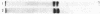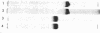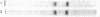Abstract
Two kinds of tubulin (α and β) have been described in microtubules from many different systems. In this study a discontinuous acrylamide-gel system containing sodium dodecyl sulfate was used to separate milligram quantities of α- and β-tubulin from microtubules of chick-embryo brain and from outer doublets of sea-urchin sperm. The isolated tubulins were characterized by peptide mapping and automated sequencing of the first 25 NH2-terminal amino acids. Our results show that α- and β-tubulin are related but distinctly different proteins and that each one has been highly conserved in the course of evolution.
Keywords: Strongylocentrotus, gel electrophoresis, protein sequencing, evolution
Full text
PDF
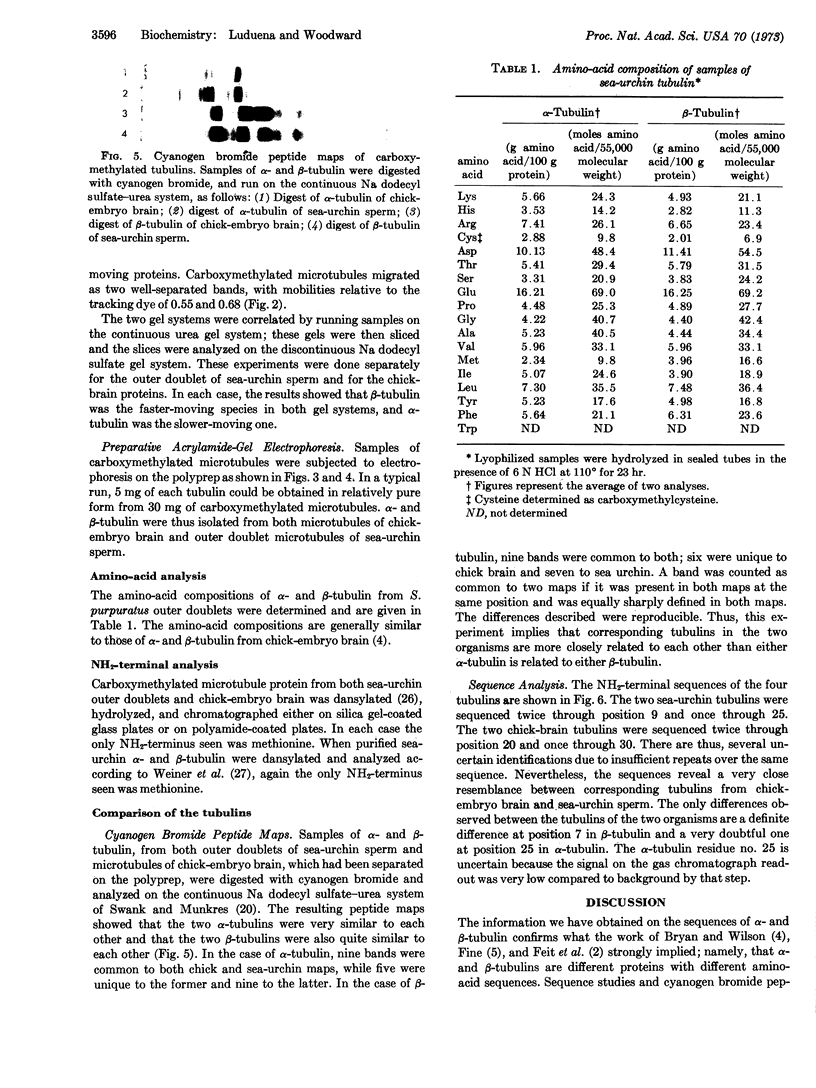


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brenner R. M. The formation of basal bodies (centrioles) in the Rhesus monkey oviduct. J Cell Biol. 1971 Jul;50(1):10–34. doi: 10.1083/jcb.50.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dales S. Concerning the universality of a microtubule antigen in animal cells. J Cell Biol. 1972 Mar;52(3):748–754. doi: 10.1083/jcb.52.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart L. P., Jr Heterogeneity of microtubule proteins from Tetrahymena cilia. J Mol Biol. 1971 Nov 14;61(3):745–748. doi: 10.1016/0022-2836(71)90078-7. [DOI] [PubMed] [Google Scholar]
- Feit H., Slusarek L., Shelanski M. L. Heterogeneity of tubulin subunits. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2028–2031. doi: 10.1073/pnas.68.9.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R. E. Heterogeneity of tubulin. Nat New Biol. 1971 Oct 27;233(43):283–284. doi: 10.1038/newbio233283a0. [DOI] [PubMed] [Google Scholar]
- Fulton C., Kane R. E., Stephens R. E. Serological similarity of flagellar and mitotic microtubules. J Cell Biol. 1971 Sep;50(3):762–773. doi: 10.1083/jcb.50.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Rasmussen H., DiBella F., Guthrow C. E., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- Kapadia G., Chrambach A. Recovery of protein in preparative polyacrylamide gel electrophoresis. Anal Biochem. 1972 Jul;48(1):90–102. doi: 10.1016/0003-2697(72)90173-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murray A. W., Froscio M. Cyclic adenosine 3':5'-monophosphate and microtubule function: specific interaction of the phosphorylated protein subunits with a soluble brain component. Biochem Biophys Res Commun. 1971 Sep;44(5):1089–1095. doi: 10.1016/s0006-291x(71)80197-3. [DOI] [PubMed] [Google Scholar]
- Nagayama A., Dales S. Rapid purification and the immunological specificity of mammalian microtubular paracrystals possessing an ATPase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):464–471. doi: 10.1073/pnas.66.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- Satir P., Satir B. A model for ninefold symmetry in alpha keratin and cilia. J Theor Biol. 1964 Jul;7(1):123–128. doi: 10.1016/0022-5193(64)90045-1. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Taylor E. W. Isolation of a protein subunit from microtubules. J Cell Biol. 1967 Aug;34(2):549–554. doi: 10.1083/jcb.34.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E. Thermal fractionation of outer fiber doublet microtubules into A- and B-subfiber components. A- and B-tubulin. J Mol Biol. 1970 Feb 14;47(3):353–363. doi: 10.1016/0022-2836(70)90307-4. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Yang S., Criddle R. S. In vitro biosynthesis of membrane proteins in isolated mitochondria from Saccharomyces carlsbergensis. Biochemistry. 1970 Jul 21;9(15):3063–3072. doi: 10.1021/bi00817a020. [DOI] [PubMed] [Google Scholar]