Abstract
Background
Dimethylamylamine (DMAA) is a component of many dietary supplements and has recently been associated with numerous adverse effects, prompting the US military and World Anti-Doping Agency to ban its use as a supplement. The current study aimed to elucidate the abuse liability profile of DMAA.
Methods
Dose-response studies of DMAA were performed with Swiss-Webster mice in locomotor and conditioned place-preference assays. The discriminative stimulus effects of DMAA were investigated in Sprague-Dawley rats trained to discriminate either cocaine or methamphetamine from saline.
Results
DMAA produced dose-dependent locomotor depression and fully substituted for cocaine and partially substituted for methamphetamine. In the conditioned place-preference assay, DMAA produced an inverted-U-shaped dose-response curve, with intermediate doses producing significant place preference.
Conclusions
The cocaine- and methamphetamine-like discriminative stimulus effects and the conditioned place preference produced by DMAA suggest that is has potential for abuse. These findings in combination with reports of substantial adverse effects of DMAA in humans suggest that control of DMAA may warrant further consideration.
Keywords: Dimethylamylamine, abuse liability, conditioned place preference, drug discrimination, locomotor activity
1. INTRODUCTION
Dimethylamylamine (DMAA) is an aliphatic amine with sympathomimetic properties that has been used for multiple purposes and referred by numerous names, including methylhexaneamine, 2-amino-4-methylhexane, forthane, and geranamine, since its inception in 1944 (Lammie, 2013). Initially developed in 1944 by Eli Lilly as a nasal decongestant (Shonle and Rohrmann, 1944), the vasoconstrictive and sympathomimetic efficacy of DMAA has been well characterized. Original reports indicated that DMAA was a less potent and longer-lasting vasoconstrictor compared to epinephrine with systemic toxicity greater than ephedrine, but less than amphetamine (Council of Pharmacy and Chemistry, 1950). Similarly, DMAA increased blood pressure in dogs (Swanson and Chen, 1946, 1948; Marsh, 1948). Whereas the sympathomimetic effects of DMAA have been characterized, its exact pharmacological mechanism has not.
Although the use of DMAA as a decongestant ended in 1983, it resurfaced in 2006 as a component of dietary supplements for weight loss and exercise, such as Jack3d, OxyELITE pro, and Hydroxystim (Lammie, 2013). The use of DMAA-containing dietary supplements is widespread in the military with 22% of Army and Air-Force personnel reporting having used these products and 10% reporting weekly use (Austin, et al., 2012). In many dietary supplements, DMAA has been marketed under the name geranamine due to the assertion that DMAA was naturally found in geranium oil (Lammie, 2013). Various reports using slightly different methodologies have yielded inconsistent and often contradictory evidence as to whether or not DMAA exists naturally in geranium oil (Gauthier, 2013). However, a recent study using ultra performance liquid chromatography mass spectrometry failed to detect any naturally occurring DMAA in geranium oils (Austin et al., 2014).
Numerous adverse effects have been associated with the use of DMAA, the most common of which include tachycardia, nausea, vomiting, agitation, tremor, dizziness, headache, and chest pain (Forrester, 2013). More serious and life-threatening effects have also been reported, such as hemorrhagic stroke (Gee, 2010; 2012; Young et al., 2012), hepatotoxicity (Foley et al., 2014), myocardial infarction (Smith et al., 2014), and death (Eliason et al., 2012). Generally, DMAA is not taken alone, but as a component of weight-loss supplements, several of which also contain caffeine (Lammie, 2013). However, nearly pure DMAA has been sold as a substitute for cocaine or 1-benzylpiperazine (Gee, 2010; 2012). Given the pressor action and widespread use of caffeine, a synergistic effect between DMAA and caffeine may be responsible for some of the adverse effects reported (Nurminen et al., 1999). As a result of these adverse effects, DMAA has caught the attention of regulatory agencies worldwide, with New Zealand fully removing it from the market in 2012 (Eliason et al., 2012), and the Food and Drug Administration banning its use as an ingredient in dietary supplements in 2013 (Foley et al., 2014).
In summary, DMAA is a synthetic, sympathomimetic aliphatic amine used in dietary supplements that has garnered significant media attention because of its numerous adverse effects. Because of the increasing popularity of synthetic drugs and “legal highs,” the widespread use of DMAA in dietary supplements for its stimulant properties, and the prior use of DMAA as a “party pill” in New Zealand (Gee, 2010; 2012), we sought to investigate the abuse liability of DMAA using conditioned place preference, drug discrimination, and locomotor activity assays, which are commonly used assays for predicting the abuse potential of drugs (Balster, 1991; Carter and Griffiths, 2009; Horton et al., 2013).
2. MATERIALS AND METHODS
2.1 Animals
Male Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN). All rats were housed individually and were maintained on a 12-/12-h light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320 to 350 g by limiting food to 15 g/day, which included the food received during operant sessions. Water was freely available. Male Swiss-Webster mice were obtained from Harlan at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group-housed in cages on a 12-/12-h light/dark cycle and were allowed free access to food and water. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
2.2 Discrimination Training
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC-compatible computers via LVB interfaces (MED Associates, St. Albans, VT). The computers were programmed in MED-PC IV (MED Associates) for the operation of the chambers and collection of data. Rats were trained to discriminate cocaine (10 mg/kg i.p.) or methamphetamine (1 mg/kg i.p.) from vehicle (saline) using a two-lever choice methodology. Food (45-mg food pellets; Bio-Serv, Frenchtown, NJ) was available as a reinforcer under a fixed-ratio 10 schedule when responding occurred on the injection-appropriate lever. There was no consequence for responses on the incorrect lever. The rats received approximately 60 training sessions before they were used in substitution experiments. Animals were selected for use in experiments when they had met the criteria of emitting 85% of responses on the injection-correct lever for both the first fixed ratio and for the remainder of the session during their last 10 training sessions. Training sessions occurred in a double alternating fashion (D-D-V-V-D, etc.), and tests were conducted between pairs of identical training sessions (i.e., between either two vehicle or two drug training sessions). Rats were tested only if they had achieved 85% drug-lever responding for both first fixed-ratio and total session on the two prior training sessions. Before each session, the rats received an injection of either vehicle or drug. Ten minutes later, the rats were placed in an operant chamber. Each training session lasted a maximum of 10 min, and the rats could earn up to 20 food pellets.
2.3 Discrimination Test Procedures
In contrast with training sessions, both levers were active during the discrimination test sessions, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until the first reinforcer was obtained or for a maximum of 20 min. At least 3 days elapsed between test sessions. DMAA was tested in ten rats trained to discriminate cocaine and ten rats trained to discriminate methamphetamine. A repeated-measures design was used, such that each rat was tested at all doses. During substitution experiments, intraperitoneal injections of saline (1 ml/kg) or DMAA (0.3, 1, 3, or 10 mg/kg) were administered 10 min before the start of the test session.
2.4 Locomotor Activity
Studies of locomotor activity were conducted using a Digiscan apparatus (model RXYZCM-16; Omnitech Electronics, Columbus, OH) and clear acrylic locomotor activity testing chambers (40.5 x 40.5 x 30.5 cm) housed in sets of two, within sound-attenuating chambers. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination. Fans provided an 80-dB ambient noise level within the chamber.
Separate groups of eight mice were injected (i.p.) with either vehicle (0.9% saline) or DMAA (0.1, 0.3, 1, 3, or 10 mg/kg) immediately before locomotor activity testing. In all studies, horizontal activity (interruption in photocell beams) was measured for 8h within 10-min periods, beginning at 08:00 h (1 h after lights on).
2.5 Conditioned Place Preference
Conditioning and preference tests were conducted in Fusion Environmental Control Chambers using Fusion v3.92 sensors (Accuscan Instruments, Inc. Columbus, OH). The sensors consisted of panels of infrared beams (16 beams per panel) and corresponding photodetectors, which were located horizontally along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination. Fans provided an 80-dB ambient noise level within the chamber. The testing arena consisted of 30.5 x 15.5 x 30.5 cm acrylic walls with two distinct floors (parallel metal rods or a perforated metal sheet).
Place conditioning, using a biased model, consisted of three phases: a pre-test for initial floor bias, four place conditioning sessions, and a final preference test. The pre-test was conducted on Day 1, during which initial floor bias was examined by injecting mice intraperitoneally with 0.9% saline (10 ml/kg) then allowing them free access to both floor types for 30 min. The amount of time spent on either floor was measured and the floor on which less time was spent was designated the drug-paired floor. Positioning of the floors alternated between chambers. On days 2 and 3, place conditioning occurred, wherein mice received one vehicle and one drug conditioning session on both days. In the mornings, mice were injected with saline and placed in the chamber with the non-drug-paired floor for 30 min, then returned to their home cage. After 4 hours, mice were injected with DMAA and immediately placed in the chambers with the drug-paired floors for 30 min. The final preference test, occurring on day 4, was identical to the pre-test. All subjects were administered 0.9% saline and the time spent on the drug-paired floor was measured. Sixteen mice were tested at each dose.
2.6 Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal inhibition of locomotor activity first appeared, as a function of dose, was used for analysis of dose–response data. Half-maximal inhibitory dose (ID50) values were then calculated by estimating the dose producing 50% of the peak ambulation from the descending linear portion of the dose–response curve. A two-way repeated-measures analysis of variance was carried out on horizontal activity counts/10-min interval. A one-way analysis of variance was carried out on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were made between each dose and the vehicle (0.9% saline) control using single degree of freedom F-tests.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Graphs for percent drug-appropriate responding and response rate were plotted as a function of the dose of the test compound (log scale). Percent drug-appropriate responding was shown only if at least three rats completed the first fixed ratio. Full substitution was defined as greater than or equal to 80% drug-appropriate responding, and was not significantly different from that of the training drug (t-test). Potencies were calculated by fitting straight lines to the linear portion of dose-effect curves for each compound by means of Origin (OriginGraph, Northhampton, MA). Data on response rate data were analyzed by one-way repeated-measures analysis of variance. Effects of individual doses were compared with those of the vehicle control value using a-priori contrasts. The criterion for significance was set a priori at P less than 0.05.
Conditioned place preference data were expressed as the mean time in seconds spent on the drug-paired floor over 30 minutes. ED50 values were then calculated by estimating the dose producing 50% of the peak preference from the ascending linear portion of the dose–response curve. These data were analyzed using a two-way analysis of variance to compare the difference in time spent on the drug-paired floor before and after conditioning with DMAA with pre-test/preference test time as a within-groups factor and dose of DMAA as a between-groups factor. Effects of individual doses on the time spent on the drug-paired floor were determined using a one-way repeated-measures analysis of variance. The criterion for significance was set a priori at P less than 0.05.
2.7 Drugs
1,3-Dimethylamylamine hydrochloride was purchased from Sigma-Aldrich (St Louis, MO) and Cayman Chemical (Ann Arbor, MI). (−)-Cocaine hydrochloride and (+)-methamphetamine hydrochloride were provided by the National Institute on Drug Abuse Supply Program. All drugs were dissolved in 0.9% saline.
3. RESULTS
3.1 Locomotor Activity
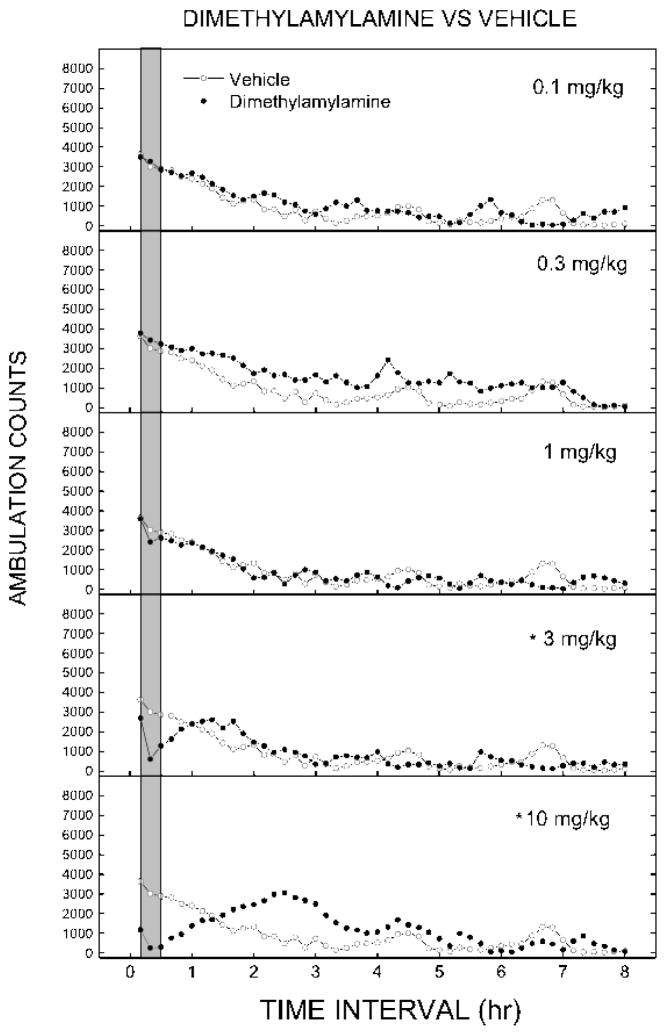
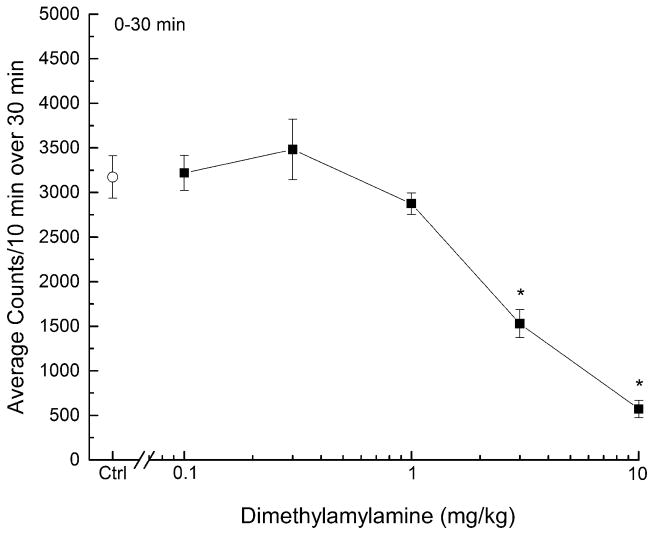
DMAA produced time and dose-dependent decreases in spontaneous locomotor activity compared to vehicle (Figure 1). A two-way mixed-model analysis of variance revealed a significant main effect of time (F47,1974=32.627, p < 0.05) and a significant time x dose interaction (F235,1974=3.214, p < 0.05), but no main effect of dose (F5,42=1.072, p > 0.3). Maximal depressant effects were seen 0 to 30 min following administration of DMAA, and lasted 50 to 70 min. Between 120 and 180 minutes, an increase in motor activity occurred in mice treated with 10 mg/kg DMAA (F5,42=3.234, p < 0.05). A dose-effect curve derived from the peak data (Figure 2) indicated depression of locomotor activity (F5, 42=31.275, p < 0.05), with significant effects following administration of 3(F1, 46=35.103, p < 0.001) and 10 mg/kg (F1, 46=173.787, p < 0.001) DMAA. The half-maximal inhibitory dose (ID50) for DMAA-induced locomotor depression was 3.21 mg/kg (95% confidence interval 0.33 – 31.12 mg/kg).
Figure 1.
Time course of locomotor activity in mice. Data are represented as mean number of ambulation counts for each 10-minute period over 8 hours for each dose of dimethylamylamine. The gray bar shows the time range of maximal effect used for analysis of dose effect (0–30 min). * indicates doses significantly different from vehicle for the period of 0–30 minutes after injection determined by a two-way analysis of variance (p < 0.05). Vehicle (0.9% saline) data were obtained from one group of mice and displayed in each panel to indicate dose-dependent differences of DMAA-induced motor activity from vehicle-treated mice.
Figure 2.
Dose-response curve for the locomotor activity assay in mice. Data are represented as the mean number of activity counts per 10 minutes for the first 30 minutes of testing. The open circle represents the activity counts after treatment with vehicle (Ctrl, 0.9% saline) and the closed squares represent activity counts after treatment with DMAA (n=8 per dose). * indicates doses significantly different from vehicle for the period of 0–30 minutes after injection (p < 0.05).
3.2 Drug Discrimination
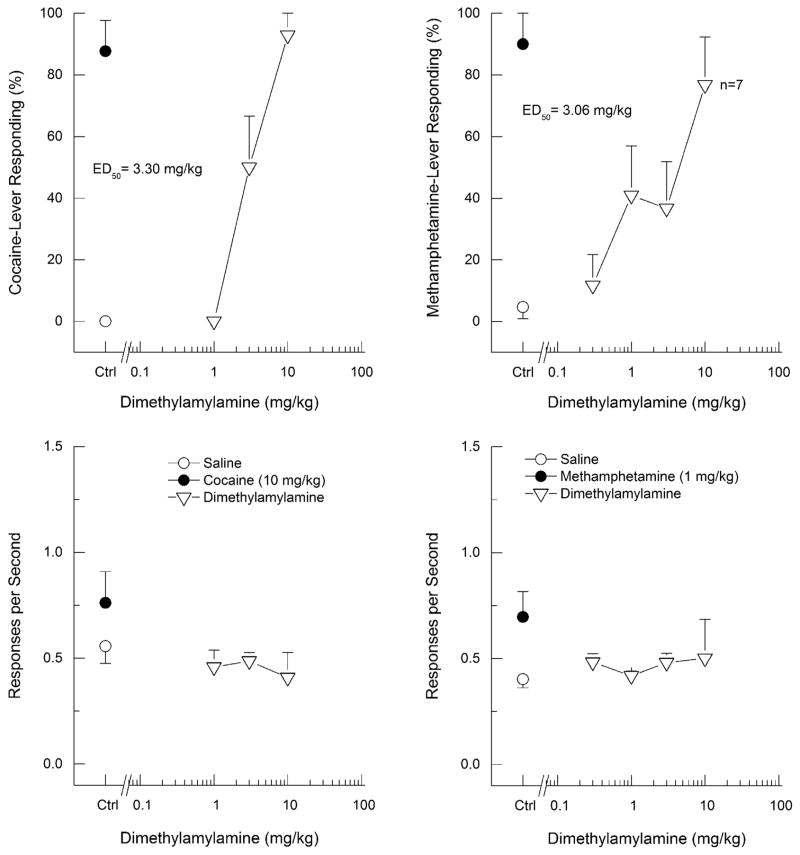
DMAA fully substituted for cocaine (ED50 = 3.30 mg/kg; 95% confidence interval 0.61 – 17.83 mg/kg), producing dose-dependent increases in drug-appropriate responding to a maximum of 93±7% in cocaine-trained rats. There was no effect of DMAA on response rate (F3, 27=0.49, p > 0.6). In contrast, DMAA produced only 77±16% drug-appropriate responding in methamphetamine-trained rats (ED50 = 3.06; 95% confidence interval 0.01 – 840.8 mg/kg). A one-way repeated-measures analysis of variance of response rate in methamphetamine-trained rats failed to detect an effect of dose on response rate (F4, 36=0.23, p > 0.9). However, three of ten rats failed to respond following the 10 mg/kg dose of DMAA.
3.3 Conditioned Place Preference
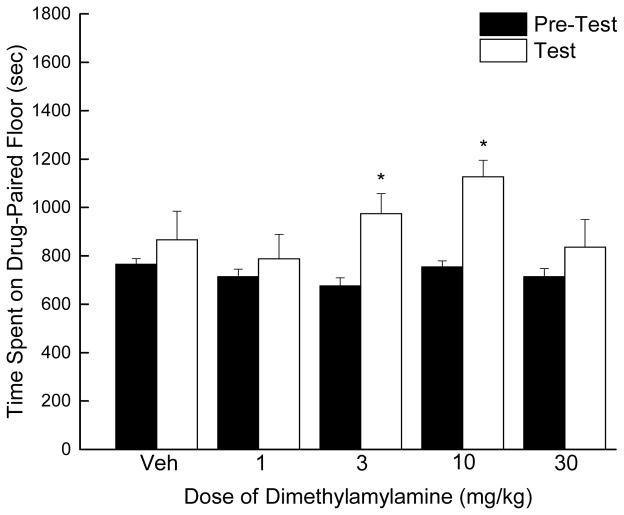
A two-way mixed model analysis of variance revealed a main effect of DMAA conditioning (F1, 73=19.986, p < 0.05) but not dose (F4, 73=1.911, p > 0.05), nor was there an interaction (F3, 60=1.929, p > 0.05). Conditioning with DMAA resulted in an inverted-U dose-response (Figure 4), with 3 (F1, 15=122.901, p = 0.003) and 10 mg/kg (F1, 15=32.483, p < 0.001) producing conditioned place preference, but not 1 or 30 mg/kg. Peak preference (1127±67 s) was observed following 10 mg/kg. The ED50 calculated for the ascending portion of the dose-effect curve was 2.33 mg/kg (95% confidence interval 0.02 – 244.04 mg/kg).
Figure 4.
Time spent on drug-paired floor in the conditioned place-preference assay after vehicle (Veh) and DMAA conditioning in mice. Pre-test data are displayed as black bars and test data for each dose are displayed as white bars (n=80 mice; n=16 per dose). Both 3 and 10 mg/kg produced conditioned place preference. * indicates doses with post-test time significantly different from pre-test time determined by a repeated-measures analysis of variance for each dose (p < 0.05).
4. DISCUSSION
In the present study, DMAA produced locomotor depression, fully substituted for the discriminative stimulus effects of cocaine, produced partial substitution for methamphetamine (77% drug-appropriate responding), and produced a conditioned place preference. The potency of DMAA was comparable in all three assays (between 2.3–3.3 mg/kg). These results indicate that DMAA produces reward-like effects and may produce subjective effects similar to that of abused psychostimulants, and therefore has potential for abuse.
Given that users state that DMAA boosts energy during exercise (Lammie, 2013), the locomotor depressant effects were unexpected, especially since DMAA produced discriminative stimulus effects similar to the psychostimulants cocaine and methamphetamine, which produce hyperlocomotion compared to vehicle controls (Carroll et al., 2009; Katz et al., 2001; Gatch et al., 2013). It should be noted that DMAA did produce an increase in motor activity between 120 and 180 minutes after 10 mg/kg DMAA. Given that the maximum increase following 10 mg/kg is equivalent to the effects of the vehicle control at 10–30 min, this is most likely a delayed onset of normal exploratory activity after the initial locomotor depression as opposed to locomotor stimulation directly due to DMAA. However, it is possible that this rebound effect may account for user reports of increases in energy during exercise (Lammie, 2013).
A possible explanation for these apparently contradictory findings is suggested by prior studies which reported that DMAA may have an adrenergic mechanism of action (e.g., Kuo et al., 2004). Similar to DMAA, adrenergic compounds such as clonidine induce locomotor depression (Geyer and Frampton, 1988; Hano et al., 1978; Mitchell et al., 2006), substitute for the discriminative stimulus effects of cocaine (Wood et al., 1985), partially substitute for methamphetamine (Munzar and Goldberg, 1999), and produce conditioned place preference (Cervo et al., 1993; Asin and Wirtshafter, 1985). These findings do not provide direct evidence that DMAA produced its behavioral effects through actions at adrenergic receptors, but do indicate that a class of compounds can produce a similar, and apparently contradictory, profile of behavioral effects, and thereby may be a starting point for investigations of the pharmacological mechanism of DMAA’s behavioral effects. These findings also do not preclude contribution of other receptor systems, in particular the other monoamines, serotonin and dopamine.
DMAA is used as a dietary supplements for weight loss and exercise, with widespread use in the military (Austin, et al., 2012; Lammie, 2013). In addition, DMAA is marketed and sold as a legal alternative to cocaine and methamphetamine (Gee et al., 2010, 2012). Because of the recreational use and reports of stimulant-like effects and concerns that it might be addictive (Lammie, 2013), the abuse liability of DMAA was tested in the current study. DMAA produced discriminative stimulus effects similar to cocaine, and to a lesser extent methamphetamine, and produced reward effects. These findings suggest that DMAA may have potential to be abused. The serious adverse effects associated with DMAA (Eliason et al., 2012; Foley et al., 2014; Forrester, 2013; Young et al., 2012) may increase the risk of recreational use. Because DMAA produced discriminative stimulus and rewarding effects similar to abused psychostimulants, vulnerable individuals may be at risk of escalating doses to dangerously toxic levels, suggesting that restricting access to this compound may be appropriate.
Further study will be necessary to confirm drug seeking (e.g., self-administration) and mechanism of action. DMAA produces behavioral effects similar to adrenergic compounds, but it will be necessary to directly test the mechanism of action of the discriminative stimulus and rewarding effects of DMAA. Adrenergic compounds are not typically abused, although clonidine has been abused by polydrug abusers (Schindler et al., 2013). Other receptors may play a role in the behavioral and toxic effects of DMAA. For example, the cardiovascular effects of DMAA are clonidine-like, but are also similar to serotonin syndrome. Further, if DMAA has dopaminergic effects, it is more likely to be abused than if its mechanism is primarily adrenergic or serotonergic.
Figure 3.
Dimethylamylamine substitution for the discriminative stimulus effects of cocaine or methamphetamine in rats. Upper panels show percentage of total responses made on the drug-appropriate lever. Bottom panels show rate of responding in responses per second (r/s). Testing of dimethylamylamine in cocaine-trained rats is shown in the left panels and testing of dimethylamylamine in methamphetamine-trained rats is shown in the right panels. Ctrl indicates vehicle (0.9% saline) control. n=10 except where shown.
Highlights.
Dimethylamylamine is a common component of dietary supplements used for weight loss and exercise and has been marketed as a party pill.
Dimethylamylamine substituted for the discriminative stimulus effects of cocaine.
Dimethylamylamine produced substantial methamphetamine-like discriminative stimulus effects.
Dimethylamine produced reward-like effects in the conditioned place preference assay.
Based on our findings, dimethylamylamine appears to have substantial potential for abuse.
Acknowledgments
Role of funding source
Funding for this study was provided by NIDA contract N01DA-13-8908. NIDA had no further role in the design, analysis or publication of this report.
The authors acknowledge Anna Sagar and Elva Flores for excellent technical assistance.
Footnotes
Contributors
SBD conducted experiments, searched the literature, and wrote initial drafts of the manuscript. SBD and MBG designed the experiments and analyzed the data. MBG wrote the final version of the manuscript. All authors approved the manuscript.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asin KE, Wirtshafter D. Clonidine produces a conditioned place preference in rats. Psychopharmacology (Berl) 1985;85:383–385. doi: 10.1007/BF00428206. [DOI] [PubMed] [Google Scholar]
- Austin KG, McGraw S, Carvey C, Lieberman HR. Use of dietary supplements containing 1,3-dimethylamylamine by military personnel. FASEB J. 2012;26:lb415. [Google Scholar]
- Austin KG, Travis J, Pace G, Lieberman HR. Analysis of 1,3 dimethylamylamine concentrations in Geraniaceae, geranium oil, and dietary supplements. Drug Test Anal. 2014;6:797–804. doi: 10.1002/dta.1491. [DOI] [PubMed] [Google Scholar]
- Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Abraham P, Mills AC, Holleman JA, Wolckenhauer SA, Decker AM, Landavazo A, McElroy KT, Navarro HA, Gatch MB, Forster MJ. Synthesis and biological evaluation of bupropion analogues as potential pharmacotherapies for cocaine addiction. J Med Chem. 2009;52:6768–6781. doi: 10.1021/jm901189z. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105S:S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Rossi C, Samanin R. Clonidine-induced place preference is mediated by alpha 2-adrenoceptors outside the locus coeruleus. Eur J Pharmacol. 1993;238:201–207. doi: 10.1016/0014-2999(93)90848-c. [DOI] [PubMed] [Google Scholar]
- Council of Pharmacy and Chemistry. New and nonofficial remedies: methylhexamine – forthane. JAMA. 1950;143:1156. [PubMed] [Google Scholar]
- Eliason MJ, Eichner A, Cancio A, Bestervelt L, Adams BD, Deuster PA. Case reports: death of active duty soldiers following ingestion of dietary supplements containing 1.3-dimethylamylamine (DMAA) Mil Med. 2012;177:1455–1459. doi: 10.7205/milmed-d-12-00265. [DOI] [PubMed] [Google Scholar]
- Foley S, Butlin E, Shields W, Lacey B. Experience with OxyELITE Pro and acute liver injury in active duty service members. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3221-4. EPub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Forrester MB. Exposures to 1,3-dimethylamylamine-containing products reported to Texas poison centers. Hum Exp Toxicol. 2013;32:18–23. doi: 10.1177/0960327112454895. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;5:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TD. Evidence for the presence of 1,3-dimethlyamylamine (1,3-DMAA) in geranium plant materials. Anal Chem Insights. 2013;8:29–40. doi: 10.4137/ACI.S11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee P, Jackson S, Easton J. Another bitter pill: a case of toxicity from party pills. N Z Med J. 2010;213:124–127. [PubMed] [Google Scholar]
- Gee P, Tallon C, Long N, Moore G, Boet R, Jackson S. Use of recreational drug 1,3-dimethylamylamine (DMAA) associated with cerebral hemorrhage. Ann Emerg Med. 2012;60:431–434. doi: 10.1016/j.annemergmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Frampton SF. Peripheral mediation of effects of clenbuterol on locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1988;30:417–420. doi: 10.1016/s0091-3057(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Hano J, Vetulani J, Sansone M, Oliverio A. Effect of clonidine, amphetamine, and their combinations on the locomotor activity of CD-1 and C57BL/6 mice. Pharmacol Biochem Behav. 1978;9:741–746. doi: 10.1016/0091-3057(78)90350-7. [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:10–36. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Katz JL, Agoston GE, Alling KL, Kline RH, Forster MJ, Woolverton WL, Kopajtic TA, Newman AH. Dopamine transporter binding without cocaine-like behavioral effects: synthesis and evaluation of benztropine analogs alone and in combination with cocaine in rodents. Psychopharmacology (Berl) 2001;154:362–374. doi: 10.1007/s002130000667. [DOI] [PubMed] [Google Scholar]
- Kuo CL, Wang RB, Shen LJ, Lien LL, Lien EJ. G-protein coupled receptors: SAR analyses of neurotransmitters and antagonists. J Clin Pharm Ther. 2004;29:279–298. doi: 10.1111/j.1365-2710.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- Lammie J. Report of the Department of Defense: 1,3-dimethylamylamine. 2013 < http://home.fhpr.osd.mil/Libraries/pdf/Report_of_the_DoD_DMAA_Safety_Review_Panel_2013.sflb.ashx>.
- Marsh DF. The comparative pharmacology of the isomeric heptylamines. J Pharmacol Exp Ther. 1948;94:225–231. [PubMed] [Google Scholar]
- Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D. The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biol Psychiatr. 2006;60:1046–1052. doi: 10.1016/j.biopsych.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Noradrenergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 1999;143:293–301. doi: 10.1007/s002130050950. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Nurminen ML, Niitynen L, Korpela R, Vapaatalo H. Coffee, caffeine and blood pressure: a critical review. Eur J Clin Nutr. 1999;53:831–839. doi: 10.1038/sj.ejcn.1600899. [DOI] [PubMed] [Google Scholar]
- Schindler EA, Tirado-Morales DJ, Kushon D. Clonidine abuse in a methadone-maintained, clonazepam-abusing patient. J Addict Med. 2013;7:218–219. doi: 10.1097/ADM.0b013e31828ab8d4. [DOI] [PubMed] [Google Scholar]
- Shonle HA, Rohrmann E. 2,350,318. U.S. Patent and Trademark Office; Washington, DC: US Patent. 1944
- Smith TB, Staub BA, Natarajan GM, Lasorda DM, Poornima IG. Acute myocardial infarction associated with dietary supplements containing 1,3-dimthethylamylamine and citrus aurantium. Tex Heart Inst J. 2014;41:70–72. doi: 10.14503/THIJ-12-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson EE, Chen KK. Comparison of pressor action of aliphatic amines. J Pharmacol Exp Ther. 1946;88:10–13. [PubMed] [Google Scholar]
- Swanson EE, Chen KK. Comparison of pressor action of alicyclic derivatives of aliphatic amines. J Pharmacol Exp Ther. 1948;93:423–429. [PubMed] [Google Scholar]
- Wood DM, Lal H, Yaden S, Emmett-Oglesby MW. One-way generalization of clonidine to the discriminative stimulus produced by cocaine. Pharmacol Biochem Behav. 1985;23:529–533. doi: 10.1016/0091-3057(85)90414-9. [DOI] [PubMed] [Google Scholar]
- Young C, Oladipo O, Frasier S, Putko R, Chronister S, Marovich M. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med. 2012;177:1450–1454. doi: 10.7205/milmed-d-11-00342. [DOI] [PubMed] [Google Scholar]