/mL. There was one observation below the LOQ for PFOA, for which the machine-read value was assigned without substitution because this approach has been shown to minimize bias (Schisterman et al., 2006).
2.3. Thyroid Function Biomarkers
Concentrations of thyrotropin (thyroid stimulating hormone, TSH), fT4, T4, and T3 in serum were determined using an immunoelectrochemiluminometric assay (Roche Elecsys 1010 system, Roche Diagnostics, U.S.A.) in 2005 at the Clinical Laboratory, Wadsworth Center, NYSDOH. The laboratory is CLIA-88 accredited. The assays make use of a competition test principle using antibodies that are specific for each analyte. Endogenous thyroid hormone in the sample competes with exogenous analog in the test, which has been labeled with a ruthenium complex for binding sites on the biotinylated antibody. The reaction mixture is exposed to a voltage to induce the chemiluminescent emission which is measured by a photomultiplier. The average inter-run coefficients of variation for TSH, fT4, T4, and T3 were 2.5% (5.1% at concentrations < 0.2 μIU/mL), 2.2%, 4.5%, and 5.9%, respectively. The laboratory reference intervals were 0.3-4.2 μIU/mL for TSH, 0.9-1.7 ng/dL for fT4, 5.1-14.1 μg/dL for T4, and 80-200 ng/dL for T3.
2.4. Statistical Analysis
Total serum lipids were calculated from serum cholesterol and triglycerides using the “short” formula (2.27 × cholesterol + triglycerides + 0.623) proposed by Phillips et al. (1989), and serum Σ PCBs, Σ PBDEs, and DDE+DDT were expressed on a lipid basis, i.e. ng/g of serum total lipids (Fitzgerald et al., 2008; Fitzgerald et al., 2010). Serum PFASs, Σ PCBs, Σ PBDEs, DDE+DDT, and TSH were natural log transformed (ln) to achieve normality. Student t-test, analysis of variance, Pearson correlation coefficients and non-parametric tests, such as Wilcoxon's two sample test and Kruskal-Wallis test, were employed to assess bivariate associations between PFASs and thyroid hormones, and with covariates, including age, sex, years of education, serum Σ PCBs and Σ PBDEs, cigarette smoking, and alcohol consumption.
To assess the associations of serum PFOS and PFOA with thyroid hormones adjusting for potential confounders, multivariable linear regression analyses were performed. Directed acyclic graphs, delineating hypothesized causal pathways between serum PFASs and thyroid hormones, and with covariates based on the findings of previous studies, were used to identify covariates for confounding adjustment (Greenland et al., 1999). The regression models included age, sex, years of education, and serum Σ PCBs (lipid basis) as covariates (Bloom et al., 2014; Davis et al., 2003; Kato et al., 2011; Tahboub and Arafah, 2009). We assessed underlying assumptions of linear regression models including linearity, homoscedasticity, normality, and independence of errors. We also evaluated influential observations in the regression models using criteria, including Cook's distance (> 1), dfbeta (≥ |1.0| for exposure), or dffit (≥ |1.5|) (Kleinbaum et al., 1998). Effects were reported as the change in thyroid hormone level per interquartile range (IQR) increase in ln PFAS.
To check potential interactions of the PFASs with covariates including age, Σ PCBs, Σ PBDEs, and DDE+DDT, we performed regression analyses including two-way product terms. In addition to Σ PCBs, we also assessed interaction by the sum of 12 PCB congeners with anti-estrogenic/dioxin-like properties (i.e., IUPAC #s 77, 81, 105, 114, 118, 123, 126, 156, 157, 167, 169 and 189 (Cooke et al., 2001, Van den Berg et al., 2006, Wolff et al., 1997)), the sum of seven PCB congeners with estrogenic properties in vivo or in vitro (i.e., IUPAC #s 52, 77, 95, 99, 101, 110 and 153 (Cooke et al., 2001)), the sum of thyroid active congeners likely to compete for binding to serum TBPs (i.e., IUPAC #s 28, 52, 60, 74, 77, 95, 99, 101, 105, 114, 118, and 126 (Bloom et al., 2014; Chauhan et al., 2000)), and the sum of non-dioxin-like/ortho- PCB congeners that may inhibit dopamine transporter (i.e., IUPAC #s 28, 52, 74, 99, 101, 110, 118, 138, 153, and 180 (Wigestrand et al., 2013)). We reported individual and joint effects for models with p-value (p) < significant product terms (Knol et al., 2009). As lipid standardization of PCBs (Phillips et al., 1989) may produce biased estimates, we repeated the analyses using PCBs or PBDEs on a wet-weight basis and adjusting for total lipids as a covariate (Schisterman et al., 2005). All the statistical tests were two-tailed, and are considered statistically significant at p < 0.05 for main effects and at p < 0.10 for product terms. All the analyses were performed using SAS version 9.3 (SAS Institute, Inc. Cary, NC, U.S.A).
3. Results
Table 1 presents the background characteristics for the study participants. The mean age (standard deviation (SD)) of participants was 63.6 (6.1) years, with an IQR of 58-69 years; 58.6% (n = 51) of whom were men and 41.4% were women. Geometric mean (GM) concentrations (SDs) of serum Σ PCBs, Σ PBDEs, and DDE+DDT were 458 (1.6) ng/g, 30.5 (3.4) ng/g, and 506 (3.1) ng/g, respectively, and their IQRs were 350-595 ng/g, 11.7-66.1 ng/g, and 303.7-965.3 ng/g, respectively.
Table 1. Background characteristics of study participants (n = 87).
| Variable | n | Mean (SD) | Median | Range |
|---|---|---|---|---|
| Age at interview (years)a | 87 | 63.57 (6.06) | 63 | 55, 74 |
| Body mass index (kg/m2)a | 87 | 28.81 (5.76) | 27.55 | 17.22, 49.6 |
| Alcohol consumption (Numbers of annual drinks in last year)a,d | 87 | 284.61 (377.36) | 116 | 0, 2184 |
| Among drinkers (Numbers of annual drinks in last year)a,d | 78 | 317.45 (385.34) | 187 | 1, 2184 |
| Cigarette smoking (Total packs in last year)a | 87 | 46.19 (144.06) | 0 | 0, 730 |
| Among smokers (Total packs in last year)a | 14 | 287.08 (250.65) | 273.75 | 0.65, 730 |
| Years of educationa | 87 | 13.92 (2.78) | 14 | 6, 20 |
| Serum Σ PBDEs (ng/g serum total lipids)b | 87 | 30.52 (3.38) | 23.51 | 4.95, 912.99 |
| Serum Σ PCBs (ng/g serum total lipids)b | 87 | 458.12 (1.57) | 445.25 | 139.3, 1638.19 |
| Serum DDE+DDT (ng/g serum total lipids)b | 87 | 506.45 (3.12) | 612.98 | 3.03, 3593.04 |
|
| ||||
| Categories | n | % | ||
|
| ||||
| Sex | ||||
| Men | 51 | 58.62 | ||
| Women | 36 | 41.38 | ||
| Income categoryc | ||||
| < $15,000 | 4 | 4.88 | ||
| ≥ $15,000 to $30,000 | 15 | 18.29 | ||
| > $30,000 to $45,000 | 23 | 28.05 | ||
| > $45,000 to $60,000 | 17 | 20.73 | ||
| > $60,000 to $75,000 | 13 | 15.85 | ||
| > $75,000 | 10 | 12.20 | ||
Abbreviations: DDE, p,p-dichlorodiphenyl dichloroethene; DDT, dichlorodiphenyl trichlorethane; Σ PBDEs, Total Polybrominated Diphenyl Ethers; Σ PCBs, Total Polychlorinated Biphenyls; Range, Minimum-Maximum; SD, Standard Deviation.
Arithmetic Mean;
Geometric Mean;
Frequency Missing=5;
A drink is defined as 12 oz. of beer, 4 oz. of wine, or 1.5 oz. of liquor.
Table 2 presents descriptive statistics for PFASs and thyroid biomarkers. GM concentrations (SDs) of serum PFOS and PFOA were 31.6 (1.7) ng/mL and 9.2 (1.7) ng/mL respectively; their IQRs were 21.7-45.2 ng/mL and 7.1-13.1 ng/mL respectively. GM concentrations (SDs) of serum TSH, fT4, T4, and T3 were 2.2 (1.7) μIU/mL, 1.2 (1.2) ng/dL, 8.6 (1.2) μg/dL, and 124.7 (1.1) ng/dL, respectively.
Table 2. Distributions for serum PFASs and thyroid biomarkers in study participants (n = 87).
| AM (SD) | GM (SD) | Median | Range | |
|---|---|---|---|---|
| PFOS (ng/mL) | 36.58 (22.8) | 31.60 (1.70) | 29.78 | 5.29, 139.53 |
| PFOA (ng/mL) | 10.42 (5.68) | 9.17 (1.72) | 9.32 | 0.58, 42.69 |
| TSH (μIU/mL) | 2.58 (1.47) | 2.25 (1.72) | 2.15 | 0.23, 9.05 |
| fT4 (ng/dL) | 1.24 (0.17) | 1.23 (1.15) | 1.26 | 0.86, 1.68 |
| T4 (μg/dL) | 8.69 (1.47) | 8.57 (1.19) | 8.66 | 6.09, 12.08 |
| T3 (ng/dL) | 125.69 (15.58) | 124.71 (1.14) | 124.60 | 82.70, 172.40 |
Abbreviations: AM, Arithmetic Mean; fT4, Free Thyroxine; GM: Geometric Mean; PFASs, Perfluoroalkyl Substances; PFOA, Perfluorooctanoic Acid; PFOS, Perfluorooctane Sulfonate; Range, Minimum-Maximum; SD, Standard Deviation; T3, Total Triiodothyronine; T4, Total Thyroxine; TSH, Thyroid Stimulating Hormone.
Table 3 presents bivariate associations between PFASs and thyroid hormones, and with covariates. Briefly, PFOS and PFOA were positively correlated (r = 0.52, p < 0.001). PFOS was positively associated with fT4 (r = 0.23, p = 0.03) and T4 (r = 0.39, p = < 0.001) and PFOA was positively associated with T4 (r = 0.27, p = 0.01) and T3 (r = 0.23, p = 0.03). Mean fT4 was significantly higher in men than in women (p = 0.02); levels of PFASs and other thyroid hormones were not significantly different between men and women (data not shown). PFASs were not significantly correlated with age.
Table 3. Pearson correlation coefficients (p-value) for unadjusted associations among serum PFASs, thyroid biomarker and covariates.
| PFOSa | PFOA a | TSHa | fT4 | T4 | T3 | |
|---|---|---|---|---|---|---|
| PFOS (ng/mL)a | 0.52 (<0.0001) | 0.05 (0.62) | 0.23 (0.03) | 0.39 (<0.001) | 0.12 (0.25) | |
| PFOA (ng/mL)a | 0.05 (0.62) | 0.17 (0.12) | 0.27 (0.01) | 0.23 (0.03) | ||
| TSH (μIU/mL)a | -0.28 (0.01) | -0.14 (0.20) | -0.19 (0.07) | |||
| fT4 (ng/dL) | 0.69 (<0.0001) | 0.15 (0.15) | ||||
| T4 (μg/dL) | 0.52 (<0.0001) | |||||
| Age at interview (years) | 0.11 (0.30) | -0.12 (0.26) | 0.14 (0.19) | -0.04 (0.69) | 0.05 (0.65) | -0.05 (0.64) |
| Years of education | -0.21 (0.05) | -0.11 (0.32) | 0.12 (0.29) | -0.03 (0.77) | -0.17 (0.11) | -0.14 (0.18) |
| Income | -0.29 (0.01) | -0.23 (0.04) | 0.03 (0.81) | 0.01 (0.93) | -0.24 (0.03) | -0.25 (0.03) |
| Cigarette smoking (Total packs in last year)a | 0.15 (0.17) | 0.28 (0.01) | -0.08 (0.48) | 0.21 (0.06) | 0.27 (0.01) | 0.22 (0.04) |
| Alcohol consumption (Numbers of annual drinks in last year)a,b | -0.09 (0.43) | -0.10 (0.38) | -0.15 (0.17) | 0.00 (0.96) | -0.22 (0.04) | -0.18 (0.10) |
| Serum Σ PCBs (ng/g serum total lipids)a | 0.30 (0.01) | 0.16 (0.15) | 0.04 (0.71) | 0.08 (0.46) | 0.09 (0.40) | -0.08 (0.48) |
| Serum Σ PBDEs (ng/g serum total lipids)a | 0.02 (0.86) | 0.04 (0.68) | -0.02 (0.85) | -0.13 (0.23) | -0.10 (0.35) | 0.05 (0.63) |
Abbreviations: Abbreviations: fT4, Free Thyroxine; Σ PBDEs, Total Polybrominated Diphenyl Ethers; Σ PCBs, Total Polychlorinated Biphenyls; PFASs, Perfluoroalkyl Substances; PFOA, Perfluorooctanoic Acid; PFOS, Perfluorooctane Sulfonate; T3, Total Triiodothyronine; T4, Total Thyroxine; TSH, Thyroid Stimulating Hormone.
Log-transformed.
A drink is defined as 12 oz. of beer, 4 oz. of wine, or 1.5 oz. of liquor.
Table 4 presents the results of multivariable analyses adjusted for age, sex, years of education, and serum Σ PCBs (lipid basis). Serum PFOS was positively associated with fT4 (β = 0.054, 95% Confidence Interval (CI) = 0.002, 0.106; p = 0.044) and T4 (β = 0.766, 95% CI = 0.327, 1.205; p = 0.001), which correspond to 4% and 9% increases in their respective means. A positive association was suggested between serum PFOS and TSH, although not of statistical significance (β = 0.129, 95% CI = -0.023, 0.281; p = 0.094). A similar association was suggested for serum PFOA and T4 (p = 0.097). When regression analyses were performed with both PFOS and PFOA in the same model, positive association between PFOS and T4 persisted whereas that between PFOS and fT4 was attenuated (data not shown).
Table 4. Final multivariable modelsa for thyroid function markers with serum PFASs (n = 87).
| Model | β | 95% LCI | 95% UCI | P-value |
|---|---|---|---|---|
| TSH (μIU/mL)b | ||||
| PFOS (ng/mL)b | 0.129 | -0.023 | 0.281 | 0.094 |
| PFOA (ng/mL)b | 0.102 | -0.047 | 0.250 | 0.176 |
| fT4 (ng/dL) | ||||
| PFOS (ng/mL)b | 0.054 | 0.002 | 0.106 | 0.044 |
| PFOA (ng/mL)b | 0.016 | -0.036 | 0.069 | 0.536 |
| T4 (μg/dL) | ||||
| PFOS (ng/mL)b | 0.766 | 0.327 | 1.205 | 0.001 |
| PFOA (ng/mL)b | 0.380 | -0.070 | 0.830 | 0.097 |
| T3 (ng/dL) | ||||
| PFOS (ng/mL)b | 2.631 | -2.248 | 7.510 | 0.287 |
| PFOA (ng/mL)b | 3.032 | -1.725 | 7.789 | 0.208 |
Abbreviations: fT4, Free Thyroxine; LCI, Lower Confidence Interval; PCBs, Polychlorinated Biphenyls; PFASs, Perfluoroalkyl Substances; PFOA, Perfluorooctanoic Acid; PFOS, Perfluorooctane Sulfonate; T3, Total Triiodothyronine; T4, Total Thyroxine; TSH, Thyroid Stimulating Hormone; UCI, Upper Confidence Interval.
Adjusted for age, sex, years of education, and serum Σ PCBs (ng/g serum total lipids).
Log transformed.
We detected statistical interactions between age and PFOA for effects on fT4 and T4 (p < 0.05 for product terms, Table 5). For example, for T4, the joint increase in age and PFOA was associated with approximately 7% increase in T4, which is greater than the sum of the individual effects of PFOA and age, suggesting that increasing age may potentiate the effects of PFOA on T4. Figure 3 presents the association between PFOA and T4 stratified by median value of age which shows increasing slope for > 63 years and no effect ≤ 63 years. Corresponding increases in T4 due to PFOA were 12% for the older and 0.7% for the younger groups (Supplemental Table 1). The joint effect of PFOA and age on fT4 corresponded to approximately 3% increase in the hormone level.
Table 5. Individual and joint effectsa of PFOA (ng/mL)b and age on thyroid hormones (n = 87).
| PFOA effect (95% CI)c | Age effect (95% CI)d | Joint effect (95% CI)e | P-valuef | |
|---|---|---|---|---|
| TSH | ||||
| (μIU/mL)b | 0.032 (-0.148, 0.212) | 0.107 (-0.107, 0.321) | 0.293 (0.035, 0.551) | 0.225 |
| fT4 (ng/dL) | -0.024 (-0.086, 0.038) | -0.034 (-0.108, 0.041) | 0.031 (-0.058, 0.121) | 0.043 |
| T4 (μg/dL) | 0.005 (-0.530, 0.540) | -0.210 (-0.847, 0.428) | 0.618 (-0.150, 1.387) | 0.029 |
| T3 (ng/dL) | 1.557 (-4.233, 7.346) | -3.301 (-10.198, 3.596) | 1.496 (-6.819, 9.812) | 0.427 |
Abbreviations: CI, Confidence Interval; fT4, Free Thyroxine; Σ PCBs, Total Polychlorinated Biphenyls; PFOA, Perfluorooctanoic Acid; T3, Total Triiodothyronine; T4, Total Thyroxine; TSH, Thyroid Stimulating Hormone
Adjusted for sex, years of education, and serum Σ PCBs (ng/g serum total lipids).
Log transformed.
PFOA effect = Change in thyroid hormone level per interquartile range increase in PFOA concentrations among the individuals with age at the first quartile.
Age effect = Change in thyroid hormone level per interquartile range increase in age among the individuals with PFOA concentrations at the first quartile.
Joint effect = Change in thyroid hormone level score per interquartile increases in both PFOA concentrations and age.
P-value for theproduct term between PFOA and age.
Figure 3.
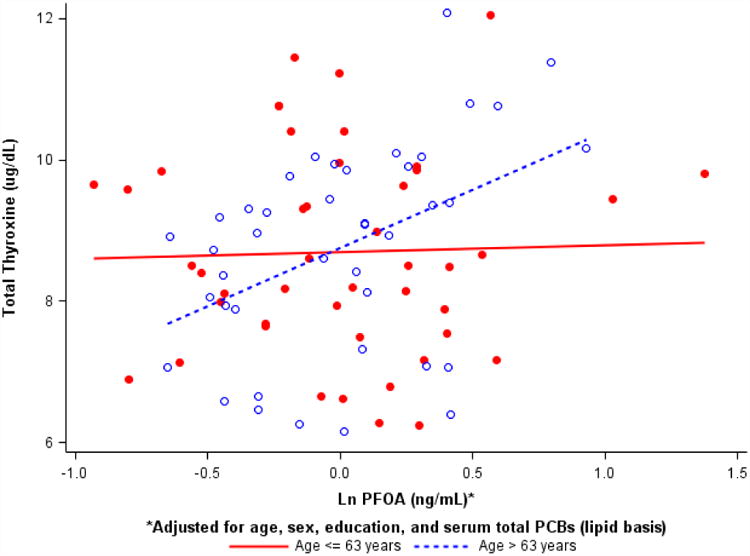
Associations between total thyroxine (μg/dL) and PFOA (ng/mL, log-transformed) stratified by age ≤ median value of 63 years (n = 87).
We also detected a sub-additive interaction between PFOS and Σ PCBs (p for a product term = 0.02) on T3 (PFOS effect = 5.145, 95% CI = 0.179, 10.111; Σ PCB effect = 2.179, 95% CI = -2.787, 7.144; and joint effect = 0.581, 95% CI = -4.668, 5.831), suggesting antagonism. The individual effects of PFOS and Σ PCBs corresponded to 4.1% and 1.7% increases in T3; however, the joint effect indicated a negligible change in T3 (i.e., 0.46%). Antagonistic effects also were detected for each PCB category – however, unlike Σ PCBs, these joint effects indicated marginal decreases in T3 (Supplemental Table 2). We also detected statistical interaction between PFOA and Σ PBDEs (p for a product term = 0.07) for the effect on TSH (PFOA effect = 0.197, 95% CI = 0.024, 0.370; Σ PBDE effect = 0.062, 95% CI = -0.096, 0.220; and joint effect = 0.093, 95% CI= -0.104, 0.291); here, the joint effect of PFOA and Σ PBDEs was smaller than the individual effect of PFOA suggesting antagonistic effect by Σ PBDEs. No interactions were detected for PFASs with sex and serum DDE+DDT, and between PFOS and PFOA.
We repeated the analyses using serum total lipids and Σ PCBs (wet weight basis) as covariates; the results were similar to those with PCBs expressed on a lipid basis (data not shown) except that p for interactions between PFOS and Σ PCBs (p = 0.07) and between PFOA and Σ PBDEs (p = 0.09) were slightly inflated. We repeated the analyses including 13 women using hormone replacement therapy (n =100). The only substantive difference detected was for the association between PFOS and T3; the association was positive and statistically significant (β = 5.154, 95% CI = 0.349, 9.960; p = 0.036).
5. Discussion
In the current study of older residents of the upper Hudson River communities, we detected positive associations between serum PFOS and fT4 and T4. We detected statistical interactions between serum PFOA and age for effects on fT4 and T4; however, the joint exposures accounted for subtle increases in levels of these hormones. We also detected statistical interactions between PFOS and Σ PCBs on T3, and between PFOA and Σ PBDEs on TSH; the concurrent exposure to PCBs and PBDEs attenuated the effects of PFOS and PFOA respectively.
This is one of few studies to focus on associations in an aging population. Although prior studies have included aging individuals in their analyses, only one elaborated associations in an aging group (Knox et al., 2011), and our results complement those findings. In men and women aged > 50 years who were exposed to background levels of PFOS (means = 25.7 ng/mL and 29.1 ng/mL for women and men respectively) and high levels of PFOA (means = 98.6 ng/mL and 124.3 ng/mL for women and men respectively), PFOS and PFOA were positively associated with T4 (Knox et al., 2011). However, Knox et al. did not measure fT4 and T3. In the same population as studied by Knox et al. (aged > 20 years), higher PFOA was associated with increased risks of hyperthyroidism among men and hypothyroidism among women (Winquist and Steenland, 2014); however, no age-dependent associations were reported.
Additional studies that examined associations between PFASs and thyroid function reported varying results. For example, serum PFOS and PFOA were not associated with fT4 and T4, and positively associated with T3 in occupational groups from two 3M Company facilities that used PFOA salt, exposing individuals to very high levels (Olsen et al., 2003). When these occupational data were reanalyzed including workers from a third facility (n = 506), serum PFOA was negatively associated with fT4 (Olsen and Zobel, 2007). However, the authors considered the associations to be clinically irrelevant. At PFAS levels lower than in our study (GMs = 14.2 ng/mL for PFOS and 4.15 ng/mL for PFOA), significant positive association between serum PFOA and T3 was detected among 502 American women ≥ 20 years of age (Wen et al., 2013). In a study of 623 Inuits, aged 18 to 73 years and residing in Nunavik, Canada, serum PFOS (GM = 18.28 ng/mL) was positively associated with fT4 and negatively associated with T3 and TSH (Dallaire et al. 2009). Other studies that focused on background levels of the chemicals reported no association with fT4 (Bloom et al., 2010; Ji et al., 2012). No association was reported for TSH by most of studies (Bloom et al., 2010; Olsen et al., 2003; Olsen and Zobel, 2007; Wen et al., 2013). Due to the fact that our analysis exclusively focused on aging men and women, and that women were mostly postmenopausal, our results may not be directly comparable to these prior studies.
In the current study, we detected main effects only for fT4 and T4, but we did not detect main effects for TSH. Increases in fT4 and T4, without a concurrent change in TSH, as detected in our study, may reflect alterations in levels of TBPs (Bloom et al., 2014). Generally, we expect that the negative feedback of increase in thyroxine levels would inhibit pituitary TSH secretion. However, it is possible that peripheral deiodination of excess thyroid hormones might restore homeostasis without influencing the HPT axis, and/or subtle increases in fT4 and T4 brought about by the current PFAS ranges might not be sufficient to suppress TSH secretion. We also detected PFOA-associated elevations in fT4 and T4 increased with age. The literature suggests that levels of TBPs as well as their binding capacity may change in older adults (Benvenga, 2012; Braverman et al., 1966), which could be one of the possible explanations for the detected age-specific associations. For instance, levels of transthyretin and albumin decrease with increasing age in elderly (Benvenga, 2012). In addition, the thyroid hormone binding capacity of thyroxine-binding globulin (TBG) is higher in older and younger people compared to the middle-aged people, and that of transthyretin is lower in younger and older people compared to the middle-aged people (Braverman et al., 1966).
Mechanisms by which PFASs may influence thyroid hormones, including interference with TBPs, are not well-understood. PFAS-influenced increases in hepatic TBP synthesis has also been implicated for higher total thyroid hormone concentrations (Knox et al., 2011). Results from a few human studies that examined associations between PFASs and measures of TBPs, including TBG, T3 uptake, and albumin, were not consistent (Dallaire et al., 2009; Knox et al., 2011; Olsen et al., 2003). In contrast to our findings, PFASs have also been shown to inhibit thyroperoxidase activity in vitro (Song et al., 2012), which may lead to decreased thyroid hormone secretion (Thalmann and Meier, 2012).
In addition to main effects for fT4 and T4, ours is the first study to report interactions between PFASs and other POPs on thyroid function. We detected antagonistic interaction between PFOS and all PCB groupings for the effect on T3. These antagonistic effects were strongest for the thyroid active congeners and weakest for Σ PCBs. While it may be a chance occurrence, we suspect that this difference may be due to an obscuring of the effect resulting from the inclusion of biologically impertinent PCB congeners in Σ PCBs. Prior literature indicates that PCBs decrease serum thyroid hormones through several mechanisms that influence hormone synthesis, and metabolism and transport proteins (Boas et al., 2012; Liu et al., 2012), which could explain the antagonistic effect we observed. Similarly, interaction between PFOA and Σ PBDEs on TSH could be ascribed to potential involvement of serum TBPs (Boas et al., 2012; Meerts et al., 2000; Ren and Guo, 2012).
Experiments in rats suggested that observed associations between PFOS and fT4 might be an experimental artifact. Specifically, reduced fT4 observed in animal studies following PFOS exposure may result from a negative bias induced by analog hormone assays used in the presence of compounds that interfere with binding to serum protein analog, including PFOS (Chang et al., 2007; Chang et al., 2008). However, more recent work in a human population did not report a negative fT4 bias in the presence of PFOS and PFOA at levels in a similar to and higher than ours, respectively (Lopez-Espinosa et al., 2012b). When fT4 was determined using the gold-standard equilibrium dialysis-radioimmunoassay method, transient increase in fT4 and decrease in TSH concentrations, and subsequent steady decrease in T4 and T3 concentrations following exposure to PFOS was reported (Chang et al., 2008). The subsequent reductions in T4 and T3 concentrations, which contradict our findings, were hypothesized to be due to increased tissue uptake and turnover of thyroid hormones. Yet, the study animals had mean serum PFOS levels more than 1000 times greater than those measured in our study participants. Additionally, rodent and human thyroid physiology differ in several ways, which include longer half-lives of thyroid hormones in humans, and different concentrations and binding capacities of TBPs across species (Jahnke et al., 2004). Thus, we speculate that a single administered high dose of PFOS to rats increases serum fT4 in a manner sufficient to prompt negative pituitary feedback, reducing TSH, and increasing the turnover rate for these short-lived thyroid hormones. However, the comparatively low doses of PFOS experienced by our study participants may manifest only as a modest increase in fT4 insufficient to prompt negative anterior pituitary feedback.
Levels of PFOS and PFOA in the current study were 1.4 and 2.5 fold higher than those reported for the U.S. population of the same age ranges during 2003-2004 (Kato et al., 2011). Given that serum PFOS was positively correlated with serum Σ PCBs and the current study population lived near a PCB-contaminated site, we could speculate that elevated levels may be due to common environmental or occupational sources in addition to the typical sources of PFASs.
The current study was performed in aging individuals without a history of overt thyroid disease and clinical neuropsychological conditions. It is possible that the detected effects have meaningful impact on neurocognitive well-being at the population level, particularly among hyperthyroid individuals or those individuals at the upper ends of thyroid hormone distributions (Bauer et al., 2012; Winquist and Steenland, 2014).
Our results should be carefully interpreted due to several limitations. Due to cross-sectional nature of the study, we are unable to determine the temporal order of PFAS exposure and thyroid hormone change, which prevent us from making etiological inferences. We did not have information on serum albumin and TBG which would have provided additional information on detected associations. In addition, multiple statistical comparisons were made, which increased the probability for chance findings due to inflation of the Type I error rate. Our small sample size limited the analysis in several ways; for instance, we were unable to assess the joint effects of age and sex on thyroid hormones, and we were unable to examine associations with clinical thyroid disorders. Additionally, we used archived sera to estimate thyroid biomarkers and PFASs, raising the possibility for sample degradation over time. However, stability of thyroid hormones following 3-5 years of storage at -20°C, and that of the PFASs following 6-8 years of storage is not likely to be of concern (Mannisto et al., 2007; Martin et al., 2004).
The selection of the 87 participants was based on serum availability for PFAS and thyroid hormone determinations, thyroid disease status, and use of sex hormones and thyroid medications. Compared to excluded participants, a higher percentage reported alcohol consumption (90% vs. 74%) whereas the proportion of women was smaller (41% vs. 54%); this is likely to be chance given random nature of exclusion. Differential selection by alcohol consumption status is less likely to affect validity of the findings, given that PFASs are unlikely to be affected by alcohol consumption. The difference in sex composition was due to exclusion of women using sex hormone replacement therapy.
6. Conclusions
In general, higher serum PFAS levels were associated with increments in fT4 and T4. Changes in levels of thyroid hormones associated with the ranges of PFASs exposures seem to be small but may still have substantial impact on cognitive function and neurobehavior. The associated public health impact may be significant given the heightened risk of thyroid diseases in elderly (Peeters, 2008), ubiquity of PFASs (Kato et al., 2011), and rapidly growing aging populations (U.S. Administration on Aging, 2012). In addition, our results also suggest that the associations of PFASs and thyroid hormones may vary by age and levels of PCBs and PBDEs.
Overall results may be helpful in shedding light on the associations between other shorter chained PFASs and thyroid hormones which are still being produced and understudied. Further studies, both epidemiologic and toxicological, that incorporate comprehensive sets of thyroid function end points and TBPs are warranted to support the findings of this study and to elucidate possible mechanisms involved.
Supplementary Material
Highlights.
Associations between PFASs and thyroid hormones were assessed in older adults.
Free and total thyroxine were positively associated with serum PFOS.
Age, PCBs, and PBDEs may modify the associations of PFASs with thyroid hormones.
Acknowledgments
This work was supported in part by grants provided by the National Institute on Aging (Grant # R15/AG0333700A1) and the Agency for Toxic Substances and Disease Registry (Grant # H75/ATH298312).
Footnotes
Conflict of Interest: The authors declare that they have no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR. Toxicological Profile for Perfluoroalkyls. Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2009. [PubMed] [Google Scholar]
- Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. http://dx.doi.org/10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Samuels MH, Whybrow PC. Behavioral and psychiatric aspects of thyrotoxicosis. In: Braverman LE, Cooper DS, editors. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. 10th. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. pp. 475–480. [Google Scholar]
- Benvenga S. Thyroid hormone transport proteins and the physiology of hormone binding. In: Braverman LE, Cooper DS, editors. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. 10th. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. pp. 92–101. [Google Scholar]
- Bloom MS, Kannan K, Spliethoff HM, Tao L, Aldous KM, Vena JE. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol Behav. 2010;99:240–245. doi: 10.1016/j.physbeh.2009.02.005. http://dx.doi.org/10.1016/j.physbeh.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Jansing RL, Kannan K, Rej R, Fitzgerald EF. Thyroid hormones are associated with exposure to persistent organic pollutants in aging residents of upper Hudson River communities. Int J Hyg Environ Health. 2014;217:473–482. doi: 10.1016/j.ijheh.2013.09.003. http://dx.doi.org/10.1016/j.ijheh.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cellular Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. http://dx.doi.org/10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Braverman LW, Dawber NA, Ingbar SH. Observations concerning the binding of thyroid hormones in sera of normal subjects of varying ages. J Clin Invest. 1966;45:1273–1279. doi: 10.1172/JCI105434. http://dx.doi.org/10.1172/jci105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. Fourth national report on human exposure to environmental chemicals. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Environmental Health; 2009. [Google Scholar]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, et al. Negative bias from analog methods used in the analysis of free thyroxine in ratserum containing perfluorooctanesulfonate (PFOS) Toxicology. 2007;234:21–33. doi: 10.1016/j.tox.2007.01.020. http://dx.doi.org/10.1016/j.tox.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, et al. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS) Toxicology. 2008;243:330–339. doi: 10.1016/j.tox.2007.10.014. http://dx.doi.org/10.1016/j.tox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Chauhan KR, Kodavanti PR, McKinney JD. Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transportprotein. Toxicol Appl Pharmacol. 2000;162:10–21. doi: 10.1006/taap.1999.8826. http://dx.doi.org/10.1006/taap.1999.8826. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Sato T, Buchanan DL. Disruption of steroid hormone signaling by PCBs. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: University Press of Kentucky; 2001. pp. 257–263. [Google Scholar]
- Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect. 2009;117:1380–1386. doi: 10.1289/ehp.0900633. http://dx.doi.org/10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Stern RA, Flashman LA. Cognitive and neuropsychiatric aspects of subclinical hypothyroidism: significance in the elderly. Curr Psychiatry Rep. 2003;5:384–390. doi: 10.1007/s11920-003-0073-6. http://dx.doi.org/10.1007/s11920-003-0073-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Hwang SA, Jansing RL, Hicks HE. Environmental exposures to polychlorinated biphenyls (PCBs) among older residents of upper Hudson River communities. Environ Res. 2007;104:352–360. doi: 10.1016/j.envres.2007.01.010. http://dx.doi.org/10.1016/j.envres.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, et al. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ Health Perspect. 2008;116:209–215. doi: 10.1289/ehp.10432. http://dx.doi.org/10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Fletcher BA, Belanger E, Tao L, Kannan K, Hwang SA. Fish consumption and concentrations of polybrominated diphenyl ethers (PBDEs) in the serum of older residents of upper Hudson River communities. Arch Environ Occup Health. 2010;65:183–190. doi: 10.1080/19338241003730929. http://dx.doi.org/10.1080/19338241003730929. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Shrestha S, Gomez MI, McCaffrey RJ, Zimmerman EA, Kannan K, et al. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and neuropsychological status among older adults in New York. Neurotoxicology. 2012;33:8–15. doi: 10.1016/j.neuro.2011.10.011. http://dx.doi.org/10.1016/j.neuro.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Geller AM, Zenick H. Aging and the environment: a research framework. Environ Health Perspect. 2005;113:1257–1262. doi: 10.1289/ehp.7569. http://dx.doi.org/10.1289/ehp.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. http://dx.doi.org/10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. Renal elimination of perfluorocarboxylates (PFCAs) Chem Res Toxicol. 2012;25:35–46. doi: 10.1021/tx200363w. http://dx.doi.org/10.1021/tx200363w. [DOI] [PubMed] [Google Scholar]
- Jahnke GD, Choksi NY, Moore JA, Shelby MD. Thyroid toxicants: assessing reproductive health effects. Environ Health Perspect. 2004;112:363–368. doi: 10.1289/ehp.6637. http://dx.doi.org/10.1289/ehp.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Kim S, Kho Y, Paek D, Sakong J, Ha J, et al. Serum concentrations of major perfluorinated compounds among the general population in Korea: dietary sources and potential impact on thyroid hormones. Environ Int. 2012;45:78–85. doi: 10.1016/j.envint.2012.03.007. http://dx.doi.org/10.1016/j.envint.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem. 2003;22:2639–2649. doi: 10.1897/02-553. http://dx.doi.org/10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. http://dx.doi.org/10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ Sci Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. http://dx.doi.org/10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. http://dx.doi.org/10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE, Nizam A. In: Applied Regression Analysis and Other Multivariable Methods. Kleinbaum DG, Kupper LL, Muller KE, Nizam A, editors. Pacific Grove, CA: Duxbury Press; 1998. pp. 212–280. [Google Scholar]
- Knol MJ, Egger M, Scott P, Geerlings MI, Vandenbroucke JP. When one depends on the other: reporting of interaction in case-control and cohort studies. Epidemiology. 2009;20:161–166. doi: 10.1097/EDE.0b013e31818f6651. http://dx.doi.org/10.1097/EDE.0b013e31818f6651. [DOI] [PubMed] [Google Scholar]
- Knox SS, Jackson T, Frisbee SJ, Javins B, Ducatman AM. Perfluorocarbon exposure, gender and thyroid function in the C8 Health Project. J Toxicol Sci. 2011;36:403–410. doi: 10.2131/jts.36.403. http://dx.doi.org/10.2131/jts.36.403. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang C, Yan M, Quan C, Zhou J, Yang K. PCB153 disrupts thyroid hormone homeostasis by affecting its biosynthesis, biotransformation, feedback regulation, and metabolism. Horm Metab Res. 2012;44:662–669. doi: 10.1055/s-0032-1311569. http://dx.doi.org/10.1055/s-0032-1311569. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect. 2012a;120:1036–1041. doi: 10.1289/ehp.1104370. http://dx.doi.org/10.1289/ehp.1104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Fitz-Simon N, Bloom MS, Calafat AM, Fletcher T. Comparison between free serum thyroxine levels, measured by analog and dialysis methods, in the presence of perfluorooctane sulfonate and perfluorooctanoate. Reprod Toxicol. 2012b;33:552–555. doi: 10.1016/j.reprotox.2011.04.002. http://dx.doi.org/10.1016/j.reprotox.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Mannisto T, Surcel HM, Bloigu A, Ruokonen A, Hartikainen AL, Jarvelin MR, et al. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem. 2007;53:1986–1987. doi: 10.1373/clinchem.2007.091371. http://dx.doi.org/10.1373/clinchem.2007.091371. [DOI] [PubMed] [Google Scholar]
- Martin JW, Kannan K, Berger U, de Voogt P, Field J, Franklin J, et al. Analytical challenges hamper perfluoroalkyl research. Environ Sci Technol. 2004;38:248A–255A. doi: 10.1021/es0405528. http://dx.doi.org/10.1021/es0405528. [DOI] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. http://dx.doi.org/10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, Mandel JH. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med. 2003;45:260–270. doi: 10.1097/01.jom.0000052958.59271.10. http://dx.doi.org/10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health. 2007;81:231–246. doi: 10.1007/s00420-007-0213-0. http://dx.doi.org/10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- Peeters RP. Thyroid hormones and aging. Hormones. 2008;7:28–35. doi: 10.14310/horm.2002.1111035. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated Hydrocarbon Levels in Human Serum: Effects of Fasting and Feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. http://dx.doi.org/10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. http://dx.doi.org/10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Ren XM, Guo LH. Assessment of the binding of hydroxylated polybrominated diphenyl ethers to thyroid hormone transport proteins using a site-specific fluorescence probe. Environ Sci Technol. 2012;46:4633–4640. doi: 10.1021/es2046074. http://dx.doi.org/10.1021/es2046074. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. http://dx.doi.org/10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. Am J Epidemiol. 2006;163:374–383. doi: 10.1093/aje/kwj039. http://dx.doi.org/10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Kim YJ, Park YK, Ryu JC. Changes in thyroid peroxidase activity in response to various chemicals. J Environ Monit. 2012;14:2121–2126. doi: 10.1039/c2em30106g. http://dx.doi.org/10.1039/c2em30106g. [DOI] [PubMed] [Google Scholar]
- Tahboub R, Arafah BM. Sex steroids and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:769–780. doi: 10.1016/j.beem.2009.06.005. http://dx.doi.org/10.1016/j.beem.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Thalmann S, Meier CA. Effects of drugs on TSH secretion, thyroid hormones absorption, synthesis, metabolism, and action. In: Braverman LE, Cooper DS, editors. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. 10th. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. pp. 187–202. [Google Scholar]
- U.S. EPA. [accessed 11/25 2013];Hudson River PCBs Superfund site: Working Together to Cleanup a Historic Region. 2011 Available: http://www.epa.gov/superfund/accomp/success/hudson.htm.
- U.S. Administration on Aging. [accessed 6/13 2013];A Profile of Older Americans: 2012. 2012 Available: http://www.aoa.gov/Aging_Statistics/Profile/2012/docs/2012profile.pdf.
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. http://dx.doi.org/10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol Sci. 2009;109:206–216. doi: 10.1093/toxsci/kfp055. http://dx.doi.org/10.1093/toxsci/kfp055. [DOI] [PubMed] [Google Scholar]
- Wen LL, Lin LY, Su TC, Chen PC, Lin CY. Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007-2010. J Clin Endocrinol Metab. 2013;98:E1456–1464. doi: 10.1210/jc.2013-1282. http://dx.doi.org/10.1210/jc.2013-1282. [DOI] [PubMed] [Google Scholar]
- Winquist A, Steenland K. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology. 2014;25:255–264. doi: 10.1097/EDE.0000000000000040. http://dx.doi.org/10.1097/EDE.0000000000000040. [DOI] [PubMed] [Google Scholar]
- Wigestrand MB, Stenberg M, Walaas SI, Fonnum F, Andersson PL. Non-dioxin-like PCBs inhibit [(3)H]WIN-35,428 binding to the dopamine transporter: a structure-activity relationship study. Neurotoxicology. 2013;39:18–24. doi: 10.1016/j.neuro.2013.07.005. http://dx.doi.org/10.1016/j.neuro.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105:13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM, Brent GA. Genomic and nongenomic actions of thyroid hormones. In: Braverman LE, Cooper DS, editors. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. 10th. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. pp. 127–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


