Abstract
Many pathogenic enveloped viruses, including HIV-1, escape complement-mediated virolysis by incorporating host cell regulators of complement activation into their own viral envelope. The presence of complement regulators including CD59 on the external surface of the viral envelope confers resistance to complement-mediated virolysis, which may explain why human pathogenic viruses such as HIV-1 are not neutralized by complement in human fluids, even in the presence of high Ab titers against the viral surface proteins. In this study, we report the development of a recombinant form of the fourth domain of the bacterial toxin intermedilysin (the recombinant domain 4 of intermedilysin [rILYd4]), a 114 aa protein that inhibits human CD59 function with high affinity and specificity. In the presence of rILYd4, HIV-1 virions derived from either cell lines or peripheral blood mononuclear cells of HIV-1–infected patients became highly sensitive to complement-mediated lysis activated by either anti–HIV-1 gp120 Abs or by viral infection-induced Abs present in the plasma of HIV-1–infected individuals. We also demonstrated that rILYd4 together with serum or plasma from HIV-1–infected patients as a source of anti–HIV-1 Abs and complement did not mediate complement-mediated lysis of either erythrocytes or peripheral blood mononuclear cells. These results indicate that rILYd4 may represent a novel therapeutic agent against HIV-1/AIDS
The complement system, a main effector of innate and acquired immunity, has the capacity to lyse and thereby inactivate pathogenic microorganisms, including enveloped viruses in the circulation of infected hosts. Complement-mediated lysis of pathogenic microorganisms including bacteria, viruses, yeasts and of cells infected by these pathogens is mediated by formation of a transmembrane pore, the membrane attack complex (MAC), which is formed by C9 polymerization triggered by activation of the complement cascades. Mammalian cells are protected against the devastating effect of complement activation and MAC formation by an array of complement regulators, including several membrane proteins such as CD55 and CD59. CD59 is a GPI-linked complement regulatory protein, which specifically inhibits MAC formation and is universally expressed on the surface of mammalian cells (1, 2). It is well established that many pathogenic enveloped viruses, including HIV-1, CMV, herpes virus, Ebola virus, and influenza virus, escape complement-mediated virolysis by incorporating host cell complement-regulatory proteins into their own viral envelope (3–9). The presence of complement regulators such as CD59 on the external surface of the viral envelope confers resistance to Ab-dependent, complement-mediated lysis. This resistance to the lysis provides a likely explanation for the evidence that certain human pathogenic viruses are not neutralized by complement in human fluids even when they induce a strong Ab response. In the specific case of HIV-1, sera from patients with HIV-1 infection contain anti–HIV-1 envelope Abs, but these Abs fail to induce complement-mediated virolysis of HIV-1 virions and cytolysis of the virus-infected cells (3, 10, 11). This protection against complement-mediated lysis is conferred by the presence of CD59 in either the HIV-1 envelope or the membrane of the infected cells (10). Deficiency or inhibition of CD59 in the surface of either the viral envelope or the infected cell membrane sensitizes them to the lytic effect of complement (3, 10, 12).
HIV-1 infection leading to AIDS is still a major public health challenge (13). Current treatment (highly active antiretroviral therapy [HAART]) can successfully control plasma levels of HIV-1 RNA below the limits of detection, but cannot eliminate infected cells and trace levels of free virions. If HAART is discontinued because of serious adverse effects or becomes ineffective because of development of drug resistance, HIV-1 contained in stable reservoirs rapidly rebounds and disease progression resumes (14). Further complicating problem of HIV-1 treatment and prevention is the fact that several anti–HIV-1 vaccine candidates have failed to show significant clinical efficacy, although they induced vigorous Ab responses (14). For these reasons, a therapeutic inhibitor of CD59 that would sensitize HIV-1 virions or HIV-infected cells to the lytic effect of complement has been actively sought by us and others (11).
In this study, we report the development of rILYd4, a high-affinity specific inhibitor of human CD59 (hCD59). rILYd4 is the recombinant form of the 114 aa domain 4 (D4) of intermedilysin (ILY), a cell lytic toxin secreted by Streptococcus intermedius. ILY is a pore-forming toxin that exclusively lyses human cells, because it binds with high affinity and specificity to hCD59 but not to CD59 from other species (15, 16). Binding of ILY to hCD59 occurs through D4, whereas the three other domains (domains 1, 2, and 3) of ILY form the lytic transmembrane pore (15). Because D4 of ILY binds to a region of hCD59 to encompass its active site (aa 42–58) (15, 17), we reasoned that rILYd4 would inhibit hCD59 function (2) and thereby enhance Ab-dependent complement-mediated virolysis of HIV-1. Our results show that rILYd4 potently enhances complement-mediated HIV-1 virolysis activated by anti–HIV-1-specific Abs with no or minimal bystander effects. We conclude that rILYd4 has strong potential as an anti–HIV-1 therapeutic agent, a notion that warrants further testing in animal studies and in human clinical trials.
Materials and Methods
Preparation of rILY
For the generation of a truncated rILYd4 fragment, sequences encoding the fragments were cloned into an expression vector pTrcHis A with a HisX6 tag. The 114 aa rILYd4 fragment contains only D4 of ILY: GALTLNHDGAFVARFYVYWEELGHDADGYETIRSRSWSGNGYNRGAHYSTTLRFKGNVRNIRVKVLGATGLAWEPWRLIYSKNDLPLVPQRNISTWGTTLHPQFEDKVVKDNTD. His-tagged rILYd4 was expressed in Escherichia coli and purified with the His•Bind purification kit (Novagen, San Diego, CA) as described previously (15).
FACS analysis
Erythrocytes from human and hCD59RBC transgenic mouse expressing hCD59 only on the mouse erythrocytes published previously (16) were preincubated with rILYd4 (1 µg/ml) or PBS for 10 min at room temperature, incubated with mouse anti-hCD59 monoclonal Ab (0.2 µg/ml; BRIC 229; IBGRL Office, Bristol, U.K.) at room temperature for 30 min, washed, and incubated with a FITC-conjugated corresponding secondary Ab. The cells were washed with PBS three times before analyzing the fluorescence intensity using a FACScan (Becton Dickinson, Franklin Lakes, NJ).
Hemolytic assay
Human or mouse blood was obtained by venipuncture into a syringe containing sodium citrate (105 mM) as an anticoagulant (blood:buffer = 9:1 v/v). The erythrocytes were washed four times by PBS, stored in Alsever’s solution at 4°C, and used for hemolytic assays, as described previously (16). The amount of hemoglobin released from lysed erythrocytes was determined by the absorbance of the supernatant at 414 nm, and the percent lysis was calculated as follows: [(experimental OD414 − blank OD414)/(total lysis OD414 − blank OD414)]×100. The total lysis sample was obtained by adding pure water to the erythrocyte pellet.
Complement-mediated lysis on human erythrocytes
The sensitivity of human erythrocytes to human complement-mediated lysis in the presence or absence of rILYd4 was assessed by two different methods: cobra venom factor (5 mg/L) lysis assay and anti-human erythrocyte Ab-sensitized erythrocyte method, as described previously (16). As the source of complement, we used human serum (HS; 50% v/v diluted in GVB++ [CompTech, Tyler, Texas]) controlled by heat-inactivated HS (50%, v/v diluted in GVB++). In both cases, hemoglobin in the supernatant of lysed erythrocytes was measured by the absorbance at 414 nm, and percent lysis was calculated as follows: % lysis = (test OD414 − blank OD414)/(total lysis OD414 − blank OD414)×100.
Complement-mediated cytolysis on human nuclear cells
Complement-mediated cytolysis of human nuclear cells was assessed by the Alamar blue assay (Serotec, Raleigh, NC) as described previously (18). Briefly, 5 × 104 human non-Hodgkin lymphoma RL cells purchased from the American Type Culture Collection (Manassas, VA) were suspended in 100 µl RPMI 1640 medium supplemented with 10% heat-inactivated FBS and plated in 96-well plates. After an additional 100 µl of medium containing different concentration of rILYd4, rituximab (2 µg/ml), and 10% HS as the source of complement was added to the wells, the cells were incubated for 4 h at 37°C. Next, 70 µl culture medium plus 30 µl Alamar blue solution (Serotec) was added to the wells and incubated at 37°C overnight. Cell lysis was assessed by reading the plates in an F-2000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan; excitation, 560 nm; emission, 590 nm). Percent cell lysis in each well was calculated as: (reading from the well without any treatment − reading from testing well)/(reading from the well without any treatment)×100. All pooled HS used as a source of complement in this study was purchased from Complement Technology (Tyler, TX).
Preparation of HIV-1 from HIV-1 chronically infected or permissive human cell lines
HIV-1 chronically infected U1 monocytic (hCD59-negative) and OM10 T-lymphocytic (hCD59-positive) human cell lines obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Germantown, MD) were used for preparation of HIV-1 virions. Cells were cultured in RPMI 1640 medium complemented with 10% heat-inactivated FCS (Hyclone, Logan, UT), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cell viability was assessed by the trypan blue dye exclusion assay. All media and reagents used were endotoxin-free, as demonstrated by the limulus amebocyte lysate assay, and all cell lines were negative for Mycoplasma contamination, as documented using the Gen-Probe Mycoplasma T. C. Rapid Detection System (Gen-Probe, San Diego, CA). To induce viral replication, infected cells (1 × 106 cells/ml) were stimulated with TNF-α (2 ng/ml) plus PMA (2 ng/ml). After 24 h of incubation at 37°C in 5% CO2, supernatants containing the virus were harvested and used as a source of HIV-1 virions. HIV-1 titers in cell culture supernatants were quantified by HIV-1 p24 ELISA Ag assay (PerkinElmer, Waltham, MA, or XpressBio, Thurmont, MD) as described below. All ELISA determinations were performed in duplicate. Human monocytic THP-1 and human T-lymphocytic H9 (HTB 176) cell lines were obtained from the American Type Culture Collection and used to propagate the HIV-1 virus. Cells were grown in RPMI 1640 medium, supplemented with 10% FCS and antibiotics, and infected with HIV-1. Ten days after infection, cells were washed three times with culture medium and resuspended in culture medium for an additional 24 h. The fresh supernatants were harvested and used as inoculates of HIV-1 virions. HIV-1 titers in cell culture supernatants were also quantified by HIV-1 p24 ELISA Ag assay (PerkinElmer or XpressBio). The lower limit of sensitivity of the assay for HIV-1 p24 was 26 pg/ml.
Preparation of six primary isolates of HIV-1 from HIV-1-infected patients
HIV-1 primary isolates were generated by coculture of PBMCs from HIV-1–infected and healthy donors, following methods described previously (19). PBMCs were prepared from heparinized peripheral blood donated by six HIV-1–seropositive patients naive for antiretroviral therapy (patients 1–6 in Table I) and by HIV-1–seronegative donors. PBMCs from seronegative and seropositive individuals were stimulated separately for 2 d with PHA (5 µg/ml) and cocultured at a 1:3 ratio in the presence of IL-2 (10 ng/ml) in complete RPMI 1640 medium (200 µl per well) in 96-well round-bottom plates. After 7 d of coculture, supernatants were harvested, aliquoted, and stored at −80°C as HIV-1 primary isolate stocks for virolysis assay.
Table I.
Profiles of HIV-1–infected patients
| Patient No. | Clinical Stage | CD4+ T Cell (count/µl) |
Plasma HIV-1 RNA (copies/ml) |
Treatment at Times of Study |
Duration HIV-1 Diagnosis (y) |
Duration of Antivirial Therapy (y) |
p24 (pg/ml) | Anti–HIV-1 Envelope Ab Titer |
|---|---|---|---|---|---|---|---|---|
| 1 | Chronic | 184 | <50 | HAART | 4 | 3 | Undetectable | 2000 |
| 2 | Acute | 319 | 82 | HAART | NA | NA | 58.9 | 10,000 |
| 3 | Chronic | 351 | 17,799 | HAART | 17 | 12 | 153.8 | 4000 |
| 4 | Chronic | 309 | <50 | HAART | NA | 10 | Undetectable | 10,000 |
| 5 | Acute | 721 | 5,586 | HAART | 1 | 1 | 82 | 8000 |
| 6 | Chronic | 674 | <50 | HAART | 16 | NA | Undetectable | 40,000 |
| 7 | Acute | 453 | 520,909 | HAART | 1 | 1 | 74.6 | 800 |
| 8 | Chronic | 522 | <50 | HAART | 3 | 2 | Undetectable | 800 |
| 9 | Acute | 71 | 160 | HAART | 1 | 1 | 38 | 10,000 |
| 10 | Chronic | 395 | <50 | HAART | 16 | 13 | Undetectable | 4000 |
| 11 | Chronic | 198 | 9,708 | HAART | 11 | NA | 15 | 1000 |
| 12 | Chronic | 824 | <50 | HAART | 10 | 8 | Undetectable | 8000 |
| 13 | Chronic | 670 | <50 | HAART | 9 | 7 | Undetectable | 10,000 |
| 14 | Chronic | 579 | 21,990 | HAART | 17 | 17 | 55.5 | 10,000 |
| 15 | Chronic | 296 | 2,588 | HAART | 13 | 13 | 25.6 | 8000 |
| 16 | Chronic | 218 | 152,000 | HAART | 10 | 10 | 129 | 10,000 |
| 17 | Chronic | 362 | 18,836 | HAART | 2 | 1 | 163.3 | 10,000 |
Anti-gp120/gp160 Ab activated complement-mediated virolysis
HIV-1 virions (20 µl containing 5 ng HIV-1 p24/ml) were preincubated for 30 min at 37°C with or without rILYd4 (20 µg/ml) before exposure to anti–HIV-1-specific Abs (anti–HIV-1 gp120 monoclonal Ab, IgG1B12, National Institutes of Health Reagent Program or gp120/gp160 polyclonal Abs; Abcam, Cambridge, MA) and to the pooled HS as a source of complement (1:10 dilution in GVB++ buffer). Heat-inactivated HS was used as a negative control. Virolysis of HIV-1 was quantified using HIV-1 ELISA Ag assay (PerkinElmer). The ELISA procedure was the same as the manufacturer’s description, except for the use of the lysis buffer. As a consequence, we measured only the p24 released from the lysed viral particles triggered by the Ab-dependent complement-mediated virolysis. The p24 in the core of the intact HIV-1 virions was not detected; therefore, p24 release served a parameter of virolysis. HIV-1 virions were treated with Triton X-100 for determination of total virolysis. The percentage of virolysis was calculated as follows: (p24 released in the presence of complement-competent serum − p24 released in the presence of heat inactivated serum) / (p24 released from Triton X-100 treated virions − p24 released by medium only) × 100%. Means ± SD of three experiments were compared using the paired two-tailed Student t test.
Complement-mediated virolysis activated by anti–HIV-1 Abs in plasmas of HIV-1-infected patients
Viral preparations (20 µl; 5 ng HIV-1 p24/ml) derived from the chronically-infected cell line OM10 or from primary HIV-1 isolates were preincubated for 30 min at 37°C with either rILYd4 (20 µg/ml) or neutralizing anti-hCD59 monoclonal Ab (30 µg/ml; BRIC229). After preincubation, heat-inactivated plasma from either HIV-1–infected or healthy individuals (1:5 at final dilution) were individually added as a source of endogenous Abs, followed by the exposure to either complement-competent or heat-inactivated HS diluted in GVB++ buffer. Triton X-100 was used for determining the total virolysis. Experiments were conducted in duplicates and the paired two-tailed Student’s t test was used to compare the means ± SD.
Infectivity assay
Five microliters of reaction mixture from each condition in the virolysis experiment described above (patients 1–4 in Fig. 3A, 3B) were added to fresh H9 cells (0.2 × 106 cells per well in 200 µl complete RPMI 1640 medium), and cultured for 7–10 d. The infectivity was then assessed by measuring HIV-1 p24 in the culture supernatant using HIV-1 p24 ELISA Antigen Assay (PerkinElmer). The lysis buffer included in the ELISA kit was used to lyse the viral particles for measuring HIV-1 core protein p24.
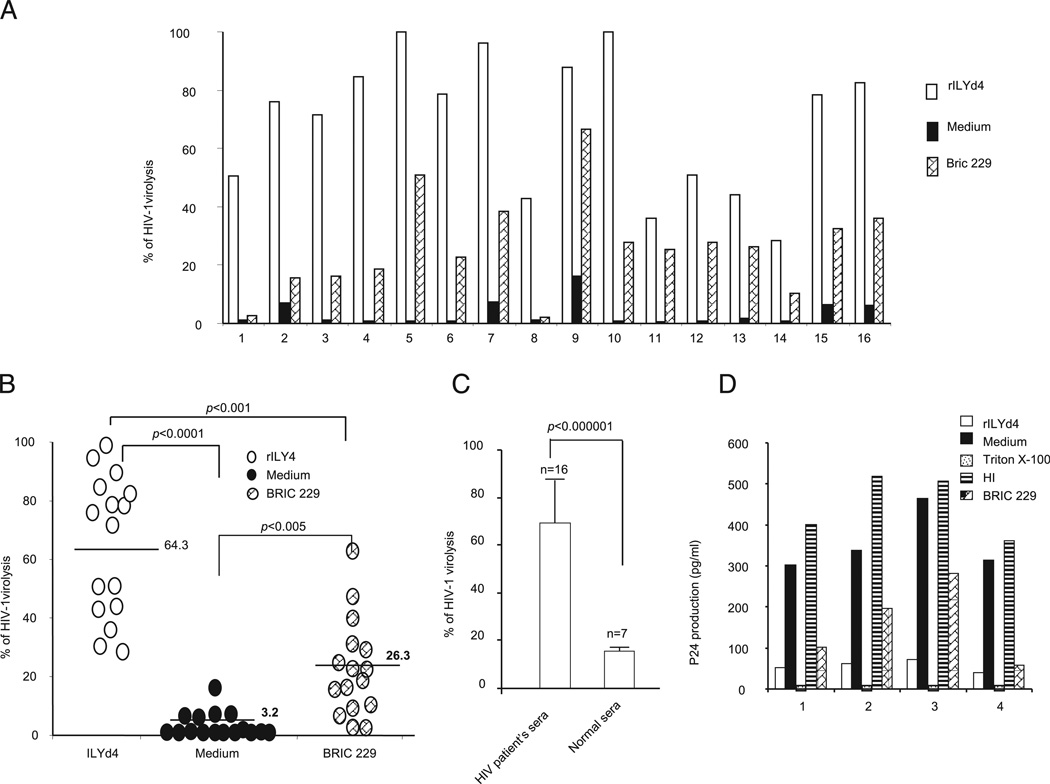
FIGURE 3.
Endogenous anti–HIV-1 Abs induce complement-mediated virolysis in the presence of rILYd4. HIV-1 CD59-positive virions preincubated with rILYd4 (20 µg/ml), medium only, or anti-hCD59 monoclonal Ab (BRIC 229) were treated with heat-inactivated plasma from 16 HIV-1–positive individuals containing anti–HIV-1 envelope Abs (individual levels of these Abs from patients 1–16 shown in Table I) followed by exposure to 10% normal HS as a source of complement (heat-inactivated normal serum was used as a negative control). A, Plasma from HIV-1–infected individuals induces complement-mediated virolysis in the presence of rILYd4. B, Pooled virolysis data from all plasma samples from HIV-1–infected individuals. Horizontal bars represent mean of virolysis values. C, rILYd4 enhances complement-mediated virolysis activated by plasma from HIV-1–infected but not from noninfected individuals. D, HIV-1 infectivity assay. Titration of produced p24 in culture supernatants from cells exposed for 10 d to conditioned medium from virions pretreated with the following conditions: medium alone, anti-CD59 Ab (BRIC 229), rILYd4, and Triton X or originally exposed to heat-inactivated serum. The experiments were repeated twice for each test. The results are represented by mean ± SD.
Plasma collection from HIV-1–infected patients
Plasma specimens were obtained from the repository at the Hawaii Center for AIDS, University of Hawaii. All samples were from patients who signed informed consent forms in accordance with the guidelines for conduction of clinical research by the University of Hawaii Institutional Review Board. Plasma HIV-1 levels (Amplicor HIV-1 Monitor Ultra Sensitive Test, Roche Diagnostics, Basel, Switzerland) and CD4 cell counts were measured in a certified clinical laboratory, as previously reported (20).
Measurement of HIV-1 p24 in plasma samples from HIV-1–infected patients
Plasma specimens were tested for HIV-1 p24 Ag using the Perkin Elmer HIV-1 ELISA kit as described above. Each plasma sample was treated with the lysis buffer included in the ELISA kit to lyse the viral particles for releasing HIV-1 core protein p24, which was then measured.
Measurement of anti–HIV-1 envelope Abs from HIV-1–infected patients
Anti–HIV-1 envelope Ab was measured using an ELISA Kit for Antibody to Human Immunodeficiency Virus 1&2 (BioChain, Hayward, CA) according to the manufacturer’s protocol. The microplates included in this kit were coated with the rHIV Ags (gp120, gp36, and gp47), which specifically capture anti–HIV envelope Abs. Test results for HIV-2 specific Ab, as measured by the FDA-licensed HIV-2 enzyme immunoassay, were negative in all subjects. Therefore, Abs measured by this ELISA kit were specifically against HIV-1 envelope.
Assessment of the nonspecific cytolytic effect of rILYd4 on erythrocytes and PBMCs from HIV-1–infected patients
Fresh whole blood from HIV-1–infected patients (patients 14 and 17 in Table I) were collected into two tubes, one containing no anticoagulation reagent for serum isolation and one containing potassium EDTA as an anticoagulant for erythrocyte preparation. The tube without anticoagulation was kept at room temperature for 30 min and subjected to centrifugation at 10,000 rpm at 4°C for 15 min to separate serum from erythrocytes. The serum was aliquoted and stored at −80°C until used as a source of complement. Some serum aliquots were heated at 56°C for 30 min to inactivate complement. Erythrocytes and PBMC from the same HIV-1-infected patients were prepared as described above and their sensitivity to complement-mediated lysis in the presence of rILYd4 was assessed as follows. Erythrocytes were washed with PBS, suspended in GVB++ (hematocrit: 2%), treated with rILYd4 (20 µg/ml) at 37°C for 15 min, and then incubated with or without rabbit anti-human erythrocyte polyclonal Abs at 37°C for 30 min. Both nonsensitized and sensitized erythrocytes were exposed to 50% serum or plasma (as a source of complement) from the same HIV-1–infected patient. The effect of rILYd4 on PBMCs from the same HIV-1–infected patient was also assessed as follows. PBMCs were prepared as described above, pretreated with rILYd4 (20 µg/ml) at 37°C for 15 min, suspended in GVB++, and exposed to the same patient’s serum or plasma (50%) as a source of complement. PBMC lysis was assessed by the release of lactate dehydrogenase measured with the CytoTox-ONE kit according to the manufacturers’ instructions (Promega, Madison, WI) (21). The fluorescent signal was recorded at 560/590 nm using Fluostar Optima (BMG Labtech, Cary, NC). The background fluorescence of the PBS buffer was subtracted. Total lysis of PBMCs was induced with Triton X-100. Percent lysis was calculated as: (experimental fluorescent signal − blank fluorescent signal)/(total lysis fluorescent signal)×100.
Results
Generation of human CD59 specific inhibitor rILYd4
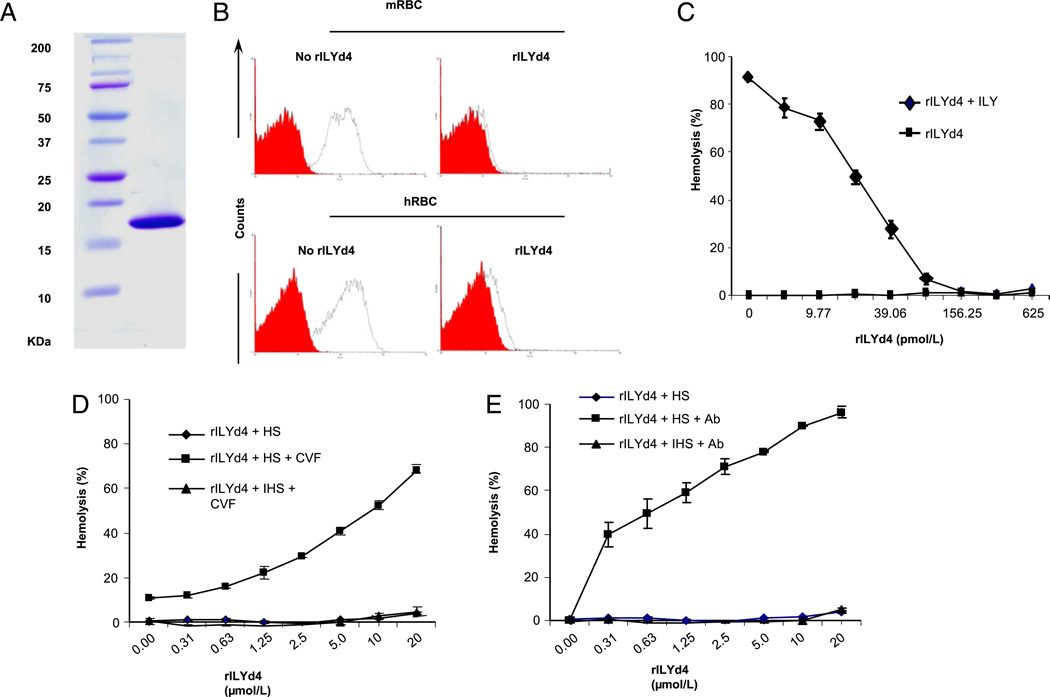
rILYd4 (MW, 18.6 kDa) was expressed in E. coli. (Fig. 1A) and purified as described in Materials and Methods. FACS analysis of both human and mouse erythrocytes that transgenically express hCD59 (16) demonstrated that rILYd4 blocked anti-hCD59 monoclonal Ab binding to membrane hCD59 (Fig. 1B). At the functional level, rILYd4 induced a significant and dose-dependent inhibition of human erythrocytes lysis mediated by the intact ILY (1.2 nM; Fig. 1C) and a significant increase of complement-mediated lysis of human erythrocytes triggered by either the alternative (Fig. 1D) or the classical activation pathways (Fig. 1E). No lysis was observed when human or hCD59 transgenic mouse erythrocytes were exposed to rILYd4 alone. Together, these experiments demonstrate that rILYd4 binds and inhibits the function of hCD59. These results confirm and extend our previous communication (22) and are comparable to those recently published by Hughes et al. (23).
FIGURE 1.
Generation of rILYd4 and characterization of its functional activity as an inhibitor of hCD59. A, SDS-PAGE separation and Coomassie Blue staining of rILYd4 after purification. B, Confirmation of rILYd4 binding to hCD59 by FACS analysis. Preincubation of rILYd4 inhibits binding of anti-hCD59 Abs to mouse erythrocytes from hCD59RBC mice (14) (top panel) and to human erythrocytes (bottom panel). The red curve represents isotype-matched Ab + FITC secondary Abs staining (negative control). The black curve represents ± rILYd4 (0 or 1 µg/ml) + anti-hCD59 Abs (0.2 µg/ml) + FITC secondary Abs. C, rILYd4 blocked the lysis of human erythrocytes induced by 1.2nM ILY, which mediates ~90% hemolysis in vitro.D and E, rILYd4 abrogates hCD59 function in complement-mediated hemolytic assays. HS = 50% HS; HIS = 50% heat-inactivated HS. D, Alternative pathway assay activated by 5 mg/l cobra venom factor. E, Classical pathway assay with human RBC sensitized with anti-human RBC Ab. Results in C, D, and E are mean ± SD from four independent experiments.
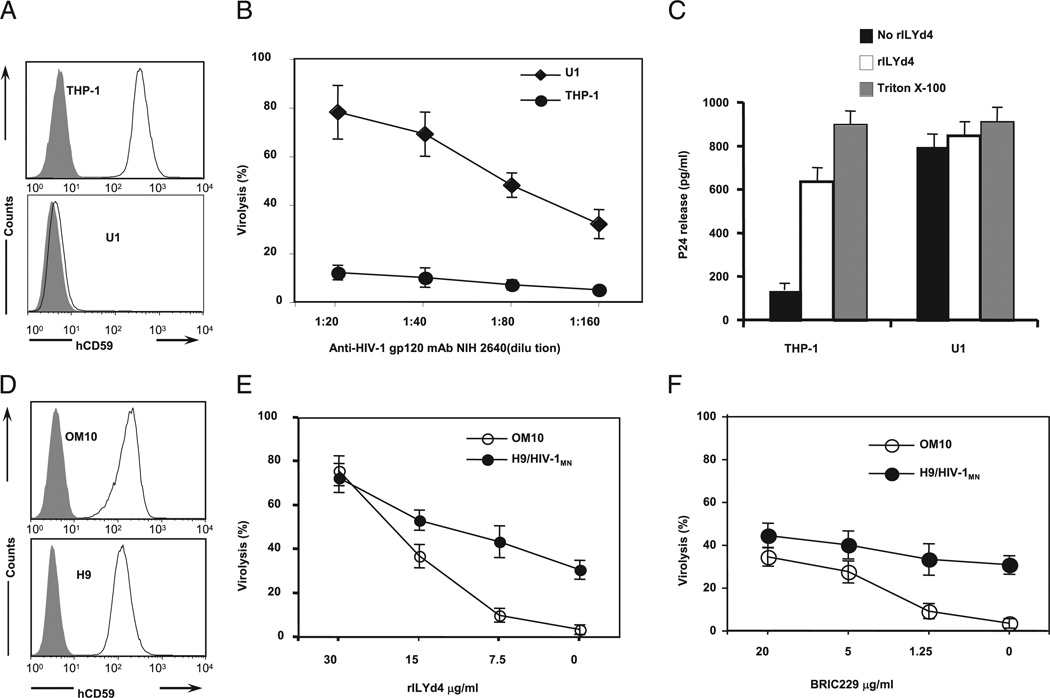
rILYd4 sensitizes the HIV-1 virions derived from CD59-positive cell lines to complement-mediated virolysis activated by anti–HIV-1 envelope Abs
As mentioned above, hCD59 protects HIV-1 from complement-mediated virolysis (3, 24). To test whether inhibition of hCD59 by rILYd4 sensitizes HIV-1 to complement-mediated virolysis, we derived HIV-1 particles from two different HIV-1–infected host human monocytic cell lines: THP-1 expresses and U1 is devoid of hCD59 in the cell membranes, as documented by FACS analysis with an anti-hCD59 specific Ab (Fig. 2A). Virolysis was quantitated by an ELISA that measured the release of HIV-1 core protein p24 from the lysed viral particles. HIV-1 virions derived from hCD59-negative U1 cells were highly sensitive to virolysis induced by anti–HIV-1 gp120 monoclonal Ab plus HS as a source of complement (Fig. 2B). In contrast, HIV-1 virions derived from hCD59-positive THP-1 cells were resistant to complement-mediated virolysis (Fig. 2B). Remarkably, preincubation of the HIV-1 virions derived from the infected THP-1 cells with rILYd4 rendered the virus sensitive to complement-mediated lysis. This virolysis enhancement did not occur in HIV-1 virions derived from hCD59-negative U1 cells (Fig. 2C). In the presence of 20 µg/ml rILYd4, THP-1 cell-derived virions were almost as sensitive to complement-mediated lysis as the virions derived from CD59 negative U1 cells (Fig. 2C). Similar dose-dependent results were obtained with 1) HIV-1 virions derived from two additional human T-lymphocytic cell lines (OM10 and H9), which express a high density of hCD59 on their cell membranes (Fig. 2D), and 2) with a different anti–HIV-1 Ab (anti-gp120/160 polyclonal Abs; Fig. 2E). Blocking hCD59 function with a neutralizing anti-hCD59 Ab increased complement-mediated virolysis, albeit with less potency than with rILYd4 (Fig. 2F). In all experiments depicted in Fig. 2B, 2C, 2E, and 2F, virolysis in the presence of HS is complement-mediated because it is totally abrogated by preincubation of serum at 56°C for 1 h. These results demonstrate that inhibition of hCD59 function by rILYd4 sensitizes HIV-1 virions to complement-mediated virolysis.
FIGURE 2.
Effects of rILYd4 on complement-mediated virolysis of HIV-1 virions. A, CD59 expression in human monocytes chronically infected with HIV-1. Cells were stained with anti-hCD59 (black lines) or isotype-matched Ab (solid gray lines). B, Complement-mediated virolysis of HIV-1 derived from either CD59-positive (THP-1) or CD59-negative (U1) cell lines. Viral preparations derived from CD59-expressing THP-1 or from CD59-negative U1 cells were incubated with anti–HIV-1 gp120 monoclonal Ab plus serum as a source of complement (heat-inactivated serum is a negative control). C, Effect of rILYd4 on HIV-1 virolysis. HIV-1 virions derived from either THP-1 or U1 cells were preincubated with rILYd4 (20 µg/ml) and treated with anti–HIV-1 gp120 monoclonal Ab plus serum as a source of complement (heat-inactivated serum is a negative control). Virolysis was analyzed by ELISA titration of released viral protein p24. D, CD59 expression on OM10 cells and H9 cells. E and F, Dose-dependent effects of rILYd4 and anti-hCD59 monoclonal Ab BRIC229 on HIV-1 virolysis activated by anti–HIV-1 gp120/gp160 polyclonal Abs. In each experiment, treatments with culture medium alone or with Triton X-100 were used as blank and 100% virolysis, respectively. Mean ± SD of three experiments performed in duplicate.
Abs in the plasma from the HIV-1–infected subjects exhibit their anti–HIV-1 activity in the presence of rILYd4
The presence of anti–HIV-1 envelope Abs in the blood of HIV-1–infected subjects fostered the notion that a hCD59 inhibitor would not protect and thereby sensitize circulating HIV-1 virions to complement-mediated virolysis. We tested this predication ex vivo by exposing HIV-1 virions derived from a hCD59 positive cell line to heat-inactivated plasma samples from either HIV-1–infected (HIV-1plasma) or control plasmas from HIV-1 seronegative donors (Controlplasma), followed by incubation with a pool of normal HS as a source of complement. Table I shows that the plasma levels of Abs against HIV-1 envelope measured by ELISA. Although the Ab titer varied among HIV-1 patients, every plasma sample from this cohort contained anti–HIV-1 envelope Abs. Preincubation with rILYd4 dramatically increased complement-mediated virolysis of CD59-positive virions exposed to HIV-1plasma, but not to the Controlplasma (Fig. 3A–C). This effect of rILYd4 was comparable with, albeit much stronger than, the effect mediated by the anti-hCD59 monoclonal Ab BRIC229 (Fig. 3B).
To understand the functional consequence of complement-mediated virolysis we used an HIV-1 infectivity assay. HIV-1–permissive H9 cells were exposed for 10 d to each conditioned medium from the virolysis experiments depicted in Fig. 3A and 3B (samples 1–4). The culture supernatants were then collected to determine HIV-1 infection intensity by measuring HIV-1 p24 (ELISA). The higher the level of p24 in the culture supernatant, the higher the number of infectious viral particles that remained in the conditioned medium of the virolysis experiments. Fig. 3D shows that p24 was undetectable in the supernatant from H9 cells exposed to conditioned medium from Triton X-100 treatment (total lysis), indicating that potentially infective particles were totally lysed and no infectious viral particles remained. The supernatants from cells exposed for 10 d to control conditioned medium (i.e., conditioned by the virions not treated with rILYd4 before exposure to endogenous anti–HIV-1 Ab and complement) had high titers of p24, indicating that the viral particles were not lysed and their infectivity was preserved. In contrast, supernatants from cells exposed for 10 d to conditioned medium from rILYd4 pretreated virions (treated with rILYd4 before exposure to endogenous anti–HIV-1 Ab and complement) showed low levels of p24, an indication that rILYd4 allowed the anti–HIV-1 Abs present in the plasmas of HIV-1–infected patients to regain their activity in triggering complement-mediated virolysis and thereby reduce the infective potential of HIV-1 virions.
rILYd4 sensitizes primary HIV-1 isolates to complement-mediated virolysis activated by anti–HIV-1 Abs present in the plasmas of HIV-1–infected patients
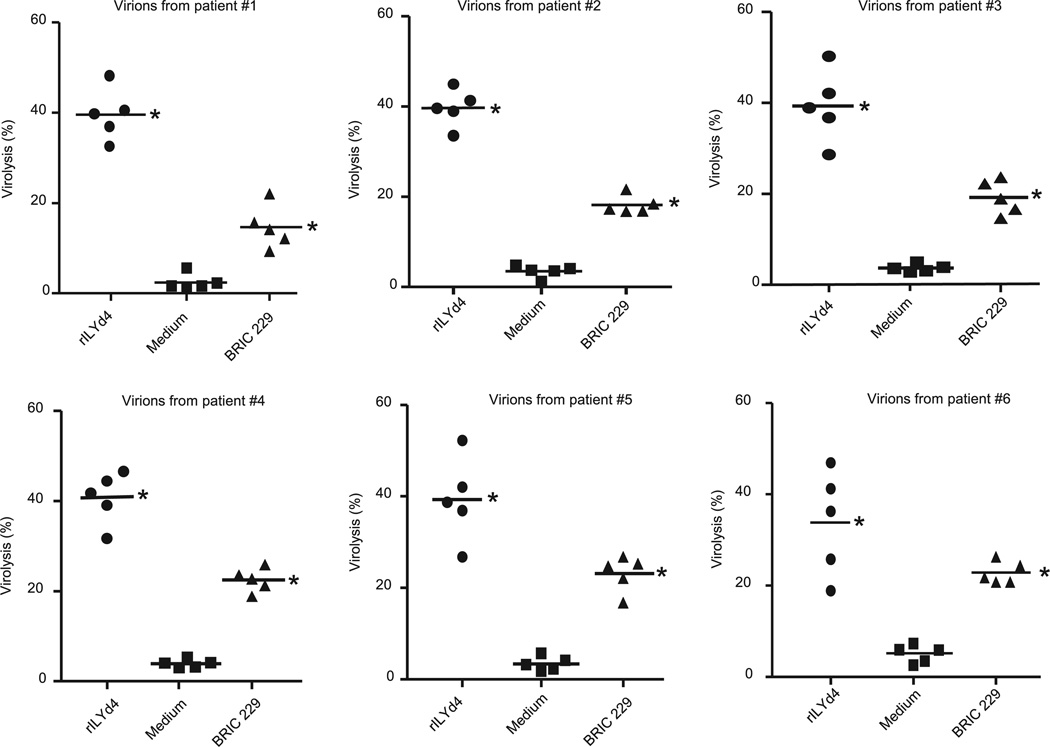
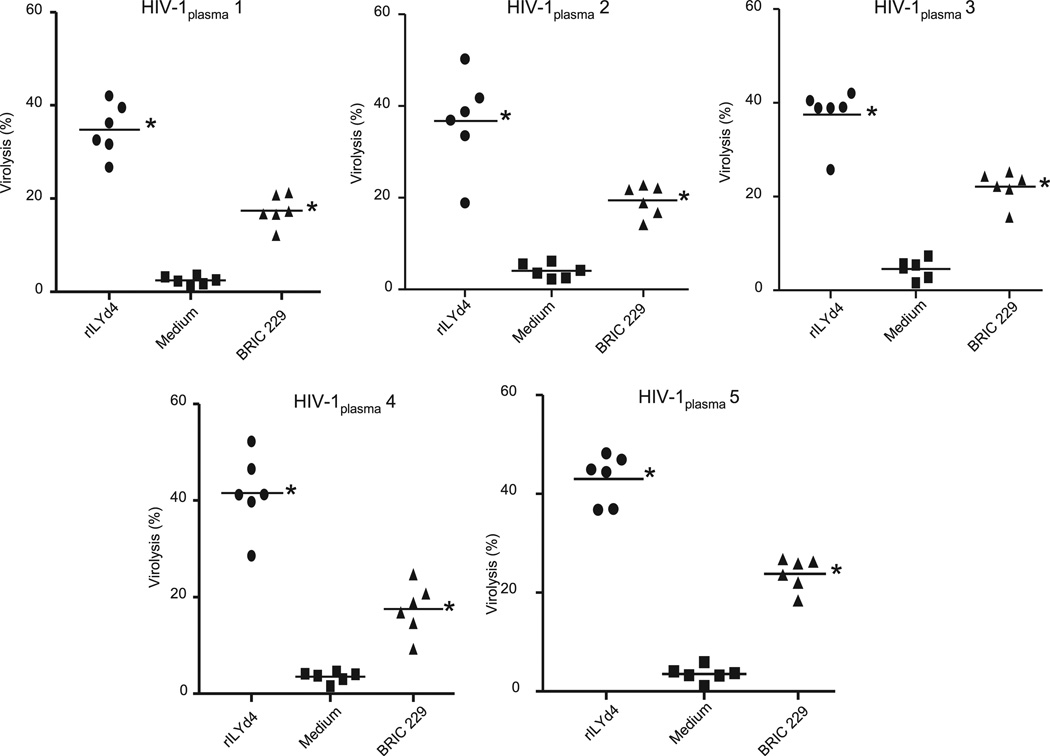
Results obtained from primary HIV-1 isolates are considered more representatives of the physiologic conditions of HIV-1–infected patients than those obtained from cultured cell lines. In the specific case of hCD59, primary HIV-1 isolates derived from PBMC of HIV-1–infected patients are likely to carry a load of membrane-derived human CD59, which confers resistance to the Ab-dependent complement-mediated virolysis. For these reasons, we generated primary HIV-1 isolates from six HIV-1–seropositive individuals who were naive for antiretroviral therapy, and we tested whether rILYd4 sensitizes these virions to complement-mediated virolysis. In the same experiment, we assessed the relative potency of endogenous anti–HIV-1 Abs developed by HIV-1–infected patients to promote complement-mediated virolysis of PBMC-derived HIV-1 primary isolates in the presence and absence of rILYd4. To this end, we pretreated the primary HIV-1 isolates with or without rILYd4 and exposed them to heat-inactivated HIV-1plasma (patients 1 to 5 in Table I), followed by incubation with pooled normal HS as a source of complement. The results showed that rILYd4 sensitized each of the six primary HIV-1 isolates to complement-mediated virolysis activated by HIV-1plasma (Fig. 4). In the presence of rILYd4, each of the five different HIV-1plasma samples tested significantly increased complement-mediated lysis of each of the six primary HIV-1 isolates (Fig. 5). These effects of rILYd4 were comparable with, albeit much stronger than, those mediated by the anti-hCD59 monoclonal Ab BRIC229 (Figs. 4 and 5). These results confirm that rILYd4 sensitizes HIV-1 to complement-mediated virolysis not only under experimental conditions using cell lines and commercially available Abs, but also of primary HIV-1 isolates sensitized by the endogenous anti–HIV-1 Abs naturally present in the blood of HIV individuals. These results also indicate that inhibition of hCD59 with rILYd4 unprotects HIV-1, unleashing the ability of complement to lyse the virions sensitized by anti–HIV-1 Abs present in the circulation of patients with HIV-1.
FIGURE 4.
HIV-1 primary isolates become sensitive to complement-mediated virolysis in the presence of rILYd4. HIV-1 primary isolates derived from six HIV-1–infected patients. PBMCs preincubated with rILYd4 (20 µg/ml), medium only, or anti-hCD59 monoclonal Ab (BRIC 229) were treated with heat-inactivated plasma from 5 HIV-1–positive individuals containing anti–HIV-1 envelope Abs (patients 1–5 shown in Table I) followed by exposure to 10% normal HS as a source of complement (heat-inactivated normal serum was used as a negative control). Each panel represents the sensitivity of HIV-1 virions derived from one patient to complement-mediated virolysis activated by the endogenous anti–HIV-1 Abs developed in five HIV-1–infected patients who were naive for antiretroviral therapy. Horizontal lines represent the mean. Statistical significance (p < 0.01 versus medium treatment group) is indicated by an asterisk.
FIGURE 5.
In the presence of rILYd4, the endogenous anti–HIV-1 Abs destruct the HIV-1 virions through complement-mediated virolysis. Another method to analyze the results shown in Fig. 4 documents that in the presence of rILYd4, the endogenous anti–HIV-1 Abs developed in six HIV-1–infected patients are capable of destroying HIV-1 virions through complement-mediated virolysis. Each panel represents the ability of the endogenous anti–HIV-1 Abs developed in one patient to destroy the HIV-1–infected PBMC-derived virions. Horizontal lines represent the mean. Statistical significance (p < 0.01 versus medium treatment group) is indicated by an asterisk.
rILYd4 does not mediate cytolytic effect on erythrocytes and PBMC in the blood from HIV-1–infected patients
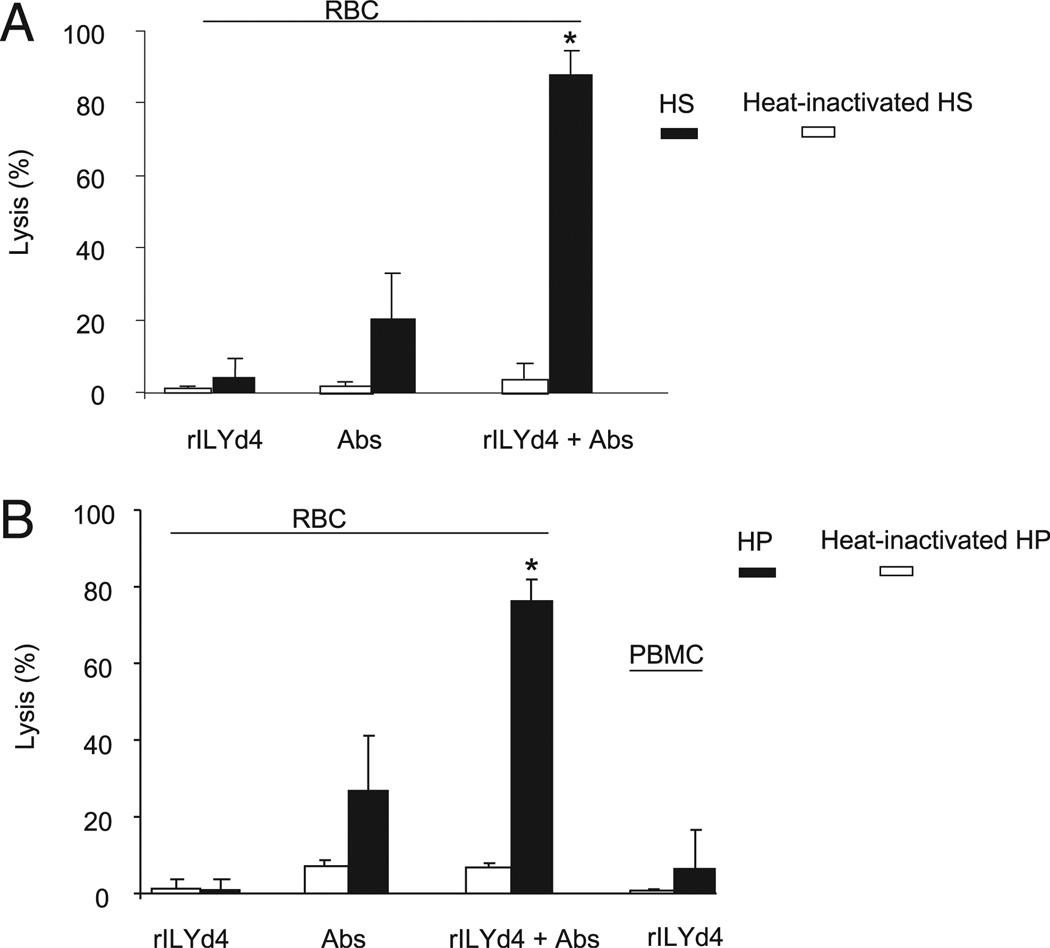
Patients infected with HIV-1 are well known to mount a vigorous and sustained Ab response to the virus (25–30). We consistently detected anti–HIV-1 envelope Abs in all 16 plasma samples from HIV-1–infected patients (Table I). When hCD59 activity is inhibited by rILYd4, these Abs could conceivably trigger unwanted complement-mediated effects, such as hemolysis. To address this issue, we investigated the potential lytic effect of rILYd4 on erythrocytes and PBMC from the HIV-1–infected patients with high levels of plasma HIV-1 RNA (patients 14 and 17 in Table I). We found that incubation of either erythrocytes or PBMC from HIV-1–seropositive individuals with 50% of their own serum (patient 17) or plasma (patient 14) in the presence of rILYd4 at the concentration that completely blocked hCD59 function did not result in any lytic effect of either cell type (Fig. 6A, 6B). This experiment indicates that rILYd4 does not induce complement-mediated lysis of cells not infected by HIV-1, such as erythrocytes. Although the infected CD4+ T cells in PBMC are susceptible to HIV-1 infection and might be lysed in the presence of rILYd4, they represent a small proportion of the overall PBMC population (0.001%–10%) (31), and their potential complement-mediated lysis in the presence of anti–HIV-1 Abs and rILYd4 is probably undetectable with the methods used for these experiments. These results suggest that bystander lytic effects induced by rILYd4 in the circulation of HIV-1 infected individuals are unlikely.
FIGURE 6.
Incubation of HIV-1–infected patient’s erythrocytes and PBMCs with 50% HIV-1–infected patient’s serum or plasma in the presence of rILYd4 did not result in a nonspecific lytic effect on the erythrocytes and PBMCs. A, The HS from and HIV-1–infected patient (patient 17 in Table I) was used as a source of complement and anti–HIV-1 Abs to investigate the bystander effect of rILY4 on the erythrocytes. B, The HP from an HIV-1-infected patient (patient 14 in Table I) was used as a source of complement and anti–HIV-1 Abs to investigate the bystander effect of rILYd4 on the RBCs or PBMCs. The patient’s erythrocytes were sensitized by anti-human erythrocyte Abs and exposed to HP or heat-inactivated HP. The results are the mean values ± SD from three experimental results. rILYd4, in the presence of rILYd4 (20 µg/ml), HIV-1–infected patients’ cells were exposed to 50% HS or HP and heat-inactivated HS or HP; Abs, HIV-1 infected patients’ erythrocytes were sensitized by anti-human erythrocyte Abs and then exposed to 50% HS or HP as well as heat-inactivated HS or HP; rILYd4 + Abs, in the presence of rILYd4 (20ug/ml), HIV-1–infected patients’ erythrocytes were sensitized by anti-human erythrocyte Abs and then exposed to 50% HS or HP and heat-inactivated HS or HP. *p < 0.01 versus heat-inactivated HS or HP treatment in rILYd4 + Abs.
Discussion
In this study, we report the development of rILYd4, a potent and specific inhibitor of hCD59. We show that rILYd4, in conjunction with anti–HIV-1 Abs, either exogenous like the anti-gp120/160 polyclonal Abs or endogenous such as those in plasma from HIV-1–infected patients, efficiently abrogates hCD59 function and renders complement-resistant laboratory strains of HIV-1 sensitive to Ab-dependent, complement-mediated lysis. We also show that, in the presence of rILYd4, anti–HIV-1 Abs in the circulation of HIV-1–infected individuals are capable of triggering complement-mediated virolysis of primary HIV-1 isolates. Inhibition of hCD59 activity by rILYd4 in erythrocytes or PBMC from HIV-1–infected individuals does not induce unwanted lytic effects.
The complement system, a key member of innate immunity, is a first-line defender against foreign pathogens such as HIV-1. However, HIV-1 in the circulation escapes complement-mediated attack and remains highly infective, even though there is strong experimental evidence that both the virus itself and anti–HIV-1 Abs in the blood of HIV-1–infected individuals are capable of activating the complement cascades (32). Indeed, HIV-1 virions from infected individuals accumulate C3 on their surface, an indication of complement activation (33). Normally, component C3 activation generates C3a and C3b, which then trigger a cascade of activation events that eventually result in formation of the MAC, an end-product of all the three complement activation pathways (2, 34). The MAC forms a lytic pore in the lipid bilayer membrane that destroys membrane integrity, allows the free passage of solutes and water, and eventually kills pathogens including viruses and/or infected cells. In HIV-1 infection, however, activation of complement, as evidenced by accumulation of C3 on the surface of the HIV-1 virions, fails to induce the HIV-1 lysis and enhances the viral infectivity by facilitating the interaction of the HIV-1 particles with complement-receptor–positive cells, including B and dendritic cells (35). Incomplete activation of complement enabling HIV-1 to escape Ab-dependent, complement-mediated lysis is due in part to the presence of hCD59 in the viral envelope, which the virus recruits from the host cell in the budding process (3, 10, 11). In addition, binding of the fluid phase complement regulator factor H to HIV-1 confers onto the virus further protection from complement attack (36). In summary, the HIV-1 virus, like other pathogens such as Schistosoma mansoni (37), manipulates the delicate balance between complement activation and restriction in a manner that is favorable to the virus.
For these reasons, it has long been suggested that an inhibitor of hCD59 or an agent that would abrogate factor H binding to the HIV-1 envelope could have a beneficial therapeutic effect against HIV-1 infection and AIDS (11, 33). The experiments reported in this paper indicate that rILYd4, a high affinity inhibitor of hCD59, could represent such a sought after therapeutic tool. Whether rILYd4 also abrogates the protective effect of factor H binding to HIV-1 is not known at present. More importantly, our experimental results show that rILYd4 abrogates hCD59 function and fosters complement-mediated cytolysis in human nucleated cells such as lymphocytes, which are the natural reservoir of HIV-1 in infected individuals (Supplemental Fig. 1). It has been documented that patients with HIV-1 infection also have diminished expression of the GPI-anchored cell surface proteins CD55 and CD59 on the erythrocytes and granulocytes (38). Because the density of hCD59 on the surface of HIV-1–infected cells appears to be reduced, one would expect that primary virions would carry less hCD59. Different density of CD59 in the membrane of laboratory or primary HIV-1 virions could explain different sensitivities to induction of complement-mediated lysis by rILYd4 (Fig. 3). In patients, a lower density of hCD59 in infected than in noninfected cells would enhance the efficacy of rILYd4 in specifically eliminating HIV-1 virions and HIV-1–infected cells. In summary, the results presented in this paper indicate that rILYd4 represents a preclinical candidate that deserves further investigation as a potential therapeutic agent against HIV-1 infection and AIDS.
However, in the discussion of a potential therapeutic use of rILYd4, it is important to highlight that abrogation of hCD59 function in humans has the potential of inducing complement-mediated side effects. A pertinent example of the potentially harmful effects of abrogating homologous restriction on the surface of “self” cells is illustrated by the human disease paroxysmal nocturnal hemoglobinuria (PNH), in which bone marrow–derived circulating cells are deficient in CD59 and other GPI-anchored proteins (39–43). Patients with PNH usually exhibit a mild hemolytic anemia, attributed to complement activation at its basal “tick over” rate, and develop paroxysmal hemolytic crisis when infections of other stressors trigger more massive complement activation (39, 40). Of note, patients with a variety of autoimmune disorders also have diminished expression of the GPI-anchored cell surface proteins CD55 and CD59 on erythrocytes and granulocytes (44). Whether administration of rILYd4 to humans with PNH or autoimmune disorders will trigger hemolysis and/or other unwanted complement-mediated phenomena is not known at present and raises a question that can be answered only by human studies. Our preliminary attempts to address the potential complement-mediated adverse effects of inhibiting hCD59 function ex vivo have shown that incubation of erythrocytes and PBMCs from normal or HIV-1–infected individuals in 50% HS or human plasma (HP) in the presence of rILYd4 at a concentration that maximally inhibits hCD59 function did not result in any spontaneous cytolysis. This finding indicates that the basal activity of complement in plasma/serum (as assessed ex vivo) is not sufficient to lyse human erythorcytes and PBMCs in the presence of a rILYd4 concentration that dramatically increased lysis when complement was activated by either the classical or alternative pathways (Figs. 1D, 1E, 6).
Plasma from HIV-1–infected patients, but not from healthy individuals, contains anti–HIV-1 Abs that promote complement-mediated virolysis of HIV-1 primary isolates in the presence of rILYd4 (Fig. 3). These endogenous anti–HIV-1 Abs developed by HIV-1–infected individuals in both the acute and chronic phases of infection activate complement through the classical pathway, as indicated by published reports that C1q-deficient serum as a source of complement fails to induce anti–HIV-1 Ab-mediated virolysis (45). Fig. 3B shows that plasmas from HIV-1–infected individuals segregate into two distinct populations: one with higher and one with lower capacity to induce complement-mediated virolysis in the presence of rILYd4. This finding may be attributed to the different Ab titers and/or different complement-activating capacity of the endogenous anti–HIV-1 Abs present in those plasmas. Additional specificity for complement activation on the viral surface would be provided by direct activation of complement triggered by gp41 and gp120, two complement-activating proteins present in the HIV-1 envelope (46, 47). Direct complement activation by viral proteins explains the complement-mediated virolysis seen in the presence of rILYd4, but in the absence of sensitizing anti–HIV-1 Abs (Fig. 3C).
An interesting advantage of rILYd4 over current anti–HIV-1 pharmacologic therapies derives from the fact that hCD59 present in the viral surface is not encoded in the viral genome, but rather a human cell protein recruited from the membrane of the host cell in the budding process. For this reason, it is less likely that the HIV-1 virus in infected individuals would acquire resistance to rILYd4 through a high rate of mutation and recombination of viral proteins, as it does to all currently available anti–HIV-1 drugs and vaccines (48–50).
In conclusion, it is tempting to postulate that administration of rILYd4 to HIV-1–infected individuals would allow anti–HIV-1 Abs to unleash their capacities to induce complement-mediated virolysis of both free viral particles and infected cells with no or minimal effect on other bystander cells. Further investigation of the potential therapeutic applications of rILYd4 for HIV-1 treatment certainly deserves consideration toward preclinical development and eventually clinical trials.
Supplementary Material
Acknowledgments
We dedicate this article to the memory of Dr. Daniel. C. Tosteson, Dean Emeritus of Harvard Medical School, who was an untiring mentor and role model to us.
This work was supported by the U.S. National Institutes of Health Grant RO1 AI061174 (to X.Q.), and the Harvard Technology Development Accelerator Fund (to X.Q.), and R21AI073250 (Q.Y.), the New Investigator Award from the University of Washington CFAR (AI 27757, to Q.Y.), the Research Facilities Improvement Program Grant C06 RR015481-01 from NIH to Indiana University School of Medicine, and the Bill and Melinda Gates Foundation (#53183 to Q.Y.).
Abbreviations used in this paper
- D4
domain 4
- HAART
highly active antiretroviral therapy
- hCD59
human CD59
- HP
human plasma
- HS
human serum
- ILY
intermedilysin
- MAC
membrane attack complex
- PNH
paroxysmal nocturnal hemoglobinuria
- rILYd4
recombinant domain 4 of intermedilysin
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Morgan BP, Harris CL. Complement Regulatory Proteins. London: Academic Press; 1999. [Google Scholar]
- 2.Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13:954–966. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]
- 3.Saifuddin M, Parker CJ, Peeples ME, Gorny MK, Zolla-Pazner S, Ghassemi M, Rooney IA, Atkinson JP, Spear GT. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J. Exp. Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiller OB, Hanna SM, Devine DV, Tufaro F. Neutralization of cytomegalovirus virions: the role of complement. J. Infect. Dis. 1997;176:339–347. doi: 10.1086/514050. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rautemaa R, Helander T, Meri S. Herpes simplex virus 1 infected neuronal and skin cells differ in their susceptibility to complement attack. Immunology. 2002;106:404–411. doi: 10.1046/j.1365-2567.2002.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernet J, Mullick J, Singh AK, Sahu A. Viral mimicry of the complement system. J. Biosci. 2003;28:249–264. doi: 10.1007/BF02970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoiber H, Pruenster M, Ammann CG, Dierich MP. Complement-opsonized HIV: the free rider on its way to infection. Mol. Immunol. 2005;42:153–160. doi: 10.1016/j.molimm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Fritzinger AE, Toney DM, MacLean RC, Marciano-Cabral F. Identification of a Naegleria fowleri membrane protein reactive with anti-human CD59 antibody. Infect. Immun. 2006;74:1189–1195. doi: 10.1128/IAI.74.2.1189-1195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz J, Zimmer JP, Kluxen B, Aries S, Bögel M, Gigli I, Schmitz H. Antibody-dependent complement-mediated cytotoxicity in sera from patients with HIV-1 infection is controlled by CD55 and CD59. J. Clin. Invest. 1995;96:1520–1526. doi: 10.1172/JCI118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dierich MP, Stoiber H, Clivio A. A “complement-ary” AIDS vaccine. Nat. Med. 1996;2:153–155. doi: 10.1038/nm0296-153. [DOI] [PubMed] [Google Scholar]
- 12.Saifuddin M, Hedayati T, Atkinson JP, Holguin MH, Parker CJ, Spear GT. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J. Gen. Virol. 1997;78:1907–1911. doi: 10.1099/0022-1317-78-8-1907. [DOI] [PubMed] [Google Scholar]
- 13.Beck EJ, Santas XM, Delay PR. Why and how to monitor the cost and evaluate the cost-effectiveness of HIV services in countries. AIDS. 2008;22(Suppl 1):S75–S85. doi: 10.1097/01.aids.0000327626.77597.fa. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MS, Hellmann N, Levy JA, DeCock K, Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J. Clin. Invest. 2008;118:1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giddings KS, Zhao J, Sims PJ, Tweten RK. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 2004;11:1173–1178. doi: 10.1038/nsmb862. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Ferris SP, Tweten RK, Wu G, Radaeva S, Gao B, Bronson RT, Halperin JA, Qin X. Rapid conditional targeted ablation of cells expressing human CD59 in transgenic mice by intermedilysin. Nat. Med. 2008;14:98–103. doi: 10.1038/nm1674. [DOI] [PubMed] [Google Scholar]
- 17.Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T, Rambaldi A, Introna M. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–3389. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- 19.van Doorn LJ, Kleter B, Pike I, Quint W. Analysis of hepatitis C virus isolates by serotyping and genotyping. J. Clin. Microbiol. 1996;34:1784–1787. doi: 10.1128/jcm.34.7.1784-1787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partha R, Lackey M, Hirsch A, Casscells SW, Conyers JL. Self assembly of amphiphilic C60 fullerene derivatives into nanoscale supramolecular structures. J. Nanobiotechnology. 2007;5:6. doi: 10.1186/1477-3155-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Weiguo, Giguel FF, Kuritzkes DR, Halperin JA, Qin X. Domain 4 of ILY sensitizes antibody therapy on cancer and HIV through abrogating human CD59 function. [Accessed: November 18, 2009];FASEB J. 2008 22:Ib522. Available at: http://www.fasebj.org/cgi/content/meeting_abstract/22/2_MeetingAbstracts/522. [Google Scholar]
- 23.Hughes TR, Ross KS, Cowan GJ, Sivasankar B, Harris CL, Mitchell TJ, Morgan BP. Identification of the high affinity binding site in the Streptococcus intermedius toxin intermedilysin for its membrane receptor, the human complement regulator CD59. Mol. Immunol. 2009;46:1561–1567. doi: 10.1016/j.molimm.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear GT, Olinger GG, Saifuddin M, Gebel HM. Human antibodies to major histocompatibility complex alloantigens mediate lysis and neutralization of HIV-1 primary isolate virions in the presence of complement. J. Acquir. Immune Defic. Syndr. 2001;26:103–110. doi: 10.1097/00042560-200102010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat. Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 26.Willey S, Aasa-Chapman MM. Humoral immunity to HIV-1: neutralisation and antibody effector functions. Trends Microbiol. 2008;16:596–604. doi: 10.1016/j.tim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Huber M, Trkola A. Humoral immunity to HIV-1: neutralization and beyond. J. Intern. Med. 2007;262:5–25. doi: 10.1111/j.1365-2796.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 28.Morris L. Neutralizing antibody responses to HIV-1 infection. IUBMB Life. 2002;53:197–199. doi: 10.1080/15216540212656. [DOI] [PubMed] [Google Scholar]
- 29.Humbert M, Dietrich U. The role of neutralizing antibodies in HIV infection. AIDS Rev. 2006;8:51–59. [PubMed] [Google Scholar]
- 30.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy JA. HIV and the Pathogenesis of AIDS: Features of HIV Transmission. 2nd Ed. Washington: ASM press; 1998. [Google Scholar]
- 32.Stoiber H, Clivio A, Dierich MP. Role of complement in HIV infection. Annu. Rev. Immunol. 1997;15:649–674. doi: 10.1146/annurev.immunol.15.1.649. [DOI] [PubMed] [Google Scholar]
- 33.Stoiber H, Banki Z, Wilflingseder D, Dierich MP. Complement-HIV interactions during all steps of viral pathogenesis. Vaccine. 2008;26:3046–3054. doi: 10.1016/j.vaccine.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Qin X, Gao B. The complement system in liver diseases. Cell. Mol. Immunol. 2006;3:333–340. [PubMed] [Google Scholar]
- 35.Stoiber H, Kacani L, Speth C, Würzner R, Dierich MP. The supportive role of complement in HIV pathogenesis. Immunol. Rev. 2001;180:168–176. doi: 10.1034/j.1600-065x.2001.1800115.x. [DOI] [PubMed] [Google Scholar]
- 36.Stoiber H, Pintér C, Siccardi AG, Clivio A, Dierich MP. Efficient destruction of human immunodeficiency virus in human serum by inhibiting the protective action of complement factor H and decay accelerating factor (DAF, CD55) J. Exp. Med. 1996;183:307–310. doi: 10.1084/jem.183.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parizade M, Arnon R, Lachmann PJ, Fishelson Z. Functional and antigenic similarities between a 94-kD protein of Schistosoma mansoni (SCIP-1) and human CD59. J. Exp. Med. 1994;179:1625–1636. doi: 10.1084/jem.179.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terpos E, Sarantopoulos A, Kouramba A, Katsarou O, Stavropoulos J, Masouridi S, Karafoulidou A, Meletis J. Reduction of CD55 and/or CD59 in red blood cells of patients with HIV infection. Med. Sci. Monit. 2008;14:CR276–CR280. [PubMed] [Google Scholar]
- 39.Parker CJ. Molecular basis of paroxysmal nocturnal hemoglobinuria. Stem Cells. 1996;14:396–411. doi: 10.1002/stem.140396. [DOI] [PubMed] [Google Scholar]
- 40.Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T, Kinoshita T. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–711. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 41.Qin X, Krumrei N, Grubissich L, Dobarro M, Aktas H, Perez G, Halperin JA. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity. 2003;18:217–227. doi: 10.1016/s1074-7613(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 42.Qin X, Hu W, Song W, Grubissich L, Hu X, Wu G, Ferris S, Dobarro M, Halperin JA. Generation and phenotyping of mCd59a and mCd59b double-knockout mice. Am. J. Hematol. 2009;84:65–70. doi: 10.1002/ajh.21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin X, Hu W, Song W, Blair P, Wu G, Hu X, Song Y, Bauer S, Feelisch M, Leopold JA, et al. Balancing role of nitric oxide in complement-mediated activation of platelets from mCd59a and mCd59b double-knockout mice. Am. J. Hematol. 2009;84:221–227. doi: 10.1002/ajh.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Delgado GJ, Vázquez-Garza E, Méndez-Ramírez N, Gómez-Almaguer D. Abnormalities in the expression of CD55 and CD59 surface molecules on peripheral blood cells are not specific to paroxysmal nocturnal hemoglobinuria. Hematology. 2009;14:33–37. doi: 10.1179/102453309X385089. [DOI] [PubMed] [Google Scholar]
- 45.Aasa-Chapman MM, Holuigue S, Aubin K, Wong M, Jones NA, Cornforth D, Pellegrino P, Newton P, Williams I, Borrow P, et al. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 2005;79:2823–2830. doi: 10.1128/JVI.79.5.2823-2830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebenbichler CF, Thielens NM, Vornhagen R, Marschang P, Arlaud GJ, Dierich MP. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J. Exp. Med. 1991;174:1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoiber H, Speth C, Dierich MP. Role of complement in the control of HIV dynamics and pathogenesis. Vaccine. 2003;21(Suppl 2):S77–S82. doi: 10.1016/s0264-410x(03)00203-2. [DOI] [PubMed] [Google Scholar]
- 48.Matsumi S, Kosalaraksa P, Tsang H, Kavlick MF, Harada S, Mitsuya H. Pathways for the emergence of multi-dideoxynucleoside-resistant HIV-1 variants. AIDS. 2003;17:1127–1137. doi: 10.1097/00002030-200305230-00003. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Picado J, Martínez MA. HIV-1 reverse transcriptase inhibitor resistance mutations and fitness: a view from the clinic and ex vivo. Virus Res. 2008;134:104–123. doi: 10.1016/j.virusres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 50.Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, Arnold E. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 2009;385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.