Abstract
In utero ethanol exposure causes Fetal Alcohol Spectrum Disorders, associated with reduced brain plasticity; the mechanisms of these effects are not well understood, particularly with respect to glial involvement.
Astrocytes release factors that modulate neurite outgrowth. We explored the hypothesis that ethanol inhibits neurite outgrowth by increasing the release of inhibitory chondroitin sulfate proteoglycans (CSPGs) from astrocytes.
Astrocyte treatment with ethanol inhibited the activity of arylsulfatase B (ARSB), the enzyme that removes sulfate groups from chondroitin-4-sulfate (C4S) and triggers the degradation of C4S, increased total sulfated glycosaminoglycans (GAGs), C4S, and neurocan core-protein content and inhibited neurite outgrowth in neurons co-cultured with ethanol-treated astrocytes in vitro, effects reversed by treatment with recombinant ARSB.
Ethanol also inhibited ARSB activity and increased sulfate GAG and neurocan levels in the developing hippocampus after in vivo ethanol exposure.
ARSB silencing increased the levels of sulfated GAGs, C4S, and neurocan in astrocytes and inhibited neurite outgrowth in co-cultured neurons, indicating that ARSB activity directly regulates C4S and affects neurocan expression.
In summary, this study reports two major findings: ARSB modulates sulfated GAG and neurocan levels in astrocytes and astrocyte-mediated neurite outgrowth in co-cultured neurons; and ethanol inhibits the activity of ARSB, increases sulfated GAG, C4S, and neurocan levels, and thereby inhibits astrocyte-mediated neurite outgrowth.
An unscheduled increase in CSPGs in the developing brain may lead to altered brain connectivity and to premature decrease in neuronal plasticity and therefore represents a novel mechanism by which ethanol can exert its neurodevelopmental effects.
Keywords: Astrocytes, arylsulfatase B, fetal alcohol, chondroitin sulfate, neurocan
Introduction
Ethanol profoundly affects the developing brain, causing structural and functional abnormalities associated with cognitive and behavioral dysfunctions, characteristic of fetal alcohol spectrum disorders (FASD) (Guerri 1998; Riley et al. 2011). Growing evidence indicates that altered plasticity mediates the effects of in utero alcohol exposure, particularly in the hippocampus (Lebel et al. 2012; Medina 2011).
Chondroitin sulfate proteoglycans (CSPGs) are extracellular matrix (ECM) proteins that, in the central nervous system (CNS), act as barriers preventing cell migration, axonal growth, and neuronal plasticity (Carulli et al. 2005). CSPGs are highly expressed in the developing brain, where they localize in proximity to growing axons (Bovolenta and Fernaud-Espinosa 2000). During postnatal development, CSPGs accumulate around synapses as a component of the perineuronal net, which stabilizes synapses and reduces the plasticity of the mature brain (Wang and Fawcett 2012).
CSPGs consist of core-proteins attached to linear chain(s) of glycosaminoglycans (GAGs); the inhibitory properties of CSPGs depend on both the core protein and the GAG chains. GAG chains are formed by repeated disaccharides, which in the case of CS are D-glucuronic acid and D-N-acetylgalactosamine modified by sulfation (Prydz and Dalen 2000). Astrocytes are major producers of CS-GAGs (Johnson-Green et al. 1991; Powell and Geller 1999). Removal of CSPG GAG chains by the enzyme chondroitinase ABC (cABC) or the inhibition of GAG polymerization by silencing chondroitin polymerizing factor in astrocytes attenuates the inhibition of neurite outgrowth and guidance by astrocyte-derived CSPG (Laabs et al. 2007; Snow et al. 1990). In particular, chondroitin 4-sulfate (C4S) has been associated with inhibition of axonal guidance and growth (Wang et al. 2008).
Neurocan is a CSPG, which is expressed only in the nervous system and is formed by a core-protein covalently bound to three CS chains (Grumet et al. 1996; Rauch et al. 2001). Neurocan is produced by glial cells in vivo and in vitro, and is an inhibitor of neurite outgrowth; indeed, neurocan is a component of the “glial boundaries” responsible for preventing developing neurons from extending axons or dendrites in improper directions and of the glial scars, which inhibit axonal regeneration after brain injury (Asher et al. 2000; Fitch and Silver 1997; Rauch et al. 2001). Degradation of core-protein neurocan by extracellular proteases increases neurite outgrowth and axonal growth (Bukhari et al. 2011; Cua et al. 2013).
Arylsulfatase B (ARSB) removes sulfate groups from C4S. Although this enzyme was initially identified as only a lysosomal enzyme, we and others reported ARSB expression in extralysosomal cell compartments, including the plasma membrane (Bhattacharyya et al. 2010; Bhattacharyya et al. 2009; Mitsunaga-Nakatsubo et al. 2009; Prabhu et al. 2009). We have also reported that the expression of syndecan-1, decorin, and versican is modified by ARSB activity in a malignant mammary cell line (MCF-7) and human prostate cells (Bhattacharyya et al. (in press); Bhattacharyya et al. 2008b). Although the role of ARSB in regulating GAG and CSPG core-protein levels in brain cells has not been reported, a recent study indicates that ARSB treatment after spinal cord injury decreases C4S immunoreactivity at the site of the injury and improves locomotor function (Yoo et al. 2013).
We have reported that astrocyte-induced neurite outgrowth of hippocampal neurons is inhibited when astrocytes are treated with ethanol, which strongly alters the ECM composition and inhibits the release of pro-neuritogenic factors (Guizzetti et al. 2010).
In this study, we demonstrated for the first time that both ARSB silencing and ethanol inhibit ARSB activity and increase the levels of C4S and neurocan in astrocytes and inhibit hippocampal neuron neurite outgrowth mediated by astrocyte-neuron adhesion. The effects of ethanol on sGAGs and neurite outgrowth are in part prevented by recombinant (r)ARSB supplementation. Furthermore, ethanol inhibited ARSB activity and increased sGAG and neurocan levels in the developing rat hippocampus in vivo.
This study describes a novel mechanism by which ethanol increases the levels of inhibitory cues released by astrocytes, which may have profound effects on neuronal development and may be involved in the reduced neuronal plasticity observed in FASD (Lebel et al. 2012).
Materials and Methods
Astrocyte cultures and astrocyte-neuron co-cultures
Time-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). Cortical and hippocampal astrocytes were prepared from rat fetuses at gestational day 21 as previously described (Guizzetti et al. 1996). Astrocytes were grown in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% Fetal Bovine Serum (FBS), 100 units/ml penicillin, and 100 µg/ml streptomycin under a humidified atmosphere of 5% CO2-95% air at 37 °C. After 9 days in culture, astrocytes were plated in the appropriate vessel. Four days later, cells were serum-deprived for 24h followed by treatments. All treatments were carried out in serum-free DMEM medium supplemented with 0.1% Bovine Serum Albumin (BSA) and antibiotics.
For astrocyte-neuron co-cultures, astrocytes were sub-cultured in glass coverslips placed into 24-well plates at a 2.5 × 105 astrocytes/ coverslip density and maintained for 4 days in complete medium followed by 24 h serum deprivation (in DMEM/BSA medium) before ethanol treatments. Hippocampal neurons were prepared from rat fetuses at gestational day 21 as described by us (Guizzetti et al. 2008; Guizzetti et al. 2010). Freshly isolated neurons resuspended in DMEM/BSA medium were immediately plated on top of control, ethanol pre-treated, or ARSB siRNA-transfected astrocytes in glass coverslips (1×104 neurons/coverslip) for an additional 18 h.
Ethanol and rARSB incubations
Treatments with 25, 50, or 75 mM ethanol were carried out in serum-free medium for 24 h; these exposures did not cause toxicity in astrocytes as previously reported by us (Guizzetti and Costa 1996). To reduce ethanol evaporation, cultures were placed in sealed chambers with a reservoir tray containing water supplemented with ethanol at the same concentration used in the culture medium (25, 50, or 75 mM) and gassed with a 5% CO2/95% air gas mixture; under these conditions no significant ethanol loss was observed at the end of the incubations (Guizzetti et al. 2007). Human rARSB (purchased from R&D Systems; 1 ng/ml) was added to astrocyte cultures for 24 h in the presence and in the absence of ethanol.
Silencing of ARSB by siRNA
Astrocyte transfection was carried out using lipofectamine RNAiMAX Transfection Reagent in Opti-MEM I medium according to the manufacturer’s instructions (Invitrogen; Carlsbad, CA) using ARSB siRNA and non-target siRNA (Qiagen, Valencia, CA). On the day of transfection primary rat astrocytes were switched to antibiotic-free medium (DMEM with 10%FBS) and supplemented with 50 nM ARSB siRNA or 50 nM non-target control, lipofectamine RNAiMAX Transfection Reagent, and Opti-MEM I according to the manufacturer's instructions for 24 h; the medium was then replaced with DMEM/BSA medium for an additional 24 (for a total of 48 h after transfection); in co-culture experiments neurons are plated on top of silenced astrocytes for an additional 18 h. The specific silencing of ARSB in astrocytes was confirmed by measuring ARSB activity and ARSB mRNA.
Enzymatic activities
Measurements of ARSB, galactose-6-sulfatase (GALNS), arylsulfatase A (ARSA), steroid sulfatase (STS), and alkaline phosphatase (AP) activities in astrocyte homogenates were carried out as previously reported (Bhattacharyya and Tobacman 2007).
Sulfated (s)GAG and C4S-GAG determinations
Total sulfated GAG content (which includes chondroitin 4-sulfate, chondroitin 6-sulfate, keratan sulfate, dermatan sulfate, heparan sulfate, and heparin) was measured in cell lysates by sulfated GAG assay (Blyscan™, Biocolor Ltd., Newtownabbey, Northern Ireland), using 1,9-dimethylmethylene blue. This method measures the sulfated polysaccharide component of sulfated GAG chains, but not the degraded disaccharide fragments or hyaluronan. The reaction was performed in the presence of excess unbound dye. The cationic dye and GAG at acid pH produce an insoluble dye-GAG complex; the GAG content was determined spectrophotometrically (at 656 nm absorbance) after dye recovery from the test sample by exposure to Blyscan dissociation reagent and expressed as µg/mg of protein of cell lysate. C4S was measured in samples immunoprecipitated with antibodies against C4S. A mouse monoclonal IgM, clone 4D1; cat.# sc-47718 from Santa Cruz Biotechnology (Santa Cruz, CA; discontinued) was used in most experiments. The specificity of this antibody was previously published (Bhattacharyya et al. 2008b). Some results (Fig. 7 D) were also confirmed using a mouse monoclonal IgM, clone LY111, cat. # 270421 purchased from Amsbio (Lake Forest, CA). The precipitate was eluted with dye-free elution buffer and subjected to sulfated GAG assay as previously described (Bhattacharyya et al. 2008a; Feferman et al. 2013).
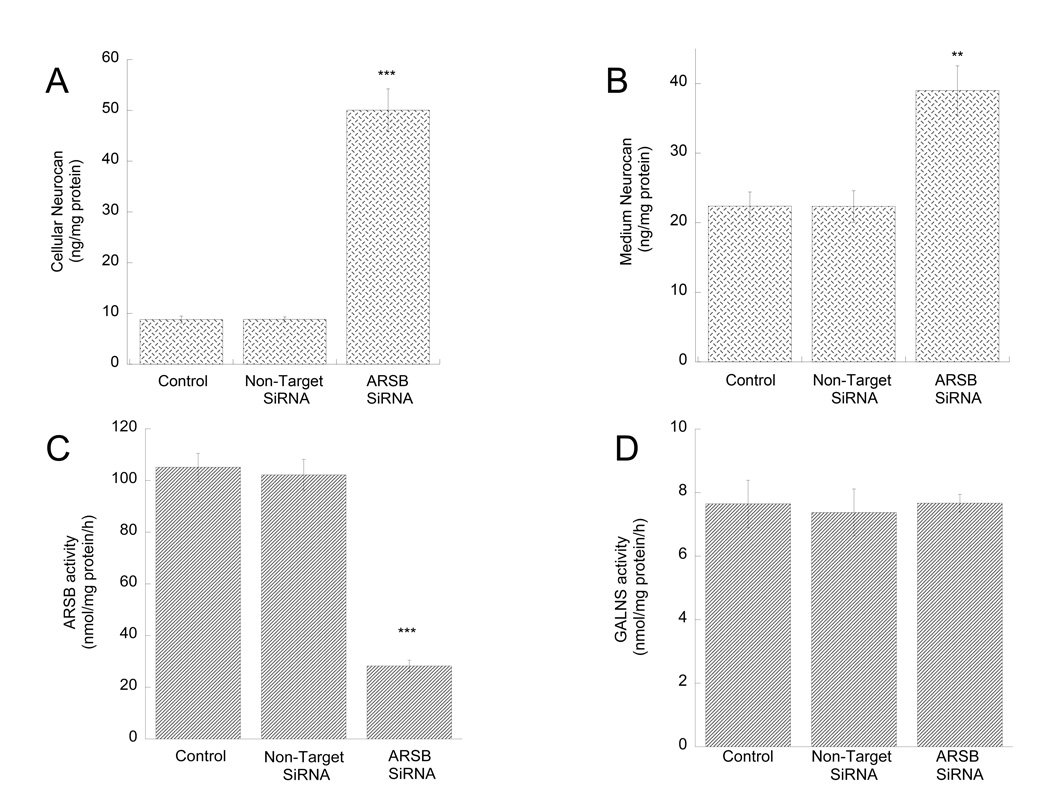
Figure 7. Effect of ARSB silencing on neurocan levels in cell lysates and conditioned media and on ARSB and GALNS activity in astrocytes.
Primary rat cortical astrocytes were transfected with a non-target (NT siRNA) or an ARSB specific siRNA (ARSB siRNA) using lipofectamine RNAiMAX. Forty-eight hours after transfection, neurocan levels were quantified in astrocyte cell lysates (A) and astrocyte-conditioned media (B); ARSB (C) and GALNS (D) activities were quantified in astrocyte homogenates as described in “Methods”. **: p< 0.01; ***: p< 0.001 compared with control by the Student’s t-test (n=3).
Quantitative PCR
RNA was isolated from astrocytes using RNeasy Plus Mini Kit (Qiagen; Valencia, CA). Total RNA was transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Carlsbad, CA) as previously described (Chen et al. 2013). ARSB and neurocan mRNA levels in astrocytes were quantified by SYBR Green and TaqMan qRT-PCR respectively; primers used for ARSB analysis were: forward: CCTCCTGGACGAAGCAGTGGG; reverse: CCCGCCGTTGTCTGTGGAGA. Messenger RNA levels were normalized to levels of HPRT1 measured in the same samples (forward primer: TCCTCAGACCGCTTTTCCCGC; reverse primer: TCATCATCACTAATCACGACGCTGG).
ELISA
Neurocan protein levels in the culture medium and cell extracts of astrocytes were measured by ELISA using a commercially available kit (Cederlane, Burlington, NC) following the directions of the manufacturer.
Protein extraction and sample preparation
Cell extracts for Western blot, ELISA, total sGAG and C4S determinations were prepared by solubilizing cells in lysis buffer (Cell Signaling) supplemented by a protease inhibitor cocktail (Roche, Indianapolis, IN); for ARSB activity, cell homogenates were prepared by sonication. Released neurocan was determined in astrocyte-conditioned medium centrifuged at 800g for 10 min to remove detached cells.
Western blot analysis
Astrocyte proteins were extracted and Western blot analysis was carried out as previously described (Guizzetti et al. 2007; Yagle et al. 2001). Total cellular protein content was quantified by the Bradford method. Samples were treated with 0.01U/µg protein (cell lysate) or 0.01 U/µl (astrocyte conditioned-medium) ABC chondroitinase (Roche) for 3 h at 37°C and 30 µg proteins (cell lysate) or 30 µl (astrocyte-conditioned medium) were loaded on SDS-PAGE, subjected to electrophoresis, transferred to polyvinylidene difluoride membranes, and labeled overnight with polyclonal antibodies against neurocan (Chemicon/ Millipore; Tamecula, CA) followed by an HRP-conjugated secondary antibody.
Morphometric analysis of neurite outgrowth
Astrocyte-neuron co-cultures were fixed, permeabilized, labeled with a neuronal-specific anti-βIII-tubulin antibody followed by an Alexa Fluor-555 secondary antibody. Coverslips were mounted on microscope slides; images were acquired using a Zeiss Axiovision fluorescence microscope attached to a digital camera. Measurements of the length of the longest neurite (axons) and minor neurites of pyramidal neurons were carried out using ImageJ software as described by us (Guizzetti et al. 2010). At least 30 neurons/treatment were measured.
In vivo neonatal alcohol exposure
Neonatal male rats were intragastrically intubated daily with 5.25 g/kg ethanol in milk formula (Similac Advance Early Shield with iron) divided into 2 feedings 2 h apart between postnatal days 4 and 9. Two additional milk formula-only feedings were given to ethanol-treated pups (2 h apart) to supplement the lack of sucking while intoxicated as previously described (Tran et al. 2007). The total ethanol volume in the formula was 11.9%. Each intubation volume was 0.0278 ml/g. The control groups received 2 sham intubations 2 hour apart. Animals were sacrificed 2 h after the last intubation; in some animals, hippocampi were collected and homogenized for ARSB activity and total sulfated GAG analysis; other animals were perfused for immunohistochemistry analysis, as described below.
Neurocan immunohistochemistry by gold immunolabeling
The levels of neurocan were determined by immunohistochemistry in the CA1 region of the hippocampus of male pups at postnatal day 9 as previously described (Moonat et al. 2010). Rats were deeply anaesthetized and perfused transcardially with normal saline (100 ml), followed by 400 ml of 4% ice-cold paraformaldehyde fixative. Brains were dissected and placed in fixative for 20 hrs at 4°C, soaked subsequently in 10%, 20%, and 30% sucrose and then frozen. 20-µm coronal sections were prepared using a cryostat, placed in 0.01 M phosphate-buffered saline (PBS) at 4°C and used for gold-immunolabeling. Sections were incubated for 18 h with a neurocan antibody (rabbit polyclonal antibody; EMD Millipore), washed, and incubated for 1 h in gold particle-conjugated anti-rabbit secondary antibody (Nanoprobes) and developed using silver enhancements solution (Ted Pella). Protein levels in gold-immunolabeled sections were quantified using the Loats Image Analysis System (Loats Associates Inc.) at high magnification (100×).
Statistical Analysis
Student’s t-test (for one-to-one comparisons) or 1-way ANOVA followed by Tukey’s post-hoc test (for multiple comparisons) were used to determine significant differences from controls.
Results
Ethanol inhibited ARSB activity in astrocytes
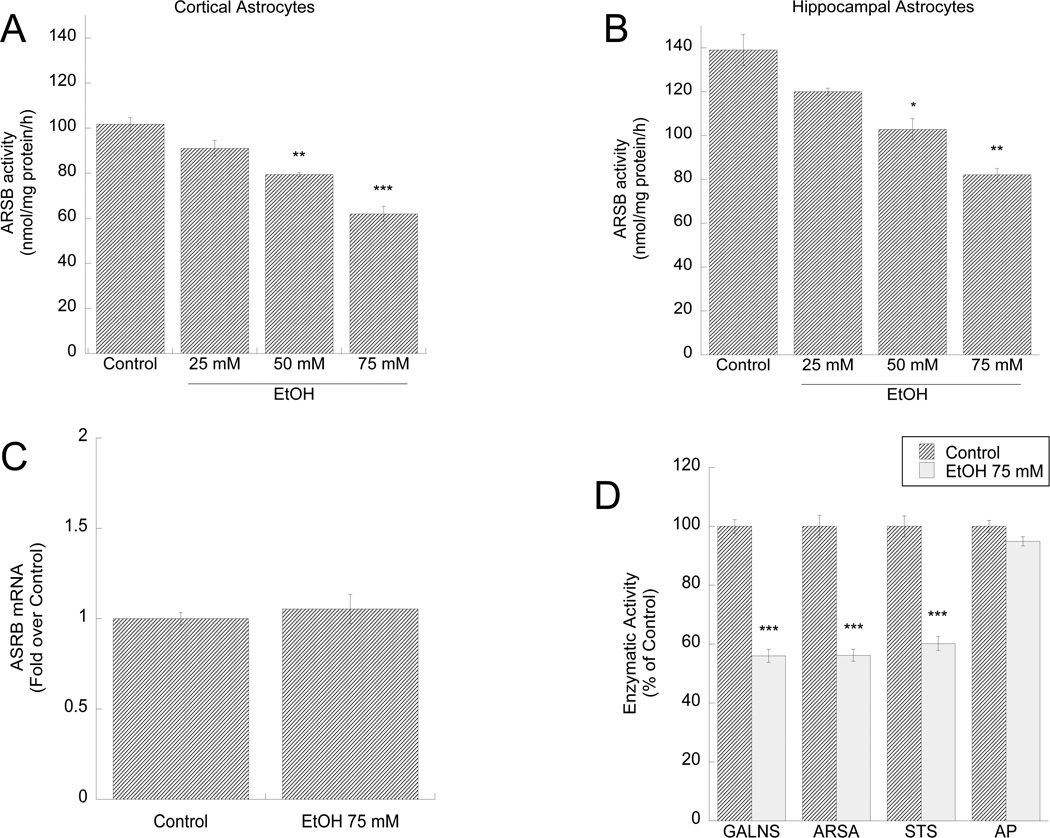
ARSB activity in primary astrocytes prepared from rat neocortex and hippocampus was inhibited by ethanol in a dose-dependent manner (Fig. 1 A,B); ethanol (75 mM) did not affect ARSB mRNA levels (Fig. 1 C). Ethanol (75 mM) also inhibited the activity of other sulfatase enzymes, namely galactose-6-sulfatase (GALNS), arylsulfatase A (ARSA), and steroid sulfatase (STS) (Fig. 1 D), but did not inhibit the activity of alkaline phosphatase (AP) (Fig. 1 D) and lactate dehydrogenase (not shown), indicating a specific inhibitory effect of alcohol on sulfatase enzymes. Previously we reported that ethanol up to 200 mM does not affect astrocyte survival (Guizzetti and Costa 1996), indicating that ethanol specifically affects sulfatase activity without causing cytotoxicity.
Figure 1. Effect of ethanol on ARSB, GALNS, ARSA, STS, and AP activity and ARSB mRNA levels in astrocytes.
Primary rat cortical (A) and hippocampal (B) astrocytes were incubated for 24 h in the presence or absence of 25, 50, or 75 mM ethanol. ARSB activity was quantified in astrocyte homogenates and expressed as nmol/mg protein. C, D: cortical astrocytes were treated with 75 mM ethanol for 24 h. C: RNA was extracted and ARSB mRNA levels were quantified by qPCR, normalized to HPRT1 levels and expressed as fold over control. D: GALNS, ARSA, STS, and AP activities were measured in astrocyte homogenates and expressed as % of control. *: p< 0.05; **: p< 0.01; ***: p< 0.001 compared with control by the Tukey’s post-hoc test (A, B) or Student’s t test (D) (n=6).
Ethanol increased sulfated GAG levels in astrocytes
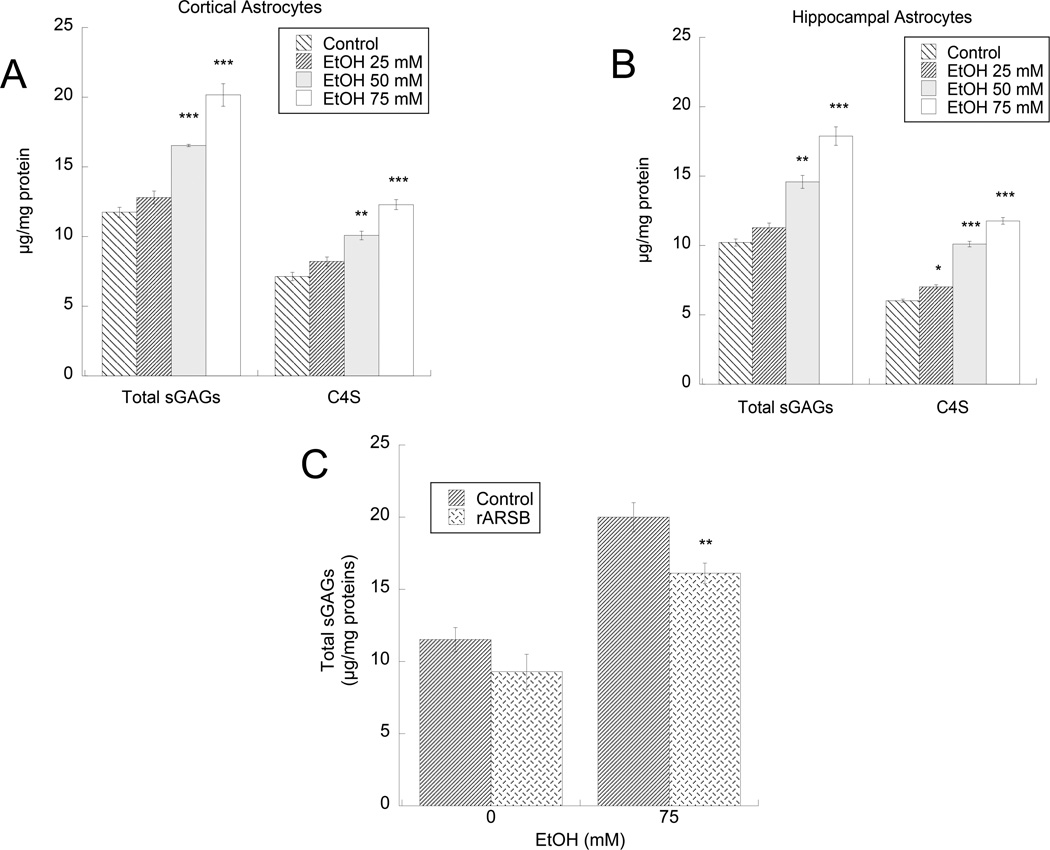
In agreement with the notion that ARSB removes sulfate groups from C4S and triggers GAG degradation, we found that ethanol increased total sulfated GAGs as well as C4S in the cell lysate of hippocampal and cortical astrocytes in a concentration-dependent manner (Fig. 2 A,B). Under basal conditions ARSB activity was significantly higher (p = 0.0077) and total GAGs and C4S were significantly lower (p = 0.026 and 0.026 respectively) in hippocampal astrocytes compared to cortical astrocytes, consistent with regulation of C4S degradation.
Figure 2. Effect of ethanol on sGAGs and C4S-GAGs in cortical and hippocampal astrocytes.
Primary rat cortical (A) and hippocampal (B) astrocytes were incubated for 24 h in the presence or absence of 25, 50, or 75 mM ethanol. Sulfated GAG and C4S-GAG levels were quantified as described in “Methods”. *: p< 0.05; **: p< 0.01; ***: p< 0.001 compared with control by the Tukey’s post-hoc test (n=6). C: Primary rat cortical astrocytes were incubated for 24 h in the presence of 75 mM ethanol, 1ng/ml rARSB, or ethanol plus rARSB; total sGAGs were quantified. **: p< 0.01compared with ethanol alone by the Student’s t-test (n=3).
The treatment with 1 ng/ml rARSB for 24 h significantly reversed the effect of ethanol on sGAG levels (Fig. 3 C).
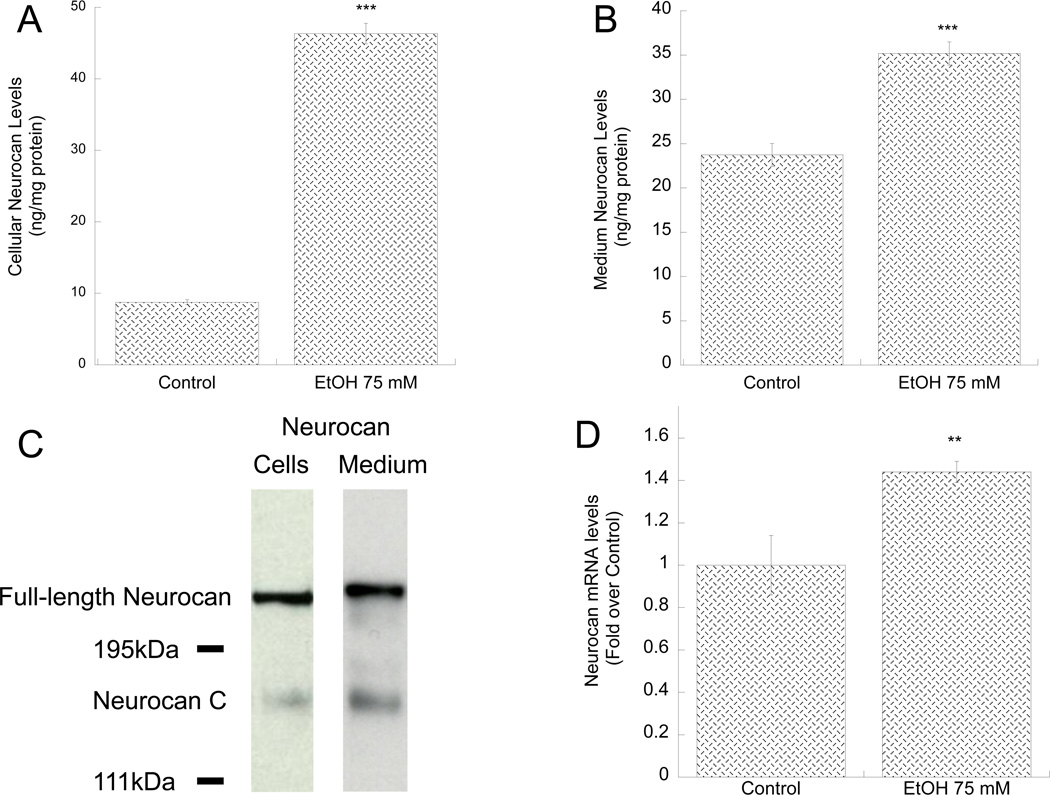
Figure 3. Effect of ethanol on neurocan protein and mRNA levels in primary astrocytes.
A, B, D: Primary rat cortical astrocytes were incubated for 24 h in the presence or absence of 75 mM ethanol. Neurocan protein levels were quantified in cell lysates (A) and astrocyte-conditioned media (B) by ELISA. C: Representative immunoblots of neurocan forms in astrocyte cell lysate (left) and astrocyte-conditioned medium (right). D: Neurocan mRNA levels were quantified by qPCR using TaqMan probes and primers; results were normalized to β-actin and expressed as fold over control. **: p< 0.01; ***: p< 0.001 compared with control by Student’s t test (n=6).
Ethanol increased neurocan expression and release in astrocytes
The proteoglycan neurocan is formed by a core protein covalently bound to three chondroitin sulfate chains mainly sulfated in position 4 (C4S) of the D-N-acetylgalactosamine residues (Rauch et al. 2001) and is produced abundantly by cultured astrocytes (Asher et al. 2000; Oohira et al. 1994). As we have previously shown that ARSB activity modulated the levels of two proteoglycans, decorin and syndecan-1, in epithelial cells (Bhattacharyya et al. 2008b), we tested whether ethanol-induced inhibition of ARSB activity affected neurocan expression in astrocytes. We found that ethanol increased the levels of neurocan measured in astrocyte cell lysate (Fig. 3 A) and astrocyte-conditioned medium (Fig. 3 B) respectively.
Neurocan has been reported to exist as the full-length, intact form of 270 kDa and as two smaller forms derived from proteolytic cleavage: 160 kDa neurocan-C and 130 kDa neurocan-N, which also have GAG attachments (Matsui et al. 1994; Rauch et al. 1992). Because the ELISA method used to quantify neurocan levels does not provide information on whether astrocytes produced and released the full-length or the proteolytically processed forms, Western blot analysis was carried out on astrocyte cell lysate and astrocyte-conditioned medium with an antibody that recognized full-length and neurocan-C forms only. Both forms were present in astrocyte cell lysate and medium, but the predominant form was the full-size neurocan (Fig. 3 C), in agreement with a previous report (Asher et al. 2000). Neurocan mRNA levels were also significantly upregulated by ethanol (Fig. 3 D).
Ethanol treatment in astrocytes inhibited neurite outgrowth of hippocampal neurons
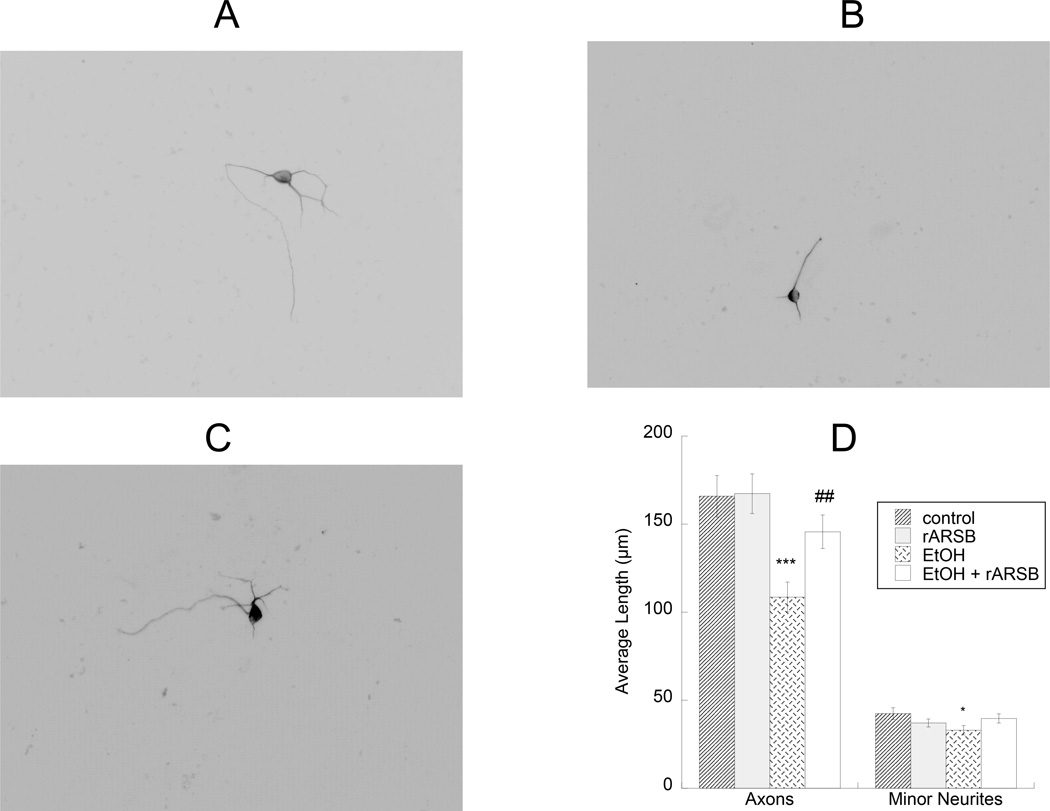
To test the hypothesis that ethanol-treated astrocytes inhibit neurite outgrowth, we plated neurons on top of astrocytes that were pre-treated with 75 mM ethanol in the presence or in the absence of rARSB (1 ng/ml) for 24 h. We observed that neurons co-cultured with ethanol-pre-treated astrocytes developed shorter axons and minor neurites compared to neurons co-cultured with control astrocytes; this effect was attenuated by rARSB (Fig. 4 A–D).
Figure 4. Effect of ethanol- and rARSB-treated astrocytes on hippocampal neuron neurite outgrowth.
Astrocytes were treated for 24 h with 75 mM ethanol, 1 ng/ml rARSB, or ethanol plus rARSB. Hippocampal neurons were plated on top of pre-treated astrocytes for an additional 18h. Cultures were then fixed and stained with neuron-specific βIII-tubulin antibody and a fluorescent secondary antibody. Neurite length was measured using the software Image J. Shown are representative neurons incubated with control (A), ethanol-treated (B), ethanol- and rARSB-treated (C) astrocytes. D: Morphometric quantification of axons and minor neurite length in 50 cells per treatment. ***, p<0.001; *, p<0.05 vs. control; ##, p<0.01 by Student’s t test.
Ethanol inhibited ARSB activity and increased sulfated GAGs and neurocan levels after in vivo ethanol exposure
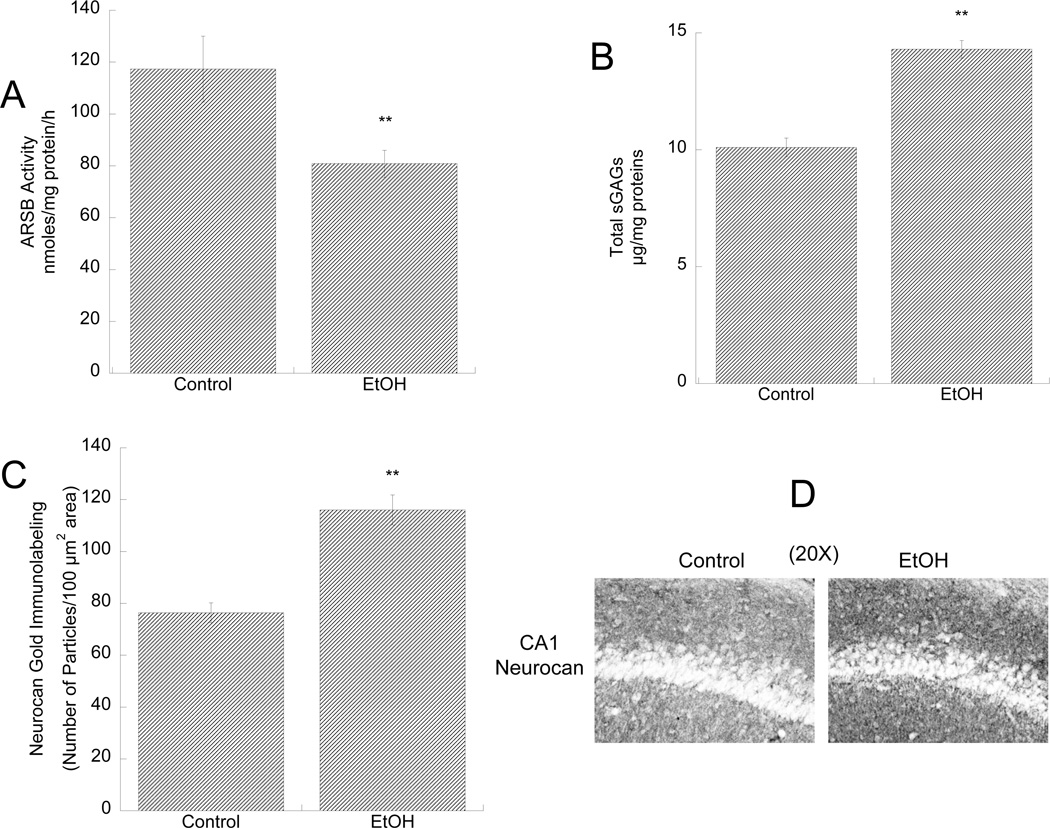
We investigated whether ethanol inhibited ARSB activity and increased sGAG and neurocan levels in vivo in the developing hippocampus of male pups intragastrically intubated between postnatal days 4 and 9 with 5.25 g/Kg alcohol. The paradigm of ethanol exposure used in this study represent an established neonatal rat model of alcohol exposure that mimics heavy alcohol exposures during the third trimester of human gestation (Tran et al. 2007). We found that ARSB activity was significantly reduced and that sGAG levels were significantly increased in the hippocampus of ethanol-treated animals compared to sham intubated controls (Fig.5 A,B). Furthermore, neurocan levels in the statum oriens of the CA1 region of the hippocampus, measured by immunohistochemistry, were significantly increased (Fig. 5 C,D)
Figure 5. Effect of in vivo ethanol exposure on ARSB activity, sGAG levels, and neurocan expression in the developing hippocampus.
Male rat pups were intubated with 5g/Kg ethanol or were sham (control) intubated from PD4 to PD9. ARSB activity (A) and sGAG levels (B) were measured in hippocampus homogenates 2 h after the last intubation. **: p< 0.01; by the Student’s t-test (n=3). C, D: Neurocan expression in the stratum oriens of the CA1 region of the hippocampus was determined by gold-immunolabeling histochemistry followed by quantification using the Loats Image Analysis System. C: Quantification of neurocan gold-immunoparticles expressed as number of immunogold particles/100 µm2 area. **: p<0.01; by the Student’s t-test; n=3. D: Representative images of neurocan gold-immunolabeling staining in control (left image) and ethanol-treated (right image) pups.
ARSB silencing increased sulfated GAG and neurocan levels in astrocytes
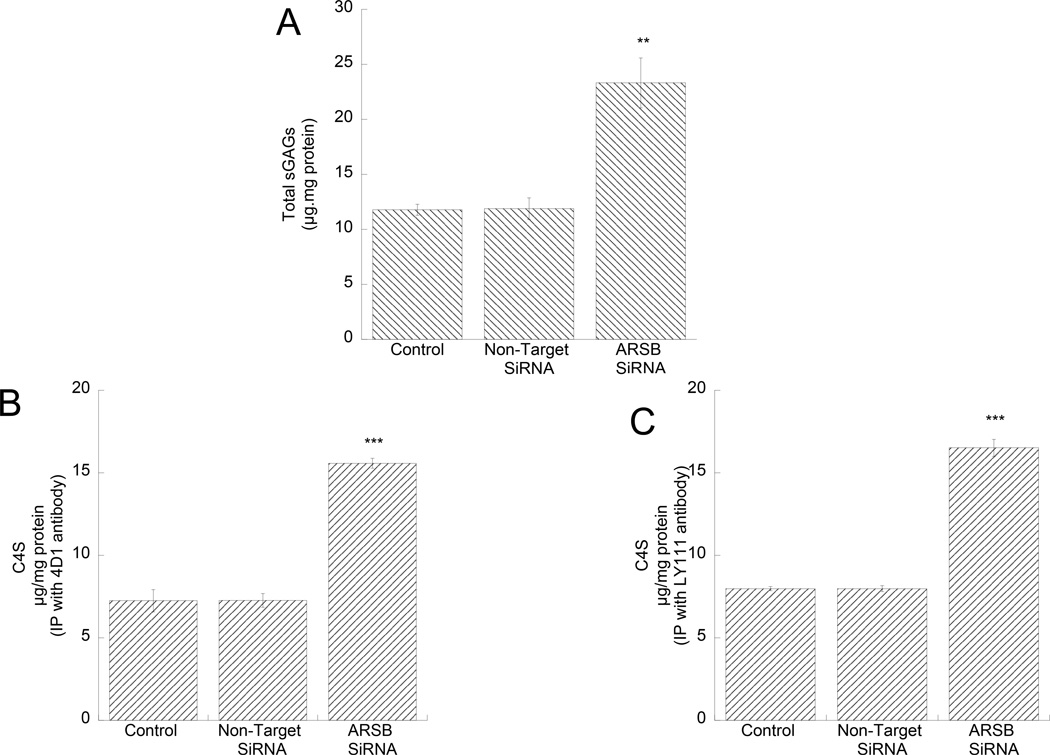
In order to investigate whether ethanol exposure and ARSB silencing produced similar effects, we silenced ARSB in astrocytes by siRNA. We found that the levels of sGAGs were increased in astrocytes transfected with ARSB siRNA (Fig. 6 A). C4S in the cell lysates of ARSB-silenced astrocytes were detected after immunoprecipitation of C4S with 2 different monoclonal antibodies: clone 4D1 (Fig. 6 B), and clone LY111 (Fig. 6 C) ARSB silencing. Similar results were obtained with both antibodies; C4S was increased after ARSB silencing and accounted for most of the increase in total sGAGs.
Figure 6. Effect of ARSB silencing on sGAG and C4S-GAG levels in astrocytes.
Primary rat cortical astrocytes were transfected with a non-target (NT siRNA) or an ARSB specific siRNA (ARSB siRNA) using lipofectamine RNAiMAX. Forty-eight hours after transfection, sGAG levels were quantified in astrocyte cell lysates (A); the levels of C4S-GAGs were quantified in astrocyte cell lysates after C4S immunoprecipitation with mouse monoclonal antibodies clone 4D1 (B) from Santa Cruz and Ly111 (C) from Amsbio. **: p< 0.01; ***: p< 0.001 compared with control by the Student’s t-test (n=3).
ARSB activity was reduced by more than 70% (Fig. 7 C) in astrocytes silenced by ARSB siRNA, while the activity of another GAG sulfatase, GALNS, was not affected (Fig. 7 D), demonstrating the specificity of the silencing. ARSB mRNA levels were reduced by approximately 50% 24 h after ARSB SiRNA transfection (not shown); we also tested another ARSB siRNA and obtained effects similar to the ones reported in Fig. 7 (not shown); together these results indicate that in our cultures we reach highly efficient and specific ARSB silencing.
ARSB silencing in astrocytes inhibited hippocampal pyramidal neuron neurite outgrowth
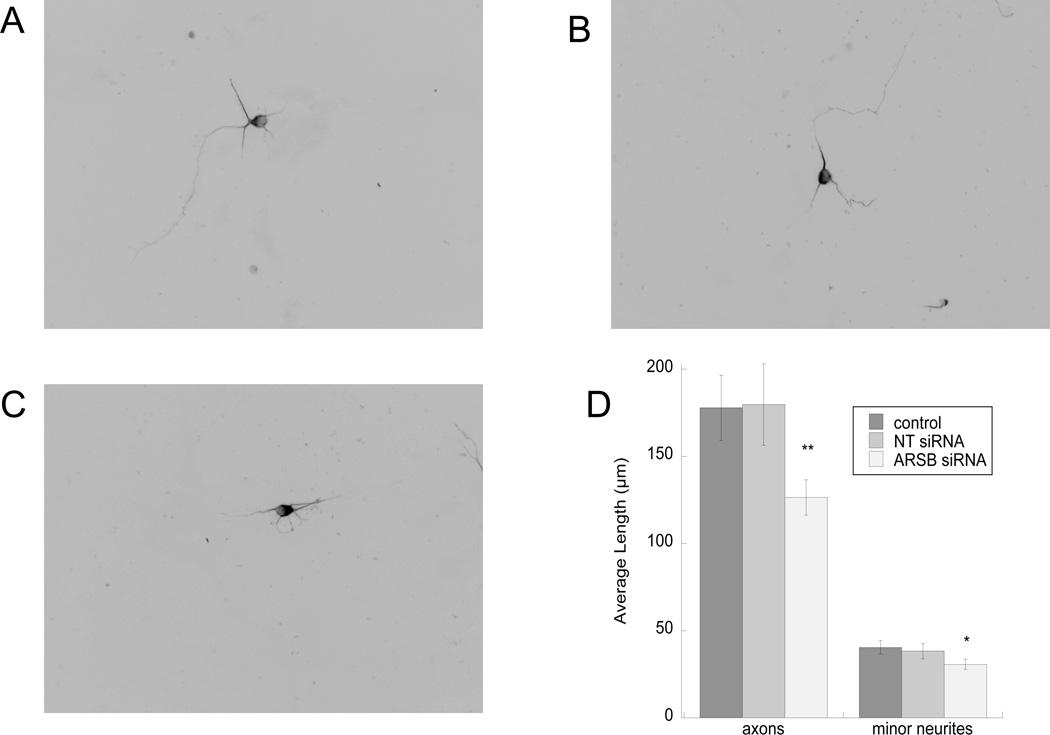
Neurite outgrowth of hippocampal pyramidal neurons co-cultured with ARSB-silenced astrocytes was reduced when compared to neurite outgrowth in neurons co-cultured with untreated astrocytes and astrocytes transfected with a non-target siRNA (Fig. 8 A–D), supporting the hypothesis that ARSB activity modulates neurite outgrowth. The same experiment was also repeated using a different siRNA with similar results (not shown).
Fig. 8. Effect of ARSB-silencing in astrocytes on neurite outgrowth.
Astrocytes were transfected for 48 h with non-target (NT) or ARSB siRNA. Neurons were plated on top of astrocytes for 16 h. Cultures were then fixed and stained with neuron-specific βIII-tubulin antibody and a fluorescent secondary. Neurite length was measured using the software Image J. Shown are representative images of neurons incubated on top of control (A), non-target siRNA-transfected (B), and ARSB siRNA-transfected (C) astrocytes. D: Morphometric quantification of neurite length in 30 cells per treatment. *, p<0.05; **: p< 0.01 by Student’s t test.
Discussion
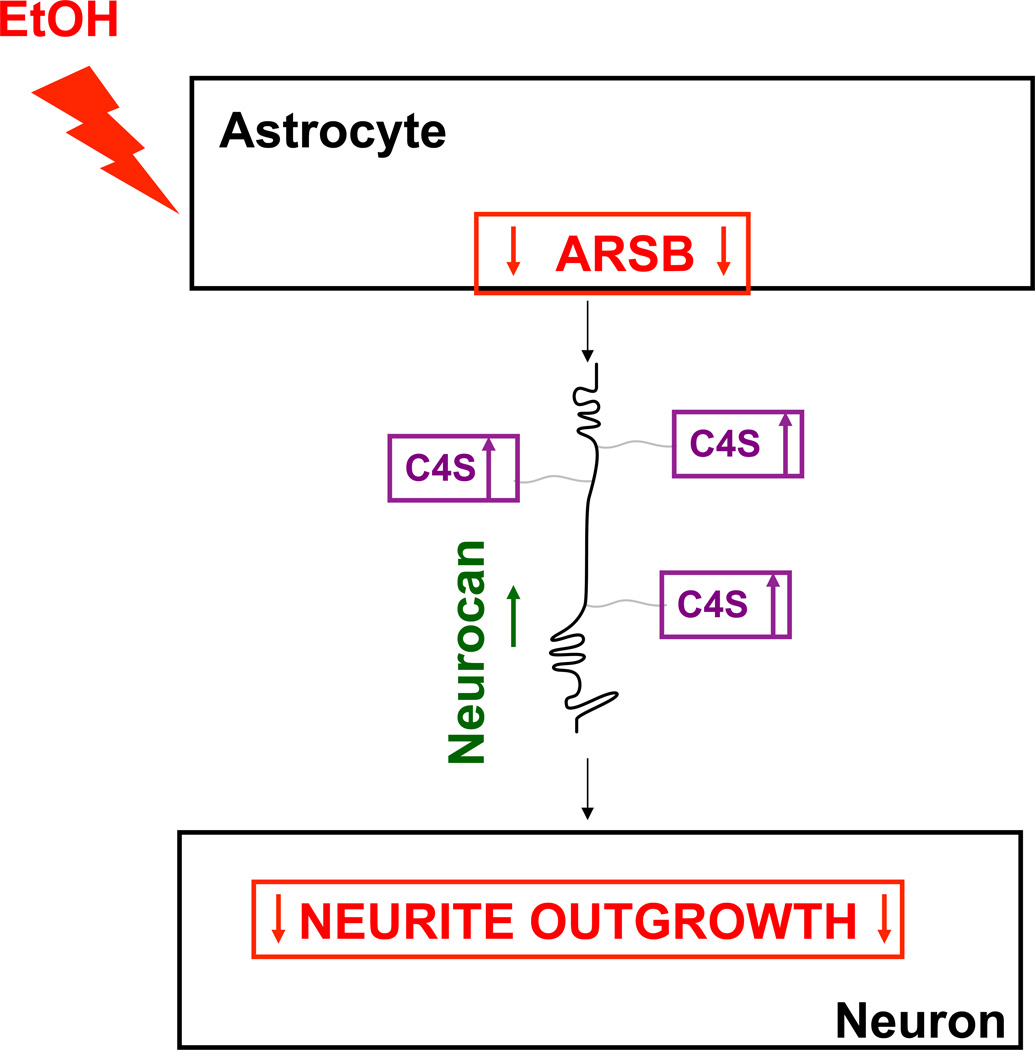
In the present study we report that ethanol inhibits ARSB activity (Fig. 1), upregulates neurocan, increases sGAGs (Figs. 2, 3), and inhibits neurite outgrowth in hippocampal pyramidal neurons plated on top of ethanol-treated astrocytes (Fig. 4). Figure 9 shows a schematic representation of the proposed model by which ethanol inhibits neurite outgrowth via ARSB inhibition with increases in C4S and neurocan. Highly sulfated CSPGs and C4S inhibit neurite outgrowth (Friedlander et al. 1994; Wang et al. 2008).
Fig. 9. Proposed mechanism of ethanol-induced inhibition of neurite outgrowth mediated by astrocyte-neuron adhesion.
This schematic indicates that ethanol, through the inhibition of ARSB activity, increases astrocyte C4S and core-protein neurocan levels leading to inhibition of neurite outgrowth mediated by astrocyte-neuron contact.
The effects of ethanol on ARSB activity (Fig. 1 A,B), total sGAGs, and C4S (Fig. 2 A,B) were dose-dependent and significant at does ranging from 25 mM to 75 mM (Fig. 2 B). The alcohol concentrations used in this study (25, 50, and 75 mM corresponding to 0.115, 0.23, and 0.35 g/dl, respectively) are clinically relevant, as they are found in the blood of individuals after moderate to high ethanol intake (Adachi et al. 1991) and are within the range of concentrations recommended for in vitro studies (Deitrich and Harris 1996).
We demonstrated that ethanol-induced inhibition of ARSB activity is involved in the upregulation of GAGs and neurocan since: a) ARSB silencing in astrocytes increased GAG and neurocan levels (Figs. 7); b) hippocampal astrocytes, which expressed higher ARSB activity than cortical astrocytes, had significantly lower levels of GAGs (Figs. 1, 2); c) rARSB significantly reduced the increase in sGAGs following ethanol (Fig. 2 C) and prevented in part ethanol-treated astrocyte-induced inhibition of neurite outgrowth induced by ethanol (Fig. 4).
Arylsulfatase B removes 4-sulfate groups from chondroitin-4-sulfate and dermatan sulfate at the non-reducing end of the sulfated glycosaminoglycan (GAG) chain and thereby is required for the degradation of these sulfated glycosaminoglycans (de Sousa Junior et al. 1990; Glaser and Conrad 1979). Indeed, in the genetic disorder Mucopolysaccharidosis VI (MPS VI; Maroteaux-Lamy Syndrome) in which ARSB activity is reduced, sulfated GAGs accumulate throughout the body (Valayannopoulos et al. 2010). While we have not yet investigated the mechanism by which ARSB might regulate neurocan expression in astrocytes, we have previously reported that ARSB regulate expression of other PGs, namely decorin, syndecan-1, and versican, in MCF-7 and prostate cells (Bhattacharyya et al. (in press); Bhattacharyya et al. 2008b).
We have also shown that neurite outgrowth mediated by astrocyte-neuron adhesion is inhibited by ethanol (Fig. 4); this effect of ethanol is due, at least in part, to the inhibition of ARSB activity and to the increase in neurocan and C4 as indicated by the fact that similar effects on neurite outgrowth were also observed when ARSB was silenced in astrocytes (Fig. 8). In the co-culture model used in these studies, neurons were plated on top of treated astrocytes and the two cell types were in contact. This model is optimal to investigate the inhibitory effect of neurocan on neurite outgrowth, which is mediated by the inhibition of cell-cell adhesion (Friedlander et al. 1994; Grumet et al. 1993; Milev et al. 1996). We have previously shown that ethanol inhibits the release of neuritogenic factors from carbachol-stimulated astrocytes in a “sandwich” co-culture system in which the two cell populations face each other without touching; these conditions are optimal to investigate the effect of ethanol on the release of neuritogenic factors not requiring cell-cell contact (Guizzetti et al. 2010). Together, our previous and current studies suggest that ethanol profoundly affects astrocyte secretion leading to the generation of an environment that inhibits neuronal development.
The reported findings demonstrate for the first time that ARSB activity plays a major role in modulating GAG and CSPG levels in brain cells. Inhibition of ARSB activity is a novel mechanism by which pathological conditions and neurotoxicant exposures may restrict neuronal plasticity. Interestingly, a recent study reported that ARSB infusion in the spinal cord after injury decreased CS-GAG levels and increased locomotor function (Yoo et al. 2013), thereby supporting the hypothesis that ARSB may be a key regulator of GAG levels and neuronal plasticity in the CNS.
Major sulfation modifications of brain CS are in position 4 (C4S) and position 6 (C6S) of the N-acetylgalactosamine residue (Ishii and Maeda 2008; Kitagawa et al. 1997). C4S-GAGs, the specific targets of ARSB, are inhibitors of axonal growth (Wang et al. 2008), while the role of C6S-GAG in neurite outgrowth remains controversial (Butterfield et al. 2010; Carulli et al. 2005; Lin et al. 2011).
In conclusion, two major findings are described in this study: 1) the inhibitory effect of ethanol on ARSB activity was identified and associated with an increase in C4S-GAG and neurocan levels both in vitro and in vivo and with the inhibition of neurite outgrowth; 2) the role of ARSB as modulator of sulfated GAG and core-protein neurocan levels in astrocytes and neurite outgrowth was established.
The hypothesis that ethanol may reduce neuronal plasticity by increasing C4S-GAGs and neurocan in the brain is novel, but is consistent with previous FASD research. Our findings are consistent with the reported effect of prenatal ethanol on the corpus callosum (Norman et al. 2009), as CSPGs are inhibitory cues involved in the formation of the corpus callosum (Lindwall et al. 2007). Also, ethanol exposure during brain development affects visual cortex plasticity (Medina et al. 2003), and GAGs and neurocan are involved in the reduction in experience-dependent plasticity in the adult visual cortex (Pizzorusso et al. 2002). Also the findings are consistent with the observation that ethanol inhibits L1CAM adhesion (Bearer et al. 1999; Chen and Charness 2012; Fitzgerald et al. 2011; Ramanathan et al. 1996), since neurocan, by binding to L1CAM in GAG-dependent and -independent manners, inhibits homophilic interactions, as well as neuronal adhesion and neurite outgrowth (Rauch et al. 2001). Therefore, by increasing CSPGs, ethanol may considerably decrease the plasticity of the developing brain, leading to some of the neurodevelopmental consequences of FASD.
Main Points.
Astrocyte ARSB modulates C4S and neurocan levels and astrocyte-mediated neurite outgrowth.
Ethanol inhibits the activity of ARSB and increases C4S and neurocan levels in astrocytes and in the hippocampus of ethanol-treated neonatal rats. Ethanol also inhibits astrocyte-mediated neurite outgrowth, an effect reversible by recombinant ARSB supplementation.
Acknowledgements
This work was supported by grant AA017180 to MG from the National Institute of Alcoholism and Alcohol Abuse and by ULI RR029879 to JKT. We thank Dr. Lucio G. Costa for helpful early discussion of the project, Dr. Subhash Pandey for his help with immunohistochemistry, and Dr. Douglas Feinstein for his help with fluorescent microscopy.
Footnotes
Conflict of Interest: The authors of this manuscript declare no conflicts of interest.
References
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52:448–453. doi: 10.15288/jsa.1991.52.448. [DOI] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Feferman L, Tobacman JK. Arylsulfatase B Regulates Versican Expression by Galectin-3 and AP-1 Mediated Transcriptional Effects. Oncogene. in press doi: 10.1038/onc.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Gill R, Chen ML, Zhang F, Linhardt RJ, Dudeja PK, Tobacman JK. Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J Biol Chem. 2008a;283:10550–10558. doi: 10.1074/jbc.M708833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Kotlo K, Shukla S, Danziger RS, Tobacman JK. Distinct effects of N-acetylgalactosamine-4-sulfatase and galactose-6-sulfatase expression on chondroitin sulfates. J Biol Chem. 2008b;283:9523–9530. doi: 10.1074/jbc.M707967200. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Solakyildirim K, Zhang Z, Chen ML, Linhardt RJ, Tobacman JK. Cell-bound IL-8 increases in bronchial epithelial cells after arylsulfatase B silencing due to sequestration with chondroitin-4-sulfate. Am J Respir Cell Mol Biol. 2010;42:51–61. doi: 10.1165/rcmb.2008-0482OC. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Solakyildirim K, Zhang Z, Linhardt RJ, Tobacman JK. Chloroquine reduces arylsulphatase B activity and increases chondroitin-4-sulphate: implications for mechanisms of action and resistance. Malar J. 2009;8:303. doi: 10.1186/1475-2875-8-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Tobacman JK. Steroid sulfatase, arylsulfatases A and B, galactose-6-sulfatase, and iduronate sulfatase in mammary cells and effects of sulfated and non-sulfated estrogens on sulfatase activity. J Steroid Biochem Mol Biol. 2007;103:20–34. doi: 10.1016/j.jsbmb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Fernaud-Espinosa I. Nervous system proteoglycans as modulators of neurite outgrowth. Prog Neurobiol. 2000;61:113–132. doi: 10.1016/s0301-0082(99)00044-1. [DOI] [PubMed] [Google Scholar]
- Bukhari N, Torres L, Robinson JK, Tsirka SE. Axonal regrowth after spinal cord injury via chondroitinase and the tissue plasminogen activator (tPA)/plasmin system. J Neurosci. 2011;31:14931–14943. doi: 10.1523/JNEUROSCI.3339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield KC, Conovaloff A, Caplan M, Panitch A. Chondroitin sulfate-binding peptides block chondroitin 6-sulfate inhibition of cortical neurite growth. Neurosci Lett. 2010;478:82–87. doi: 10.1016/j.neulet.2010.04.070. [DOI] [PubMed] [Google Scholar]
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang X, Kusumo H, Costa LG, Guizzetti M. Cholesterol efflux is differentially regulated in neurons and astrocytes: Implications for brain cholesterol homeostasis. Biochim Biophys Acta. 2013;1831:263–275. doi: 10.1016/j.bbalip.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Charness ME. Ethanol inhibits neuronal differentiation by disrupting activity-dependent neuroprotective protein signaling. Proc Natl Acad Sci U S A. 2008;105:19962–19967. doi: 10.1073/pnas.0807758105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Charness ME. Ethanol disrupts axon outgrowth stimulated by netrin-1, GDNF, and L1 by blocking their convergent activation of Src family kinase signaling. J Neurochem. 2012;123:602–612. doi: 10.1111/j.1471-4159.2012.07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cua RC, Lau LW, Keough MB, Midha R, Apte SS, Yong VW. Overcoming neurite-inhibitory chondroitin sulfate proteoglycans in the astrocyte matrix. Glia. 2013;61:972–984. doi: 10.1002/glia.22489. [DOI] [PubMed] [Google Scholar]
- de Sousa Junior JF, Nader HB, Dietrich CP. Sequential degradation of chondroitin sulfate in molluscs. Desulfation of chondroitin sulfate without prior depolymerization by a novel sulfatase from Anomalocardia brasiliana. J Biol Chem. 1990;265:20150–20155. [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Feferman L, Bhattacharyya S, Deaton R, Gann P, Guzman G, Kajdacsy-Balla A, Tobacman JK. Arylsulfatase B (N-acetylgalactosamine-4-sulfatase): potential role as a biomarker in prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:277–284. doi: 10.1038/pcan.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DM, Charness ME, Leite-Morris KA, Chen S. Effects of ethanol and NAP on cerebellar expression of the neural cell adhesion molecule L1. PLoS One. 2011;6:e24364. doi: 10.1371/journal.pone.0024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser JH, Conrad HE. Chondroitin SO4 catabolism in chick embryo chondrocytes. J Biol Chem. 1979;254:2316–2325. [PubMed] [Google Scholar]
- Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J Cell Biol. 1993;120:815–824. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Sakurai T. Functions of brain chondroitin sulfate proteoglycans during developments: interactions with adhesion molecules. Perspect Dev Neurobiol. 1996;3:319–330. [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M, Renau-Piqueras J. Glia and fetal alcohol syndrome. Neurotoxicology. 2001;22:593–599. doi: 10.1016/s0161-813x(01)00037-7. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Chen J, Oram JF, Tsuji R, Dao K, Moller T, Costa LG. Ethanol induces cholesterol efflux and up-regulates ATP-binding cassette cholesterol transporters in fetal astrocytes. J Biol Chem. 2007;282:18740–18749. doi: 10.1074/jbc.M702398200. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Inhibition of muscarinic receptor-stimulated glial cell proliferation by ethanol. J Neurochem. 1996;67:2236–2245. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. J Biol Chem. 2008;283:31884–31897. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia. 2010;58:1395–1406. doi: 10.1002/glia.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Maeda N. Spatiotemporal expression of chondroitin sulfate sulfotransferases in the postnatal developing mouse cerebellum. Glycobiology. 2008;18:602–614. doi: 10.1093/glycob/cwn040. [DOI] [PubMed] [Google Scholar]
- Johnson-Green PC, Dow KE, Riopelle RJ. Characterization of glycosaminoglycans produced by primary astrocytes in vitro. Glia. 1991;4:314–321. doi: 10.1002/glia.440040309. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Tsutsumi K, Tone Y, Sugahara K. Developmental regulation of the sulfation profile of chondroitin sulfate chains in the chicken embryo brain. J Biol Chem. 1997;272:31377–31381. doi: 10.1074/jbc.272.50.31377. [DOI] [PubMed] [Google Scholar]
- Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O'Connor MJ, Narr KL, Kan E and others. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Rosahl TW, Whiting PJ, Fawcett JW, Kwok JC. 6-Sulphated chondroitins have a positive influence on axonal regeneration. PLoS One. 2011;6:e21499. doi: 10.1371/journal.pone.0021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Matsui F, Watanabe E, Oohira A. Immunological identification of two proteoglycan fragments derived from neurocan, a brain-specific chondroitin sulfate proteoglycan. Neurochem Int. 1994;25:425–431. doi: 10.1016/0197-0186(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Medina AE. Fetal alcohol spectrum disorders and abnormal neuronal plasticity. Neuroscientist. 2011;17:274–287. doi: 10.1177/1073858410383336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci. 2003;23:10002–10012. doi: 10.1523/JNEUROSCI.23-31-10002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Maurel P, Haring M, Margolis RK, Margolis RU. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-zeta/beta, and N-CAM. J Biol Chem. 1996;271:15716–15723. doi: 10.1074/jbc.271.26.15716. [DOI] [PubMed] [Google Scholar]
- Mitsunaga-Nakatsubo K, Kusunoki S, Kawakami H, Akasaka K, Akimoto Y. Cell-surface arylsulfatase A and B on sinusoidal endothelial cells, hepatocytes, and Kupffer cells in mammalian livers. Med Mol Morphol. 2009;42:63–69. doi: 10.1007/s00795-009-0447-x. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2010;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oohira A, Matsui F, Watanabe E, Kushima Y, Maeda N. Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody IG2 in the rat cerebrum. Neuroscience. 1994;60:145–157. doi: 10.1016/0306-4522(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Pascual M, Guerri C. The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem. 2007;103:557–568. doi: 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Powell EM, Geller HM. Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia. 1999;26:73–83. doi: 10.1002/(sici)1098-1136(199903)26:1<73::aid-glia8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Prabhu SV, Bhattacharyya S, Guzman-Hartman G, Macias V, Kajdacsy-Balla A, Tobacman JK. Extra-lysosomal localization of arylsulfatase B in human colonic epithelium. J Histochem Cytochem. 2009;59:328–335. doi: 10.1369/0022155410395511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Sci. 2000;113(Pt 2):193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Wilkemeyer MF, Mittal B, Perides G, Charness ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133:381–390. doi: 10.1083/jcb.133.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U, Feng K, Zhou XH. Neurocan: a brain chondroitin sulfate proteoglycan. Cell Mol Life Sci. 2001;58:1842–1856. doi: 10.1007/PL00000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U, Karthikeyan L, Maurel P, Margolis RU, Margolis RK. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. J Biol Chem. 1992;267:19536–19547. [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Tran TD, Stanton ME, Goodlett CR. Binge-like ethanol exposure during the early postnatal period impairs eyeblink conditioning at short and long CS-US intervals in rats. Dev Psychobiol. 2007;49:589–605. doi: 10.1002/dev.20226. [DOI] [PubMed] [Google Scholar]
- Valayannopoulos V, Nicely H, Harmatz P, Turbeville S. Mucopolysaccharidosis VI. Orphanet J Rare Dis. 2010;5:5. doi: 10.1186/1750-1172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y and others. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Hoecker HL, Boys CJ, Lim KO. Global Functional Connectivity Abnormalities in Children with Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2013;37:748–756. doi: 10.1111/acer.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagle K, Lu H, Guizzetti M, Moller T, Costa LG. Activation of mitogen-activated protein kinase by muscarinic receptors in astroglial cells: Role in DNA synthesis and effect of ethanol. Glia. 2001;35:111–120. doi: 10.1002/glia.1076. [DOI] [PubMed] [Google Scholar]
- Yoo M, Khaled M, Gibbs KM, Kim J, Kowalewski B, Dierks T, Schachner M. Arylsulfatase B improves locomotor function after mouse spinal cord injury. PLoS One. 2013;8:e57415. doi: 10.1371/journal.pone.0057415. [DOI] [PMC free article] [PubMed] [Google Scholar]