Abstract
Acute kidney injury (AKI) is a common problem in hospitalized patients which enhances morbidity and mortality and promotes the development of chronic and end stage renal disease. Ischemia reperfusion injury (IRI) is one of the major causes of AKI and is characterized by uncontrolled renal inflammation and tubular epithelial cell death. Our recent studies demonstrated that regulatory T cells (Tregs) protect the kidney from IR-induced inflammation and injury. Blockade of programmed cell death 1 (PD-1) on the surface of Tregs, prior to adoptive transfer, negates their ability to protect against ischemic kidney injury. The current study was designed to investigate the role of the known PD-1 ligands, PD-L1 and PD-L2 in kidney IRI. Administration of PD-L1 or PD-L2 blocking antibodies prior to mild or moderate kidney IRI significantly exacerbated the loss of renal function, renal inflammation and acute tubular necrosis (ATN) compared to mice receiving isotype control antibodies. Interestingly, blockade of both PD-1 ligands resulted in worse injury, dysfunction and inflammation than blocking either ligand alone. Genetic deficiency of either PD-1 ligand also exacerbated kidney dysfunction and ATN after sub-threshold ischemia. Bone marrow chimeric studies revealed that PD-L1 expressed on non-bone marrow derived cells is critical for this resistance to IRI. Finally, blockade of either PD-1 ligand negated the protective ability of adoptively-transferred Tregs in IRI. These findings suggest that PD-L1 and PD-L2 are non-redundant aspects of the natural protective response to ischemic injury and may be novel therapeutic targets for AKI.
Introduction
Acute kidney injury (AKI) occurs in approximately 5% of hospitalized patients with detrimental consequences in terms of morbidity and mortality (1, 2). In addition, AKI increases the likelihood of developing chronic kidney disease and end stage renal disease (3, 4). Kidney ischemia reperfusion injury (IRI) is a common cause of AKI (5, 6). Animal models have revealed that inflammation begins as early as 30 minutes of reperfusion and inhibition of the immune response to IRI by various strategies dramatically improves renal function and histological integrity after ischemia (7–13). The innate inflammatory response, consisting of neutrophils and macrophages, is an important component of kidney IRI (8, 12, 14–17). Our recent studies have demonstrated that regulatory T cells (Tregs): A) make up a critical component of the natural intrinsic protective response to kidney IRI (18) and B) can be used therapeutically (by adoptive transfer) to protect against kidney IRI in naïve mice (18–20). Other groups have demonstrated that Tregs protect against nephrotoxic AKI (21) and promote recovery from established AKI (22, 23) in mouse models. Tregs use many different mechanisms to reduce inflammation, including TGFβ, IL-10, extracellular adenosine, CTLA-4 and programmed death -1 (PD-1) (19, 24–28).
PD-1 is a negative co-stimulatory molecule expressed by T lymphocytes, monocytes, dendritic cells and B cells (29, 30). PD-1 has two ligands: PD-L1 and PD-L2. PD-L1 is expressed by numerous immune and non-immune cells, whereas PD-L2 expression is limited primarily to antigen presenting cells (29, 30). PD-1 stimulation leads to inhibition of TCR signaling in CD4+ and CD8+ T cells (29, 30). Nonetheless, PD-1 is indispensable for Treg function, as recent studies show that Tregs lacking PD-1, or Tregs in the presence of PD-1 blocking antibodies, display impaired suppressive activity in vitro and in vivo (19, 25, 28, 31, 32).
Given that PD-1 expression on Tregs is vital for their ability to suppress kidney IRI (19) and the increasing use of PD-1 and PD-1 ligand blocking antibodies in clinical practice (33–35), we sought to determine the role of PD-L1 and PD-L2 in the natural course of kidney IRI and in Treg-mediated protection from IRI.
Materials and Methods
Mice
Six to 10 week old, male C57Bl/6 mice were obtained from Charles River Laboratories (Wilmington, MA) or The Jackson Laboratory (Bar Harbor, ME). B7-H1 KO (PD-L1 KO) mice on the C57Bl/6 background have been described previously (36) and were a generous gift from Lieping Chen (Yale University) via Victor Engelhard (University of Virginia). B7-DC KO (PD-L2 KO) mice (37) and CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) mice on the C57Bl/6 background were obtained from Jackson Laboratory. Mice were housed in a specific pathogen free facility at the University of Virginia. Routine testing confirmed the absence of murine norovirus, epizootic diarrhea in infant mice, Theiler’s murine encephalomyelitis virus, minute virus of mice, mouse parvovirus, mouse hepatitis virus, fur mites and pinworm.
Antibody treatment and adoptive transfer
Mice were injected i.p. with 300 μg isotype control or blocking antibodies purchased from BioXCell (West Lebanon, NH): rat IgG2a (clone: 2A3) – control for anti-PD-L2, rat IgG2b (clone: LTF-2) – control for anti-PD-L1, rat anti-PD-L1 (clone: 10F.9G2), rat anti-PD-L2 (clone: TY25). The antibodies were administered to the mice 24 h prior to IRI. Tregs were isolated from spleen of naïve WT mice with Dynal (Carlsbad, CA) CD4 negative selection kit and Miltenyi Biotec (Auburn, CA) CD25 positive selection kit according to the manufacturers’ protocols as previously described (18–20). One hundred thousand freshly isolated Tregs (in 200 microliters of normal saline) were administered to the mice 18 h prior to IRI (6 hours after injection of isotype or blocking antibodies).
Renal ischemia reperfusion injury model
Bilateral renal ischemia was induced as described previously (18–20). Briefly, mice were anesthetized and bilateral flank incisions were made. For sham animals renal pedicles were isolated but not clamped, in experimental animals both renal pedicles were clamped for the indicated number of minutes. After which the clamps were removed and kidneys observed to ensure reperfusion. Some kidney IRI experiments were performed using a Harvard Apparatus (Holliston, Massachusetts) mouse temperature controller (Figures 6, and 7) in the Old Medical School Building at UVA while others were performed using the Fine Science Tools (Foster City, CA) mouse temperature controller (no longer available from the manufacturer) in Jordan Hall at UVA (Figures 1, 2, 3, 4, 5 and 8) due to a laboratory move. Our experience was that when using the Harvard Apparatus temperature controller a shorter ischemic time was required for equivalent renal injury with the Fine Science Tools controller, thus 21 minutes was used with this setup to induce mild sub-threshold kidney IRI in Figures 6 and 7. Multiple factors are critical in the response to kidney IRI and changing surgery room and mouse temperature controller has been shown to affect the response to kidney IRI in mice by others (38). All animal experiments were performed in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals, and approved by the University of Virginia Institutional Animal Care and Use Committee.
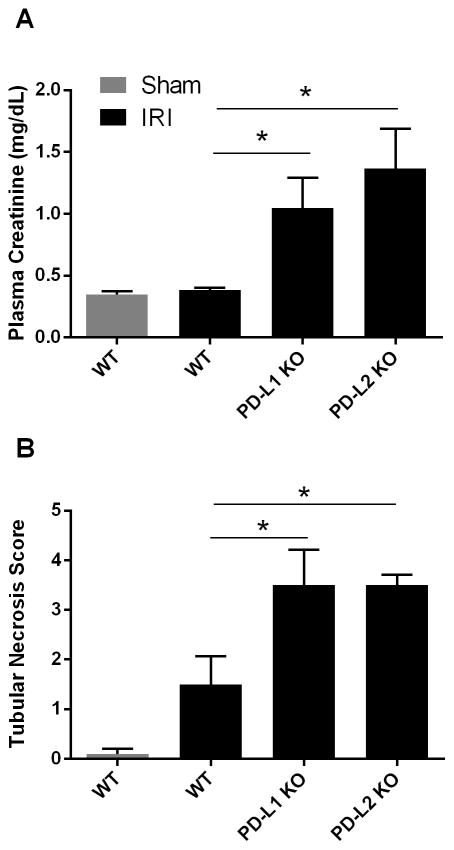
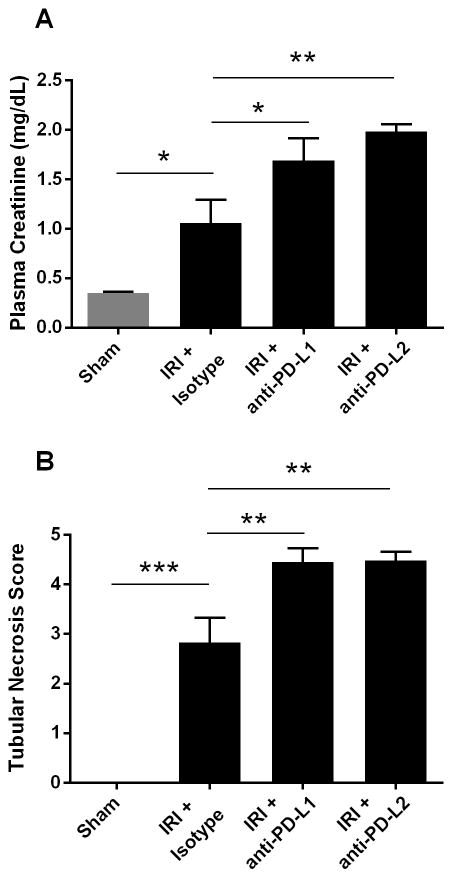
Figure 6. Genetic deficiency of either PD-L1 or PD-L2 increases kidney dysfunction and injury after mild ischemia-reperfusion injury.
Naïve C57Bl/6 (WT) mice, PD-L1 KO or PD-L2 KO mice were subjected to mild bilateral renal IR injury (21 min ischemia). Plasma creatinine level was assessed after 24 hours of reperfusion (A). ATN scores were determined using H&E stained kidney sections (B). N=5–6 per group, pooled from 3 independent experiments. Data are presented as the mean + SEM, * denotes P<0.05.
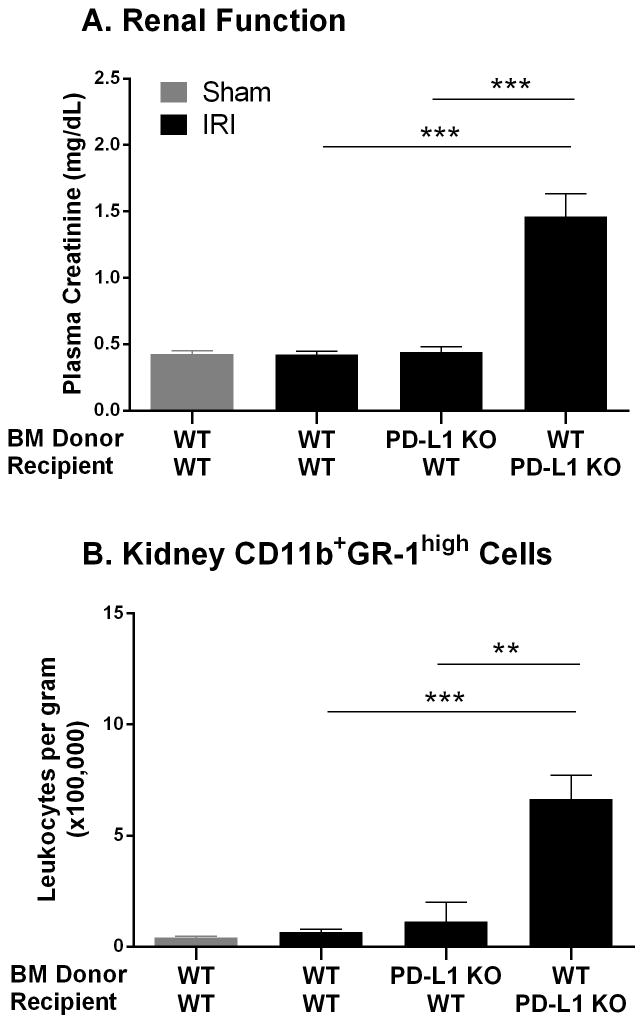
Figure 7. PD-L1 expressed on non-bone marrow derived cells promotes resistance to renal dysfunction and inflammation after mild kidney IRI.
WT and PD-L1 KO mice were irradiated to deplete bone marrow derived cells, and then reconstituted with isolated bone marrow from WT or PD-L1 KO mice as described in the Materials and Methods section. Seven to 8 weeks later mice underwent mild bilateral kidney IR surgery (21 min ischemia). At 24 hours of reperfusion plasma creatinine levels were assessed (A) and kidney cell suspensions were analyzed by flow cytometry for CD45+7AAD−CD11b+GR-1high cells (B). Data are presented as the mean + SEM. N=4–6 per group, pooled from 3 independent experiments, ** denotes P<0.01; *** denotes P<0.001.
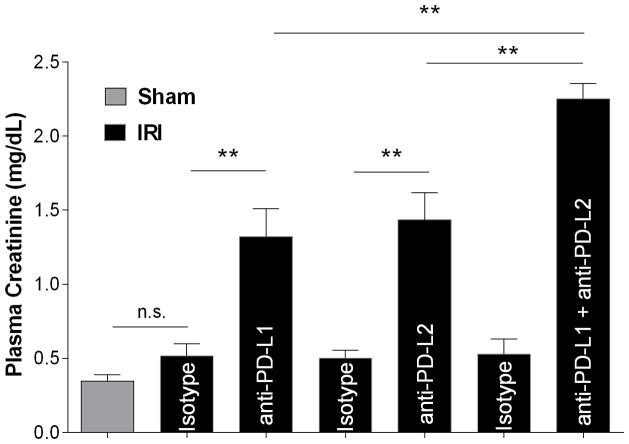
Figure 1. Blocking PD-1 ligands exacerbates renal dysfunction after mild ischemia reperfusion.
Naïve WT mice were treated with isotype control antibodies, anti-PD-L1, anti-PD-L2 or anti-PD-L1 and anti-PD-L2 together. After 24 hours sham or mild bilateral kidney IR surgery (24 min ischemia) was performed. Plasma creatinine levels were assessed after 24 hours of reperfusion. N=7–11 per group, pooled from 3 independent experiments. Data are presented as mean + SEM, ** denotes P<0.01; n.s.: not significantly different.
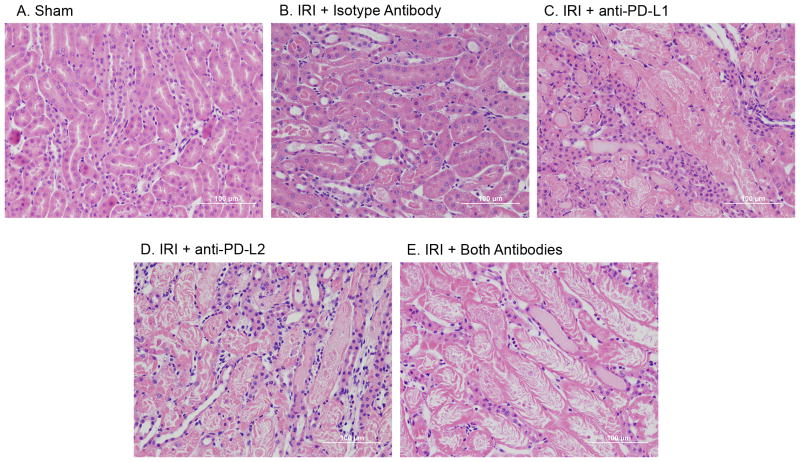
Figure 2. Blocking PD-1 ligands increases acute tubular necrosis (ATN) after kidney IRI.
Naïve WT mice were treated with isotype control antibodies, anti-PD-L1, anti-PD-L2 or anti-PD-L1 and anti-PD-L2 together. Twenty-four hours later sham or mild bilateral kidney IR surgery (24 min ischemia) was performed. After 24 hours of reperfusion, kidney sections were stained with H&E to assess outer medulla tubular necrosis. Images are representative of N=5 for the sham group and N=9–11 for IRI groups.
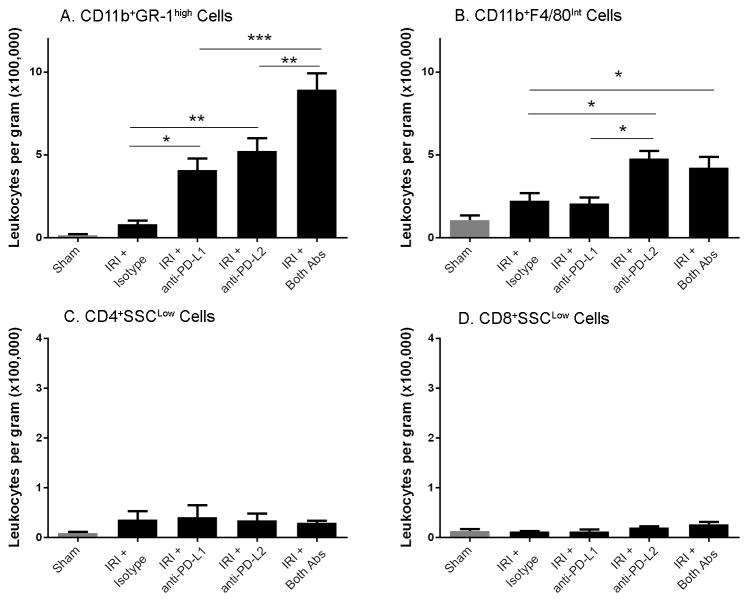
Figure 3. Blocking PD-1 ligands exacerbates innate renal inflammation after kidney IRI.
Naïve WT mice were treated with isotype control antibodies, anti-PD-L1 or anti-PD-L2 or the combination of both blocking antibodies. After 24 hours, sham or mild bilateral kidney IR surgery (24 min ischemia) was performed. At 24 hours of reperfusion kidney cell suspensions were analyzed by flow cytometry for CD45+7AAD−CD11b+GR-1high cells (A), CD45+7AAD−CD11b+F4/80int cells (B), CD45+7AAD−CD4+SSClow (C) and CD45+7AAD−CD8+SSClow (D). Absolute number of cells per gram of kidney was determined as described in the Materials and Methods section. Data are presented as mean + SEM, * denotes P<0.05; ** denotes P<0.01; *** denotes P<0.001. There are no significant differences between any group in panels C and D. N=5 for sham, 9–11 for each IRI group, pooled from 3 independent experiments.
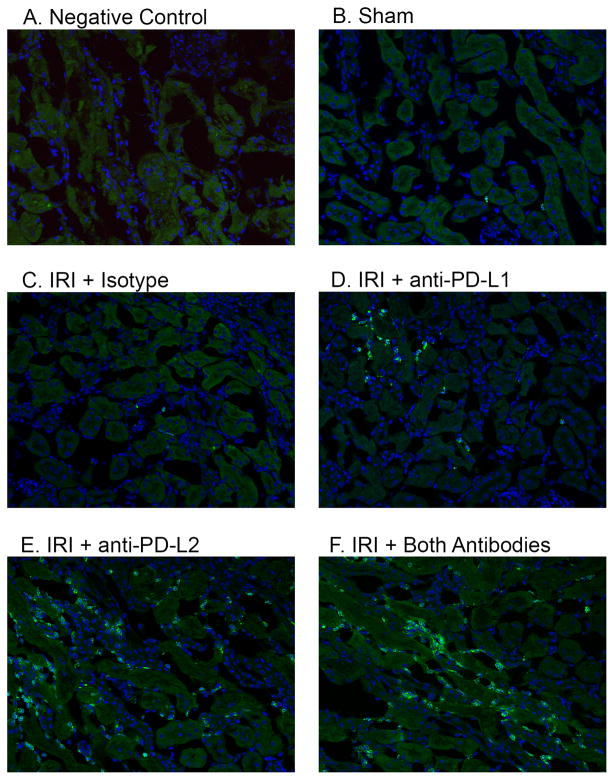
Figure 4. Blocking PD-1 ligands increases innate inflammation in the renal outer medulla after kidney IRI.
Naïve WT mice were treated with isotype control antibodies, anti-PD-L1 or anti-PD-L2 antibodies or the combination of both blocking antibodies. After 24 hours, sham or mild bilateral kidney IR surgery (24 min ischemia) was performed. Frozen kidney sections were stained with DAPI and 7/4 antibodies to detect innate leukocytes. Negative control (A) shows background green autofluorescence in an injured and inflamed mouse kidney. Pictures taken of the outer medulla region of each kidney under 200X magnification are representative of at least 5 mice per treatment group.
Figure 5. Blocking PD-1 ligands exacerbates renal dysfunction after moderate ischemia and reperfusion.
Naïve WT mice were treated with isotype control antibodies, anti-PD-L1 or anti-PD-L2 antibodies. Twenty-four hours later sham or moderate bilateral kidney IR surgery (26 min ischemia) was performed. After 24 hours of reperfusion plasma creatinine levels were assessed and kidney sections were stained with H&E to assess outer medulla tubular necrosis. N=5–7 per group, pooled from 2 independent experiments. Data are presented as mean + SEM, * denotes P<0.05; ** denotes P<0.01.
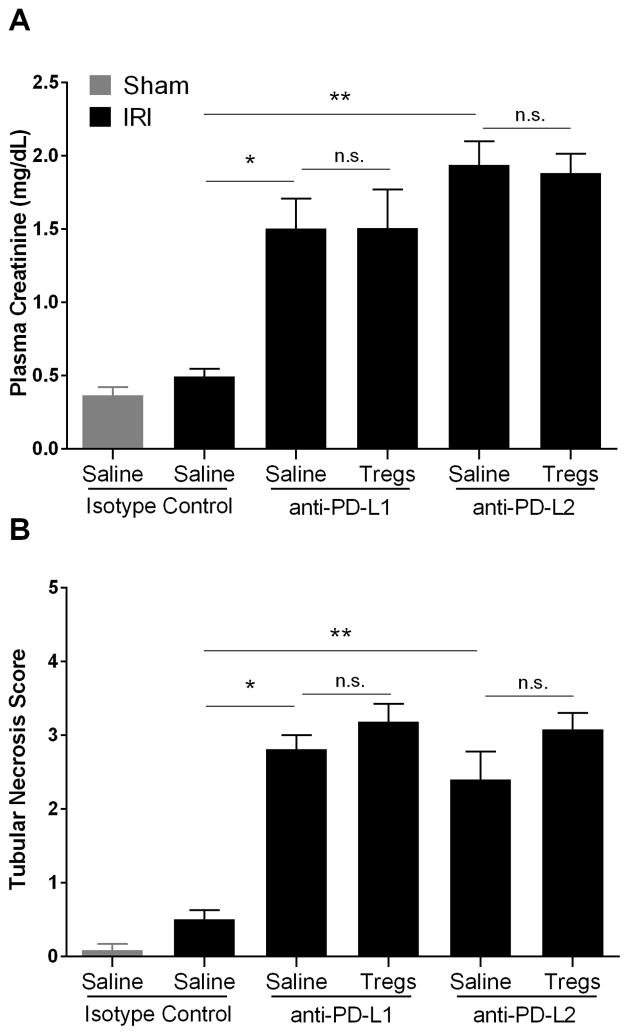
Figure 8. Blocking antibodies to either PD-1 ligand negate the protective effect of adoptively-transferred Tregs in kidney IRI.
Naïve WT mice were treated with isotype control antibodies, anti-PD-L1 or anti-PD-L2. Six hours later mice were administered either 100,000 freshly-isolated WT Tregs in normal saline or an equivalent amount of normal saline only. After 18 hours sham or mild kidney IR surgery (24 min ischemia) was performed, and the kidneys were allowed to reperfuse for 24 hours. To assess renal function, plasma creatinine was measured (A) and ATN scores were determined using H&E stained kidney sections (B). Data are presented as mean + SEM, N=5 for each group, pooled from 2 independent experiments, * denotes P<0.05; ** denotes P<0.01.
Bone marrow chimera generation
WT (CD45.1) mice and PD-L1 KO (CD45.2) mice were irradiated with 650 rad, two times 4 hours apart, then reconstituted with approximately 5 million bone marrow cells isolated from either WT (CD45.1) or PD-L1 KO (CD45.2) on the same day, as described previously (12). Seven to eight weeks after irradiation and bone marrow cell transfer the mice were subjected to mild ischemia reperfusion injury. Flow cytometery to determine the percentage CD45.1+ cells out of circulating CD11b+ cells confirmed the efficiency of the bone marrow transfer as 90.3±2.3%, (N=9) of circulating leukocytes were of bone marrow donor origin at the time of kidney IRI surgery.
Assessment of renal injury
Plasma creatinine was measured 24 h after IRI using DetectX NCal NIST-Calibrated Serum Creatinine Detection Kit, ARBOR ASSAYS (Ann Arbor, MI) according to the manufacturer’s protocol. Tubular necrosis in outer medulla region of the kidney was assessed by hematoxylin and eosin staining as reported before (18–20). Briefly, kidney sections were observed under 200X magnification and acute tubular necrosis score was assigned in a blinded manner based on the percentage of necrotic tubules in outer medulla (pink casts visible inside the tubules). The applied scoring system was as follows: 0, no damage; 1, <10%; 2, 10 to 25%; 3, 25 to 50%; 4, 50 to75%; and 5 >75%.
Flow Cytometry
AccuCheck counting beads from Invitrogen (Carlsbad, CA) were used to determine total CD45+ cell number per gram of collagenase digested kidney as reported previously (18–20). Leukocyte number was used to transform the percentages of leukocytes of specific phenotype to the leukocyte count and used to compare different groups. Fc receptors were blocked with 2.4G2 and 7-AAD Viability Staining Solution (BioLegend San Diego, CA) was applied to allow gating on live cells. The following anti-mouse fluorophore-labeled antibodies were used: GR-1 FITC (clone: RB6-8C5), Cd11b PE (M1/70), CD45 PECy7 (30-F11), F4/80 Alexa Fluor 647 (BM8) from BioLegend; CD45 PE (30-F11), CD4 PECy7 (RM4-5), CD3 FITC (145-2C11), CD8 PE (53–6.7) and NK1.1 PerCP-Cy5.5 (PK136) from BD Pharmigen, San Jose, CA. Data acquisition was performed on a FACSCalibur flow cytometer (BD, San Jose, CA) with a Cytek 8 color upgrade (Cytek Development, Inc. Freemont, CA) using FlowJo Collector’s Edition software (Tree Star, Ashland, OR). Flow cytometry analysis was performed using FlowJo Version X for Windows (Tree Star).
Immunofluorescence microscopy
Kidney samples were fixed as described previously (18). Subsequently, 7 μm frozen kidney sections were incubated 30 min in a PBS solution containing 0.3% Triton (Sigma-Aldrich, St. Louis, MO), 2.4G2 (BD Pharmigen), 1% bovine serum albumin and 10% horse serum. Afterwards the samples were labeled with a FITC-conjugated antibody (clone 7/4; Cedarlane, Burlington, Ontario, Canada) to visualize neutrophils and recently emigrated monocytes/macrophages (39, 40). ProLong Gold Antifade reagent with DAPI (Molecular Probes, Eugene, OR) was used to stain nuclei and to preserve the samples. The slides were observed and images captured under 200X magnification using an Olympus BX51 fluorescent microscope (Center Valley, PA) in the Advanced Microscopy Core Facility at the University of Virginia.
Real-Time Reverse Transcriptase–PCR
After harvesting, kidney sections were placed in RNA Later (Ambion, Austin, TX). RNA and cDNA were prepared as previously reported (19). GAPDH, KIM-1, IL-6, CXCL-1, and ICAM-1 Quantitect primers were purchased from Qiagen (Valencia, CA). Real-time Reverse Transcriptase-PCR was performed with MyIQ Single Color Real-Time PCR Detection System (BioRad, Hercules, CA) as previously described (19). No-template controls confirmed the lack of contamination and non-specific amplification (data not shown).
ELISA on Total Kidney Homogenate
Immediately after harvesting, kidney samples were frozen in liquid nitrogen and stored in −80 °C until further analysis. The samples were placed in lysis buffer consisting of Pierce RIPA Buffer (Thermo Scientific, Rockford, IL), Phenylmethansulfonyl fluoride (Sigma-Aldrich) and Protease Inhibitor Cocktail (Sigma-Aldrich) and homogenized using a Tissue Lyzer LT (Qiagen, Valencia, CA). Subsequently the samples were spun down and the supernatant was sonicated with Ultrasonic Processor GEX130 (Cole-Parmer, Vernon Hills, IL) for 10s set on 70% power. The samples were spun down once again and an aliquot of the supernatant was used to determine protein content using Pierce BCA Protein Assay Kit (Thermo Scientific) according to manufacturer’s protocol. Antibody Pair Buffer Kit and Mouse IL-6, TNF-β and IFN-γ Antibody Pairs were purchased from Life Technologies (Carlsbad, CA). Absorbances were measured with Epoch Microplate Spectrophotometer (BioTek, Winooski, VT) and cytokine concentrations were normalized to total protein concentration in the kidney extract (pg/mg).
Statistical Analysis
Data were analyzed using 1- or 2- way ANOVA with post-hoc analysis as appropriate using SigmaPlot 11.0 (San Jose, CA) or GraphPad InStat 3 (GraphPad Software Inc. La Jolla, CA). P values of <0.05 were considered statistically significant.
Results
Blockade of PD-1 ligands exacerbates renal dysfunction and tissue injury after mild ischemia-reperfusion injury
To investigate the role of PD-1 ligands in kidney IRI naïve C57Bl/6 (WT), mice were treated with isotype control (rat IgG2a and/or rat IgG2b), anti-PD-L1, anti-PD-L2 or a combination of both blocking antibodies. Twenty four hours after the treatment mice underwent sham or mild (24 min ischemia) bilateral renal ischemia surgery. Subsequently the kidneys were allowed to reperfuse for 24 hours. Whereas mild ischemia did not result in a significant increase in plasma creatinine in the isotype control antibody treated mice, administration of PD-L1 or PD-L2 blocking antibodies caused a significant increase in plasma creatinine, denoting significant renal dysfunction (Figure 1). Importantly, simultaneous administration of both anti-PD-L1 and anti-PD-L2 antibodies induced a significantly greater rise in plasma creatinine when compared to the treatment with PD-L1 or PD-L2 blocking antibodies alone (Figure 1). Acute tubular necrosis (ATN) in the outer medulla region of the kidney mirrored plasma creatinine levels confirming exacerbated renal injury in the mice in which PD-1 ligand signaling was inhibited (Figure 2 and Table 1). As an additional measure of the effect of PD-1 ligand blockade on tubular epithelial cell injury, mRNA for kidney injury molecule 1 (KIM-)1 was compared between groups. KIM-1 is not expressed in healthy proximal tubular epithelial cells, but becomes upregulated shortly after injury (41). Sham KIM-1 mRNA abundance, normalized to GAPDH, was set as 1. Kidney KIM-1 expression was elevated in isotype control treated mice after IRI (645 ± 332.7) and even further elevated in mice pre-treated with the PD-L1 blocking antibody (6,326 ± 1,489, P<0.05 vs. isotype+IRI). Administration of anti-PD-L2 blocking antibody also resulted in increased KIM-1 expression compared with administration of isotype control antibodies (3,662 ± 763.5), although this increase did not reach statistical significance.
Table 1.
ATN scores in mice treated with PD-1 ligand blocking antibodies prior to IRI
| Sham | IRI + Isotype Antibody | IRI + anti-PD-L1 | IRI + anti-PD-L2 | IRI + Both Antibodies | |
|---|---|---|---|---|---|
| ATN Score | 0.2 ± 0.1 | 0.7 ± 0.3 | 2.7 ± 0.3** | 2.8 ± 0.2** | 3.7 ± 0.1**,# |
Max ATN Score is 5. Mean ± SEM,
denotes P<0.01 vs. IRI + Isotype;
denotes P<0.01 vs. IRI + anti-PD-L1 and vs. IRI + anti-PD-L2.
ATN: acute tubular necrosis; PD-1: programmed cell death 1; IRI: ischemia reperfusion injury; PD-L1: PD-1 ligand 1; PD-L2: PD-1 ligand 2.
PD-L1 and PD-L2 blockade enhances the innate immune response in the kidney after IRI
Flow cytometry was used to assess the accumulation of different immune cells in the kidney at 24 h of reperfusion. Mild ischemia (24 min) in the isotype control treated mice led to an increase in the total CD45+ population in the kidney and an increase in the CD11b+GR-1high cells (Supplemental Figure 1 and Figure 3) that have previously been identified by ImageStream analysis as kidney-infiltrating neutrophils (11). When either PD-1 ligand was blocked the percentage of total CD45+ cells in the kidney was further increased and the percentage of CD45+ cells that were CD11b+GR-1high was markedly enhanced (Supplemental Figure 1). The absolute number of CD11b+GR-1high cells in the kidney was significantly higher in all groups that were treated with PD-1 ligand blocking antibodies (Figure 3A). Similar to plasma creatinine levels and histological damage in the kidney, blockade of both PD-1 ligands resulted in additive neutrophil accumulation (Figure 3A). We also compared the accumulation of CD11b+F4/80int leukocytes, which have properties of inflammatory monocytes/macrophages in the post ischemic kidney (12). In contrast to the effect on neutrophils, blockade of PD-L1 had no effect on the accumulation of CD11b+F4/80int leukocytes (Figure 3B). However, blockade of PD-L2 did cause a significant increase in the inflammatory monocyte/macrophage number in the post-ischemic kidney (Figure 3B). Flow cytometry revealed that there is no increase in CD4+ or CD8+ T cell infiltration after IRI in mice with PD-1 ligand blockade (Figure 3C and 3D). To confirm flow cytometry results, frozen kidney sections were stained with DAPI and the 7/4 antibody that recognizes neutrophils and monocytes/macrophages (39, 40). Immunofluorescence microscopy revealed increased innate leukocyte influx in the kidneys of mice treated with PD-L1 and PD-L2 blocking antibodies (Figure 4).
PD-L1 and PD-L2 blockade leads to increased level of IL-6, CXCL1 and ICAM-1 in post-ischemic kidney
Taking into consideration studies linking PD-1 or PD-L1 blockade/deficiency to increased IL-6 production (42–44) we measured IL-6 mRNA level in the kidney of anti-PD-L1 and/or anti-PD-L2 treated mice. Sham mRNA expression of IL-6 normalized to GAPDH levels was set as 1. The values for the other groups are reported relative to sham (Table 2). Blockade of both PD-1 ligands simultaneously resulted in a more than 1000-fold increase in IL-6 mRNA expression (Table 2). To confirm our results we performed ELISA on kidney homogenates and detected a significant increase in IL-6 protein abundance in kidneys from mice treated with the combination of PD-1 ligand blocking antibodies (Supplemental Figure 2). The qPCR analysis also revealed significant increases in CXCL1 and ICAM-1 mRNA expression in mice pretreated with both PD-1 ligand blocking antibodies (Table 2). Renal IFN-γ protein levels were not significantly different after IRI between groups (ng/mg total protein measured by ELISA: IRI + Isotype: 5.8±1.2; IRI + anti-PD-L1: 6.4±0.8; IRI + anti-PD-L2: 5.9±0.7; IRI + both blocking antibodies: 5.2±0.9; N=7 to 9 per group, pooled from 3 independent experiments).
Table 2.
Changes in inflammatory mRNA abundance in the kidney 24 hrs after mild kidney IRI.
| Gene | |||
|---|---|---|---|
| Group | IL-6 | CXCL1 | ICAM-1 |
| Sham | 1 | 1 | 1 |
| IRI + isotype antibody | 42 ± 18 | 21 ± 9 | 2 ± 1 |
| IRI + anti-PD-L1 | 342 ± 85 | 71 ± 28 | 2.7 ± 0.5 |
| IRI + anti-PD-L2 | 530 ± 220 | 89 ± 28 | 3 ± 0.4 |
| IRI + both antibodies | 1,853 ± 565* | 147 ± 36* | 4 ± 1* |
For each mRNA, expression level in sham-operated mice were set to 1 and expression in each treatment group is expressed as relative arbitrary units.
denotes P < 0.05 vs. IRI + isotype antibody group within each column.
IRI: ischemia reperfusion injury; PD-L1: programmed cell death 1(PD-1) ligand 1; PD-L2: PD-1 ligand 2; IL-6: interleukin 6; CXCL1: chemokine (C-X-C motif) ligand 1; ICAM-1: Intercellular Adhesion Molecule 1.
Blockade of either PD-1 ligand exacerbates moderate renal IRI
To investigate the role of PD-1 ligands in moderate kidney IRI, mice were treated with blocking antibodies to PD-L1 or PD-L2 or isotype controls prior to 26 min bilateral IRI and analyzed after 24 hrs of reperfusion for the degree of renal injury and dysfunction. Twenty six minutes of ischemia resulted in a significant increase in PCr at 24 hr of reperfusion compared to the sham-operated control mice and tubular necrosis (Figure 5). Blockade of either PD-1 ligand significantly exacerbated the IRI-induced renal dysfunction and histological damage as measured by plasma creatinine levels and ATN, respectively (Figure 5).
Genetic deficiency of PD-L1 or PD-L2 increases ischemic kidney injury in mice
To investigate the effect of global deficiency of PD-L1 or PD-L2, KO mice were compared to WT controls (C57Bl/6) after mild kidney IR surgery (21 min ischemia) (Figure 6). Compared to WT controls, both PD-L1 KO and PD-L2 KO mice had significant renal dysfunction and ATN when exposed to mild kidney IR (Figure 6).
Role of PD-L1 ligand expression on bone marrow-derived vs. non-bone marrow derived cells in protection from kidney IRI
Since PD-L1 is expressed on many immune cells and non-immune cells such as kidney tubular epithelial cells and vascular endothelial cells we investigated the contribution of PD-L1 in the immune and non-immune compartments by generating bone marrow chimeric mice as described in the Materials and Methods section. Seven to eight weeks after irradiation and bone marrow cell transfer the mice were subjected to mild kidney IR surgery (21 min ischemia) that was not sufficient to cause renal dysfunction or inflammation in the WT → WT control chimeras or in chimeric WT mice reconstituted with PD-L1 KO bone marrow (Figure 7). However, mild ischemia did cause marked renal dysfunction and infiltration of CD11b+GR-1high innate leukocytes in PD-L1 KO mice reconstituted with WT bone marrow, that was significantly higher than the WT → WT and PD-L1 KO → WT mice (Figure 7).
Blockade of either PD-1 ligand in recipient mice prevents protection from IRI by adoptively-transferred Tregs
To study the role of PD-1 ligands in protection from kidney IRI by adoptively-transferred Tregs, naïve wild-type mice were treated with isotype control, anti-PD-L1 or anti-PD-L2 blocking antibodies. Six hours later mice were administered either 100,000 freshly-isolated Tregs in normal saline or normal saline alone intravenously. Eighteen hours after Treg or saline injection, the mice underwent sham or mild bilateral renal IR surgery (24 min ischemia). The kidneys were allowed to reperfuse for 24 hours then plasma creatinine measurements and evaluation of outer medulla ATN scores was performed. Mice that were subjected to 24 minutes of ischemia in the presence of PD-L1 or PD-L2 blocking antibodies were not protected from kidney dysfunction or histological damage by adoptive transfer of WT Tregs (Figure 8 and Supplemental Figure 3). To confirm that the freshly-isolated Tregs used in these experiments were functional we adoptively-transferred 100,000 WT Tregs to naïve mice in the absence of PD-1 ligand blocking antibodies (mice were pre-treated with an isotype control antibody), and as described previously (18–20) the WT Tregs offered significant protection from kidney dysfunction induced by 26 minutes of ischemia (24 hr post IRI plasma creatinine [mg/dl]: Sham 0.37±0.06; IRI+saline 1.5±0.27, P<0.01 vs. sham; IRI+Tregs 0.30±0.02, P<0.01 vs. IRI+saline). Twenty six minutes of ischemia was used in this control experiment so that there would be measureable and significant renal dysfunction in the IRI+saline group for the Tregs to protect from since 24 min of ischemia in the absence of PD-1 ligand blocking antibodies does not result in a significant increase in plasma creatinine at 24 hrs of reperfusion (Figure 1). In these control experiments, adoptive transfer of Tregs inhibited CD11b+GR-1high cell (neutrophil) accumulation in the kidneys of mice that underwent 26 min of ischemia, compared to normal saline treated group, similar to our previous reports (18–20). CD45+7-AAD−CD11b+GR-1high leukocyte count per gram of kidney: Sham 105,691±64,477; IRI+saline 487,080±148,260, P<0.05 vs. sham; IRI+Tregs 108,486±73,585, P<0.05 vs. IRI+saline. However, Treg administration to the mice pretreated with antibodies against PD-L1 or PD-L2 caused an increase in the neutrophil accumulation in ischemic kidneys compared to mice that did not receive Treg adoptive transfer (CD45+7-AAD−CD11b+GR-1high leukocyte count per gram of kidney: IRI+anti-PD-L1+saline 484,151±41,982 vs. 772,828±141,740 in the IRI+anti-PD-L1+Tregs group, P<0.01; and IRI+anti-PD-L2+saline 382,915±79,245 vs. 651,583±97,049 in the IRI+anti-PD-L2+Tregs group, P<0.01).
Discussion
Based on our previous studies that revealed PD-1 expression on Tregs is required for their ability to protect the kidney from IRI, we investigated the role of the known PD-1 ligands in A) the natural protective response to IRI in naïve mice and B) in the ability of adoptively transferred Tregs to protect the kidney during IRI. Using monoclonal blocking antibodies to PD-L1 and/or PD-L2 we found that PD-L1 or PD-L2 blockade, prior to IRI, results in increased kidney damage and inflammation. Furthermore, simultaneous blockade of both PD-1 ligands caused a further increase in inflammation and renal damage when compared to blockade of only one ligand. Knockout mouse models confirmed the protective role of both PD-L1 and PD-L2 in kidney IRI. Bone marrow chimeric studies revealed that PD-L1 expressed on non-bone marrow derived cells is critical for this resistance to IRI. Finally, by treating recipient mice with either PD-L1 or PD-L2 blocking antibodies, prior to adoptive transfer, the protective action of Treg transfer was completely abolished. Thus, both PD-1 ligands contribute in a non-redundant fashion to the body’s natural tissue-sparing and anti-inflammatory response to ischemia/reperfusion injury, and in addition both ligands contribute to the therapeutic mechanisms of adoptively transferred Tregs.
Ischemia reperfusion injury (as studied in this manuscript) occurs during 24 hours, which is a time frame most consistent with an innate immune response. While the majority of studies on the PD-1 and PD-1 ligands have focused on their role in antigen-specific adaptive immune responses (29, 45), some recent studies have demonstrated a role for this pathway in innate immunity (42, 46–50). In an acute liver IRI model, PD-L1 blockade increased liver damage, and administration of a PD-L1 Ig fusion protein reduced liver inflammation and injury (48). In a murine model of tuberculosis, PD-1, PD-L1 or PD-L2 blockade augmented IFN-γ production by NK cells (46) and PD-1 KO mice are more resistant to Listeria monocytogenes infection than WT controls in the absence of T and B cells (50). To further support the role of PD-1 in suppressing innate immunity, macrophages stimulated with a PD-L1 fusion protein produce less IL-12 after LPS administration than the control group (47). In the current study, we found that blocking PD-1 ligands prior to injury only modulated the influx of innate leukocytes with properties of neutrophils and inflamed monocytes/macrophages with no effect on CD4+ or CD8+ T cells.
The finding that there is additive injury when both PD-1 ligands are blocked suggests that the two ligands play different and independent roles in protecting the kidney from IRI. Other laboratories have also found that the two PD-1 ligands operate independently in other models. Brown et al. showed that blockade of PD-L1 and PD-L2 on human dendritic cells has an additive effect and causes enhanced CD4+ T cell proliferation (51). Other studies utilizing PD-L1 and PD-L2 siRNA in DCs showed that the lack of both ligands resulted in enhanced ability of DCs to induce proliferation and cytokine production in antigen-specific CD4+ T cells (52). This may partially explain our results, as DCs are important inducers of kidney IRI pathology on one hand (53), but on the other hand, if stimulated properly, can induce tolerance against kidney IRI (54) and offer protection against cisplatin-induced AKI in mice (55).
The finding that PD-1 ligands have independent roles in protection could be due to their differential expression patterns. For example, the highest expression of KIM-1 (a marker of direct tubular epithelial cell injury) was observed in the mice where PD-L1 was blocked and PD-L1 is expressed on mouse and human kidney tubular epithelial cells (56–58). While one group has shown that activating PD-L1 on isolated tubular epithelial cells promotes cell death under certain in vitro conditions (59), PD-L1 on other cell types promotes cell survival (60). Since PD-L1 is also expressed on a variety of immune cells we generated bone marrow chimeric mice to investigate which type(s) of cells must express PD-L1 for resistance to kidney IRI to occur. The results clearly show that PD-L1 expression on bone marrow derived cells is not required for resistance to IRI, but that lack of PD-L1 on non-bone marrow derived cells (possibly tubular epithelial cells or vascular endothelial cells or others) is required. Expression of PD-L2 in mice is limited exclusively to immune cells (61, 62) and PD-L2 blockade results in a more robust immune response than PD-L1 blockade in our study. Based on the expression pattern of PD-L2 in mice and our current bone marrow chimera results, our findings suggest that PD-L1 on non-immune cells and PD-L2 on immune cells mediate intrinsic resistance to kidney IRI.
Higher renal IL-6 mRNA and protein levels, due to combined blocking antibody administration, may result from enhanced accumulation of IL-6 producing immune cells infiltrating the kidney. IL-6 producing monocytes/macrophages have been implicated by others as pathogenic in kidney IRI (63). Classically activated or M1 macrophages are the predominant macrophage phenotype in the kidney during the first several days of reperfusion (16) and are capable of producing IL-6 (64). Based on these studies and our results the PD-1 ligands may restrain M1 macrophages that respond and contribute to the initial ischemic kidney injury. Whether the PD-1 ligands influence the conversion of M1 monocyte/macrophages inside the kidney to M2 reparative macrophages several days after IRI (16, 65) has not yet been investigated.
The directionality of PD-1:PD-1 ligand signaling in kidney IRI is not known and could involve signaling into cells that express PD-1 to inhibit their inflammatory response to IRI. On the other hand, stimulating PD-1 on Tregs may enhance their anti-inflammatory properties in a currently unknown way. In support of this, we and several other laboratories have found that PD-1 KO Tregs or Tregs in the presence of antibodies that interfere with PD-1 signaling do not suppress inflammation (19, 25, 28, 31, 32). Another possibility is reverse signaling, whereby Tregs (and other cells that express PD-1) could send anti-inflammatory signals into immune cells that express the PD-1 ligands as reported previously (66) or pro-survival signals through PD-1/PD-L1 interaction into PD-L1 expressing cells such as tubular epithelial cells (60). Finally, PD-L1 may interact with CD80 (67) expressed on immune cells in a manner that is anti-inflammatory and renal-protective during kidney IRI.
Our previous study showed that incubation of isolated Tregs with a blocking antibody to PD-1 (then washing), prior to adoptive transfer to WT recipients, prevented the ability of Tregs to protect the kidney. This suggested that Tregs interact with recipient cells via PD-1 and PD-1 ligand interactions that promote protection from kidney inflammation and injury. Administration of either PD-1 ligand blocking antibody prior to Treg adoptive transfer rendered the Tregs unable to protect the kidney. Surprisingly, we found that Treg adoptive transfer in the presence of PD-1 ligand blocking antibodies significantly enhanced neutrophil accumulation in the post ischemic kidney. The reasons for this are not currently known. Because we and others (18, 68) have not observed significant trafficking of Tregs into the kidney at 1 to 24 hrs of reperfusion, in the absence of pharmacological intervention, we hypothesize that Tregs either in the circulation or at some site outside the kidney provide renal protection. How and whether the PD-1 ligands modulate the trafficking of Tregs to the kidney and other locations has not been studied, but may offer some insight on how the PD-1 ligands promote Treg function. Since blocking antibodies were injected before Treg administration, we cannot exclude the possibility that PD-L1 blocking antibodies bound to PD-L1 on the adoptively transferred Tregs. In fact, PD-L1 was shown to be required for Treg-mediated suppression of IFN-γ production by CD4+ T cells (69) and Treg-mediated protection from ischemic stroke (70). Although we have not been able to observe PD-L2 expression of Tregs by flow cytometery (data not shown), recent studies suggest that PD-L2 on Tregs may promote the ability of Tregs to suppress B cell activity (71). Thus PD-1 ligands expressed on Tregs (in addition to PD-1 on Tregs) may also be important for their function in kidney IRI. Additional studies are required to determine which cells interact with Tregs in order to mediate their protective function in kidney IRI. However, these findings demonstrate that in order to protect the kidney from acute ischemic kidney injury, Tregs require the availability of both PD-L1 and PD-L2.
In contrast to our current results and the studies from other laboratories referenced above (suggesting that PD-1 ligand signaling is anti-inflammatory), there are multiple reports of both PD-1 ligands promoting inflammation in other models (37, 72–75). Interestingly, the ability of PD-L1 and PD-L2 to promote inflammation does not require PD-1 expression on target cells, suggesting the existence of a currently unknown pro-inflammatory receptor for PD-L1 and PD-L2 (37, 75). Taken together with our previous results pointing to a key role for PD-1 expressed on Tregs in protection from kidney IRI (19), our current findings suggest that the anti-inflammatory and tissue-protective interaction between PD-L1/PD-L2 and PD-1 is more important than the interaction of PD-L1/PD-L2 with an immune-activating receptor in ischemic kidney injury.
In summary, our findings establish a protective role for both PD-1 ligands in a mouse model of ischemic AKI and shed additional light on the PD-1 signaling pathway in innate immune responses. It appears that PD-L1 on non-immune cells and PD-L2 on immune cells are key elements of the natural resistance to kidney IRI. In addition, our results indicate that PD-L1 and PD-L2 participate in Treg-mediated protection against kidney IRI. Therefore, induction of intrinsic PD-L1 and/or PD-L2 expression and signaling may constitute a novel strategy to protect the kidney from ischemic injury.
Supplementary Material
Acknowledgments
This work was supported by NIH grants K01DK088967 (GRK) and R03DK099489 (GRK).
We thank Dr. Lieping Chen and Dr. Victor Engelhard for kindly providing the PD-L1 KO mice. We acknowledge the technical assistance from the UVA Advanced Microscopy Core Facility and the UVA Research Histology Core Facility. We thank Dr. Mark Okusa for thoughtful suggestions about this project.
References
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Liangos O, Wald R, O’bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 3.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 4.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinsey GR, Okusa MD. Pathogenesis of acute kidney injury: foundation for clinical practice. Am J Kidney Dis. 2011;58:291–301. doi: 10.1053/j.ajkd.2011.02.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adybelli Z, Giuliani A, Danesi TH, Kim JC, Nayak A, Neri M, Virzi GM, Brocca A, Scalzotto E, Salvador L, Ronco C. Cardiac Surgery-Associated Acute Kidney Injury. Cardiorenal medicine. 2013;3:178–199. doi: 10.1159/000353134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’donnell MP, Rabb H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 2007;71:1223–1231. doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 10.Lee HT, Kim M, Kim M, Kim N, Billings FTT, D’agati VD, Emala CW., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–722. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 15.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinsey GR, Huang L, Jaworska K, Khutsishvili K, Becker DA, Ye H, Lobo PI, Okusa MD. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77:771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Nho D, Chung HS, Shin MK, Kim SH, Bae H. CD4+CD25+ regulatory T cells attenuate cisplatin-induced nephrotoxicity in mice. Kidney Int. 2010;78:1100–1109. doi: 10.1038/ki.2010.139. [DOI] [PubMed] [Google Scholar]
- 22.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H. Foxp3(+) regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 23.Kim MG, Koo TY, Yan JJ, Lee E, Han KH, Jeong JC, Ro H, Kim BS, Jo SK, Oh KH, Surh CD, Ahn C, Yang J. IL-2/Anti-IL-2 Complex Attenuates Renal Ischemia-Reperfusion Injury through Expansion of Regulatory T Cells. J Am Soc Nephrol. 2013;24:1529–1536. doi: 10.1681/ASN.2012080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 26.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, June CH, Murphy WJ, Munn DH, Blazar BR. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mcgee HS, Yagita H, Shao Z, Agrawal DK. Programmed Death-1 Antibody Blocks Therapeutic Effects of T-Regulatory Cells in Cockroach Antigen-Induced Allergic Asthma. American Journal of Respiratory Cell and Molecular Biology. 2010;43:432–442. doi: 10.1165/rcmb.2009-0258OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. New England Journal of Medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheridan C. Cautious optimism surrounds early clinical data for PD-1 blocker. Nat Biotech. 2012;30:729–730. doi: 10.1038/nbt0812-729. [DOI] [PubMed] [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, Mcdermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, Mcmiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, Mcdonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong H, Zhu G, Tamada K, Flies DB, Van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 37.Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol. 2012;303:F1487–1494. doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson RB, Hobbs JaR, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 40.Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–2097. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- 41.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 42.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. The Journal of Clinical Investigation. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dulos J, Carven GJ, Van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, De Haan AF, Mulders P, Punt CJ, Jacobs JF, Schalken JA, Oosterwijk E, Van Eenennaam H, Boots AM. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. Journal of Immunotherapy. 2012;35:169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 45.Mcdermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer medicine. 2013;2:662–673. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, De La Barrera SS, Garcia VE. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis. 2010;202:524–532. doi: 10.1086/654932. [DOI] [PubMed] [Google Scholar]
- 47.Cho HY, Choi EK, Lee SW, Jung KO, Seo SK, Choi IW, Park SG, Choi I, Lee SW. Programmed death-1 receptor negatively regulates LPS-mediated IL-12 production and differentiation of murine macrophage RAW264.7 cells. Immunol Lett. 2009;127:39–47. doi: 10.1016/j.imlet.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Ji H, Shen X, Gao F, Ke B, Freitas MC, Uchida Y, Busuttil RW, Zhai Y, Kupiec-Weglinski JW. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology. 2010;52:1380–1389. doi: 10.1002/hep.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rui Y, Honjo T, Chikuma S. Programmed cell death 1 inhibits inflammatory helper T-cell development through controlling the innate immune response. Proc Natl Acad Sci U S A. 2013;110:16073–16078. doi: 10.1073/pnas.1315828110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broadwater M, Choi IH, Tamada K, Chen L. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 52.Hobo W, Maas F, Adisty N, De Witte T, Schaap N, Van Der Voort R, Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8+ T cells. Blood. 2010;116:4501–4511. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30:268–277. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD. Dendritic cells tolerized with adenosine A(2)AR agonist attenuate acute kidney injury. J Clin Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tadagavadi RK, Reeves WB. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol. 2010;21:53–63. doi: 10.1681/ASN.2009040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115:184–191. doi: 10.1016/j.clim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Schoop R, Wahl P, Le Hir M, Heemann U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant. 2004;19:2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 58.Starke A, Lindenmeyer MT, Segerer S, Neusser MA, Rusi B, Schmid DM, Cohen CD, Wuthrich RP, Fehr T, Waeckerle-Men Y. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int. 2010;78:38–47. doi: 10.1038/ki.2010.97. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Zhang J, Guo G, Ruan Z, Jiang M, Wu S, Guo S, Fei L, Tang Y, Yang C, Jia Z, Wu Y. Induced B7-H1 expression on human renal tubular epithelial cells by the sublytic terminal complement complex C5b-9. Molecular immunology. 2009;46:375–383. doi: 10.1016/j.molimm.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 60.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 62.Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH, Kelley VR. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J Immunol. 2007;179:7466–7477. doi: 10.4049/jimmunol.179.11.7466. [DOI] [PubMed] [Google Scholar]
- 63.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005;16:3315–3325. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 64.Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Lech M, Grobmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders HJ. Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy. J Am Soc Nephrol. 2014;25:292–304. doi: 10.1681/ASN.2013020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuipers H, Muskens F, Willart M, Hijdra D, Van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36:2472–2482. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 67.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai L-W, Yong K-C, Lien Y-HH. Pharmacologic recruitment of regulatory T cells as a therapy for ischemic acute kidney injury. Kidney Int. 2012;81:983–992. doi: 10.1038/ki.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, Sayegh MH, Guleria I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 70.Li P, Mao L, Liu X, Gan Y, Zheng J, Thomson AW, Gao Y, Chen J, Hu X. Essential Role of Program Death 1-Ligand 1 in Regulatory T-Cell-Afforded Protection Against Blood-Brain Barrier Damage After Stroke. Stroke. 2014 doi: 10.1161/STROKEAHA.113.004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gotot J, Gottschalk C, Leopold S, Knolle PA, Yagita H, Kurts C, Ludwig-Portugall I. Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo. Proc Natl Acad Sci U S A. 2012;109:10468–10473. doi: 10.1073/pnas.1201131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. Journal of neuroinflammation. 2013;10:111. doi: 10.1186/1742-2094-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 74.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.