Abstract
Little information exists on the effect of race and ethnicity on collection of peripheral blood stem cells (PBSC) for allogeneic transplantation. We studied 10776 donors from the National Marrow Donor Program who underwent PBSC collection from 2006-2012. Self-reported donor race/ethnic information included Caucasian, Hispanic, Black/African American (AA), Asian/Pacific Islander (API), and Native American (NA). All donors were mobilized with subcutaneous filgrastim (G-CSF) at an approximate dose of 10 µg/kg/d for 5 days. Overall, AA donors had the highest median yields of mononuclear cells (MNC)/L and CD34+ cells/L blood processed (3.1 × 109 and 44 × 106 respectively) while Caucasians had the lowest median yields at 2.8 × 109 and 33.7 × 106 respectively. Multivariate analysis of CD34+/L mobilization yields using Caucasians as the comparator and controlling for age, gender, body mass index, and year of apheresis revealed increased yields in overweight and obese AA and API donors. In Hispanic donors, only male obese donors had higher CD34+/L mobilization yields compared to Caucasian donors. No differences in CD34+/L yields were seen between Caucasian and NA donors. Characterization of these differences may allow optimization of mobilization regimens to allow enhancement of mobilization yields without compromising donor safety.
Introduction
Mobilized peripheral blood stem cells (PBSC) are commonly used in allogeneic hematopoietic cell transplantation because of the relative ease of collection and rapid engraftment.1,2 However, there is significant variation in mobilization response to filgrastim (G-CSF) in healthy donors, which sometimes requires additional apheresis collections or repeat mobilization cycles to collect an adequate PBSC dose for transplant.3,4 Information on the effect of various donor demographic, laboratory, and other factors on both peak CD34+ responses and apheresis cell yields in both the related and unrelated settings is inconsistent.5–7 Racial variations have been described in the baseline cell counts, which may have an effect on the ability to adequately collect stem cells.8 Little information exists on the effect of donor race and ethnicity on mobilization, especially in the unrelated donor setting.9–11 This study analyzes the effect of donor race and ethnicity on PBSC mobilization in a large cohort of adult unrelated donors.
Methods
Data Sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplants to a centralized statistical center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in CIBMTR's capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act of 1996 (HIPPA) Privacy Rule. Additional details regarding the data source are described elsewhere.12
Study Population
The study population consisted of U.S. volunteer donors from the National Marrow Donor Program (NMDP) who underwent G-CSF, filgastrim (Neupogen®, Amgen, Thousand Oaks, CA, USA) mobilized PBSC collection from January 2006 to December 2012. Only first-time hematopoietic progenitor cell (HPC) donors for whom data were available from baseline to the first day of apheresis on the NMDP data collection forms were included. Donor race/ethnicity was self-reported. Donors were classified as Caucasian, Hispanic/Latino (Hispanic), African American (AA), Asian/Pacific Island (API), or Native American (NA). Donors who were of Other/Multiple/Unknown race were excluded from the analysis. Only donors from apheresis centers that collected from both Caucasian and minority donors were included. We identified 10,776 volunteer donors eligible for inclusion in this study. All donors provided written informed consent for participation in research studies approved by the NMDP IRB and were evaluated for medical suitability, transplantation-transmissible infectious diseases, and contraindications for PBSC donation using standardized NMDP criteria.
PBSC Donation and Data Collection
The PBSC donation processes were facilitated by 63 donor centers and 89 apheresis centers. Donor centers manage donor medical evaluations, infectious disease marker testing, and G-CSF administration. They are also responsible for coordinating the donation process, monitoring the donor's recovery, and data reporting. Apheresis centers perform PBSC collections, coordinate donor G-CSF administration in conjunction with local donor centers as needed, assist donor centers with data reporting, and are responsible for submitting PBSC Product Analysis Forms to the NMDP.
All PBSC mobilizations were performed according to the NMDP-sponsored and IRB-approved research protocol for manufacturing PBSC products, operated under an Investigational New Drug application with the United States Food and Drug Administration (FDA). Dosing of G-CSF for volunteer donors was approximately 10 µg/kg/day actual body weight rounded to combinations of 300µg and 480µg vials based on the donor's actual body weight as long as protocol defined targets of 13.3 µg/kg or less per day were not exceeded. This was done for ease of administration of G-CSF. The protocol included provisions for G-CSF dose reductions in the presence of high-grade symptoms. Typically, donors received subcutaneous G-CSF daily for 4 days prior to and on the first day of apheresis. All donors underwent one or two days of apheresis, however, only apheresis products collected on the first day were analyzed. The volume of whole blood processed was targeted to be between 12 and 24 L per collection, depending on recipient weight and/or immediate pre-procedure donor blood CD34+ cell count. The protocol allowed the maximum total blood volume processed to be 24 L, whether collected over one or two days. If the PBSC product could not be collected using peripheral veins, a central venous catheter (CVC) was used. Data collection began at the time of the donor's medical evaluation to determine suitability to donate HPCs, continued through each day of G-CSF, on the day of each apheresis procedure, and postcollection.
End Points and Statistical Analysis
The primary endpoints of this study are the number of mononuclear cells (MNC) and CD34+ cells collected after 1 day of apheresis. Secondary endpoints include, change of white blood cells (ΔWBC), MNC (ΔMNC), neutrophil (ΔNeu), and platelet (ΔPlt) counts from baseline prior to the start of G-CSF to post-G-CSF, prior to the start of apheresis. Significant variability exists among apheresis centers concerning number of days of collection and volume of blood processed per day. To avoid variability inherent in the second day of collection, we normalized the MNC and CD34+ cell counts to volume of blood processed on the first day of apheresis.
The main parameter analyzed is the self-reported race/ethnicity of the donor, classified as Caucasian, Hispanic/Latino, African American, Asian/Pacific Island, or NA. The analysis quantified other donor and collection characteristics by race/ethnicity. These variables included donor gender, age, weight, body mass index (BMI), baseline complete blood count (CBC), total G-CSF dose/kg donor actual body weight, year of donation, presence/absence of a CVC, and apheresis center. Univariate comparisons of these factors between the 5 racial/ethnic groups were performed using the Chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Linear mixed model with a random effect for apheresis center was used to analyze the effect of donor race/ethnicity on the primary and secondary endpoints. Interactions between the main effect and all other significant factors were checked. Because a number of pairwise comparisons were required we considered a difference of p<0.005 as significant using a Bonferroni correction.
Results
Donor Demographics
A total of 10776 unrelated volunteer PBSC donors were available for analysis. The baseline characteristics of the various racial/ethnic groups are shown in Table 1. The majority of donors were Caucasians (79.4%). Hispanics comprised the second largest group at 9.4%, followed by API (5.5%), AA (4.7%) and NA (1.1%). NA donors were generally the oldest donors (median age 38 years) while API donors were the youngest (median age 32 years). Interestingly, the majority of donors in all racial/ethnic groups were either overweight or obese (as defined in Table 1). Percentages vary from 50% in the API vs. 67% in Caucasians to as high as 80% in AA. Although Hispanic and AA donors were more likely to be obese (Table 1), stratification of the various races/ethnicities by BMI found Caucasians were the heaviest donors in all BMI categories while API were the lightest in the normal and obese and Hispanics were the lightest in the overweight group (Table 2). There were significantly more male donors in the Caucasian, API, and NA groups while male–female ratios in the Hispanic and AA groups were roughly equal. There was a statistically significant difference in use of CVCs, with AA having the highest percentage of insertion (19%) and Caucasians having the lowest (8%, p<0.001).
Table 1. Characteristics of unrelated PBSC donors facilitated by the NMDP, 2006-2012.
| Caucasian | Hispanic (HIS) | African American (AA) | Asian/Pacific Islander (API) | Native American (NA) | ||
|---|---|---|---|---|---|---|
| Variable | N (%) | N (%) | N (%) | N (%) | N (%) | p-value |
| Number of donors | 8560 | 1010 | 504 | 587 | 115 | |
| Male/Female | 5601/2959 (65/35) | 508/502 (50/50) | 244/260 (48/52) | 367/220 (63/37) | 68/47 (59/41) | <0.001 |
| Donor age at donation | <0.001 | |||||
| 18 to 29 | 3434 (40) | 368 (36) | 149 (30) | 230 (39) | 37 (32) | |
| 30 to 39 | 2348 (28) | 309 (31) | 172 (34) | 226 (39) | 31 (27) | |
| 40 to 49 | 1890 (22) | 241 (24) | 123 (24) | 96 (16) | 29 (25) | |
| 50 to 61 | 888 (10) | 92 (9) | 60 (12) | 35 (6) | 18 (16) | |
| Median (Range) | 34 (18-61) | 35 (18-61) | 36 (19-60) | 32 (19-60) | 38 (20-60) | <0.001 |
| Donor BMI (kg/m2) | <0.001 | |||||
| Underweight (<18.5) | 50 (1) | 8 (1) | 3 (1) | 10 (2) | 0 | |
| Normal (18.5-24.9) | 2753 (32) | 237 (23) | 87 (17) | 285 (49) | 24 (21) | |
| Overweight (25-29.9) | 3346 (39) | 376 (37) | 184 (37) | 204 (35) | 39 (34) | |
| Obese (30+) | 2408 (28) | 388 (38) | 230 (46) | 88 (15) | 52 (45) | |
| Unknown | 3 (N/A) | 1 (N/A) | 0 (N/A) | 0 (N/A) | 0 (N/A) | |
| Median (Range) | 27.0 (15.7-61.3) | 28.4 (17.0-49.5) | 29.5 (16.7-50.6) | 25.0 (16.4-43.5) | 29.4 (19.0-44.0) | <0.001 |
| Median Donor weight (kg) (Range) | 84 (41-176) | 81 (41-160) | 86 (42-138) | 72 (37-145) | 87 (47-154) | <0.001 |
| Year of donation | 0.022 | |||||
| 2006 | 931 (11) | 118 (12) | 50 (10) | 36 (6) | 11 (10) | |
| 2007 | 993 (12) | 109 (11) | 61 (12) | 64 (11) | 15 (13) | |
| 2008 | 1120 (13) | 137 (14) | 67 (13) | 81 (14) | 24 (21) | |
| 2009 | 1156 (13) | 141 (14) | 84 (17) | 93 (16) | 16 (14) | |
| 2010 | 1269 (15) | 163 (16) | 78 (15) | 76 (13) | 19 (17) | |
| 2011 | 1405 (16) | 159 (16) | 80 (16) | 106 (18) | 10 (9) | |
| 2012 | 1686 (20) | 183 (18) | 84 (17) | 131 (22) | 20 (17) | |
| G-CSF dose | ||||||
| Median 5-day total dose (µg) (Range) | 4500 (1800-6720) | 4500 (2400-6300) | 4500 (2340-6000) | 3900 (2400-6240) | 4500 (2400-6000) | <0.001 |
| Median 5-day total dose/donor weight (µg/kg) (Range) | 52.8 (27.0-93.6) | 52.9 (33.6-73.5) | 52.5 (26.8-64.1) | 54.2 (40.0-73.5) | 52.7 (30.5-62.6) | <0.001 |
| Baseline counts | ||||||
| Median WBC (x109/L) (Range) | 6.3 (0.4-18.9) | 6.7 (2.5-15.1) | 5.6 (2.4-13.2) | 6.2 (3.0-14.9) | 6.9 (4.0-11.7) | <0.001 |
| Median platelets (x109/L) (Range) | 241 (85-585) | 257 (112-548) | 251 (142-509) | 257 (112-474) | 245 (145-434) | <0.001 |
| Median neutrophils (x109/L) (Range) | 3.9 (0.2-14.7) | 4.2 (1.3-11.8) | 3.1 (1.1-10.7) | 3.8 (1.5-11.6) | 4.4 (2.2-8.6) | <0.001 |
| Median MNC (x109/L) (Range) | 2.3 (0.2-8.7) | 2.5 (0.7-5.9) | 2.4 (1.1-4.9) | 2.3 (1.0-4.6) | 2.5 (1.1-4.0) | <0.001 |
| CVC inserted | 725 (8) | 136 (13) | 98 (19) | 61 (10) | 10 (9) | <0.001 |
| Median volume of whole blood processed on collection Day 1 (L) (Range) | 19.1 (2.6-34.6) | 18.0 (3.5-32.2) | 18.0 (3.7-27.0) | 18.0 (2.7-25.7) | 18.0 (8.5-24.0) | <0.001 |
Table 2. Distribution of weight in different BMI categories according to race/ethnicity.
| BMI Category | Race/Ethnicity | N | Median (kg) | Range | p-value |
|---|---|---|---|---|---|
| Underweight/Normal | Caucasian | 2803 | 68.0 | 41.0 – 103.0 | <0.001 |
| HIS | 245 | 62.0 | 41.0 – 93.0 | ||
| AA | 90 | 66.5 | 42.0 – 91.6 | ||
| API | 295 | 61.0 | 37.0 – 92.0 | ||
| NA | 24 | 66.8 | 47.0 – 88.5 | ||
|
| |||||
| Overweight | Caucasian | 3346 | 85.0 | 56.8 – 123.0 | <0.001 |
| HIS | 376 | 78.0 | 52.6 – 108.0 | ||
| AA | 184 | 81.7 | 61.8 – 112.7 | ||
| API | 204 | 80.0 | 56.0 – 96.0 | ||
| NA | 39 | 83.6 | 64.0 – 96.0 | ||
|
| |||||
| Obese | Caucasian | 2408 | 102.1 | 69.9 – 175.5 | <0.001 |
| HIS | 388 | 97.6 | 68.0 – 160.1 | ||
| AA | 230 | 99.6 | 67.6 – 138.0 | ||
| API | 88 | 92.8 | 72.1 – 145.5 | ||
| NA | 52 | 100.3 | 73.2 – 154.2 | ||
Response to G-CSF
Baseline counts are shown in Table 1 while donor response to G-CSF is seen in Table 3. In summary, AA donors had the lowest median baseline WBC and neutrophil counts while NA donors had the highest baseline WBC counts. After 5 days of G-CSF stimulation, however, AA donors had the largest ΔWBC (median 38.7 × 109/L, range 6.1-79.3), ΔNeu (median 33.6 × 109/L, range 5.6-68.1) and ΔMNC (median 4.5 × 109/L, range -0.7 to 16.8) after five days of G-CSF stimulation, while Caucasians had the smallest ΔWBC (median 30.9 × 109/L, range 1.7-97.4), ΔNeu (median 27.0 × 109/L, range -0.9 to 91.7) and ΔMNC (median 3.4 × 109/L, range -2.4 to 28.3).
Table 3. Donor response after 5 days G-CSF.
| Caucasian | HIS | AA | API | NA | ||
|---|---|---|---|---|---|---|
| Variable | Median (Range) | Median (Range) | Median (Range) | Median (Range) | Median (Range) | p-value |
| ΔWBC (x109/L) | 30.9 (1.7, 97.4) | 35.1 (1.7, 83.3) | 38.7 (6.1, 79.3) | 35.9 (4.8, 90.4) | 33.5 (9.3, 81.0) | <0.001 |
| ΔPIatelets (x109/L) | -19 (-280, 211) | -27 (-210, 228) | -23 (-290, 120) | -30 (-162, 155) | -26 (-115, 57) | <0.001 |
| ΔNeutrophils (x109/L) | 27.0 (-0.9, 91.7) | 30.3 (1.2, 78.7) | 33.6 (5.6, 68.1) | 31.8 (3.4, 85.8) | 28.4 (5.7, 76.6) | <0.001 |
| ΔMNC (x109/L) | 3.4 (-2.4, 28.3) | 4.1 (-1.8, 20.7) | 4.5 (-0.7, 16.8) | 3.7 (-0.7, 21.5) | 4.1 (-0.9, 17.4) | <0.001 |
| CD34+ (x106/L) pre-apheresis | 78.3 (0.3, 2236) | 89.7 (4.7, 1117) | 112 (3.4, 1089) | 87.3 (0.3, 1145) | 79.5 (17.4, 292) | <0.001 |
Cell counts of the apheresis product after 1 day of collection are shown in Table 4. AA were the most robust mobilizers with the highest median CD34+ cells collected per liter of blood processed at 44.0 × 106/L (range 0.6-210) as well as the highest median total number of CD34+ cells collected at 704 × 106 (range 7.9-3985). Conversely, Caucasians had the lowest median CD34+ cells collected per liter at 33.7 × 106 CD34+/L (range 0.4-285) while API had the lowest median total number of CD34+ cells collected at 555.8×106 CD34+ cells (range 72.4-2795). Yield of stem cells, expressed as percentage of CD34+ cells/MNC, were the highest in AA donors with median 1.25% (range 0.19-16.9). Caucasians and NA were essentially the same with median 1.19% (range 0.01-43.0) and 1.18% (range 0.28-6.68) respectively.
Table 4. Collection efficiency after day 1 of apheresis.
| Caucasian | HIS | AA | API | NA | ||
|---|---|---|---|---|---|---|
| Variable | Median (Range) | Median (Range) | Median (Range) | Median (Range) | Median (Range) | p-value |
| Total MNC (x109) | 50.7 (0.6-177) | 49.4 (4.2-184) | 50.7 (6.9-161) | 46.2 (4.1-123) | 51.4 (19.7-235) | <0.001 |
| MNC/L of blood processed (x109/L) | 2.8 (0.1-13.1) | 3.0 (0.2-12.1) | 3.1 (0.6-9.3) | 2.8 (0.3-7.1) | 3.1 (1.0-11.7) | <0.001 |
| Total CD34+ (x106) | 616.2 (7.9-4677) | 615.3 (54.2-3321) | 704.0 (7.9-3985) | 555.8 (72.4-2795) | 651.3 (101.1-2986) | <0.001 |
| CD34+/L of blood processed (x106/L) | 33.6 (0.4-285) | 36.0 (3.1-151) | 44.0 (0.6-210) | 34.6 (4.4-155) | 36.8 (6.8-149) | <0.001 |
| CD34+/MNC (x103) | 11.9 (0.1-430.0) | 12.2 (1.1-230.0) | 13.6 (0.2-150.0) | 12.5 (1.9-169.4) | 11.8 (2.8-66.8) | <0.001 |
Multivariate Analysis
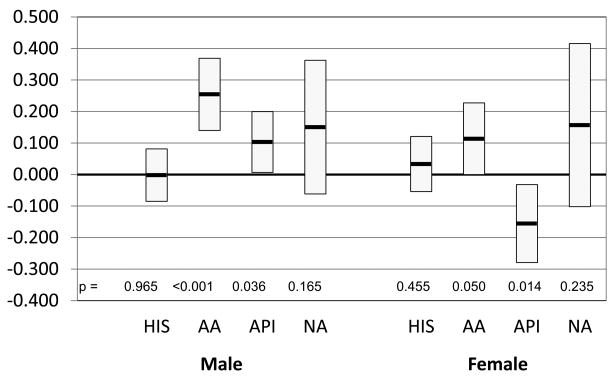
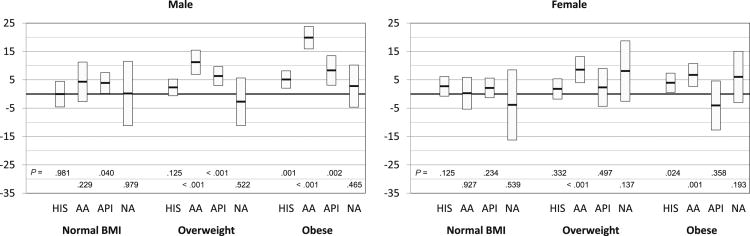
A linear mixed model with a random effect for apheresis center was used to assess the effect of donor race/ethnicity on collection efficiency after adjusting for significant effects among donor gender, age, BMI, and year of donation. For CD34+ cells collected/L of blood processed, we found both gender and BMI had a significant interaction with race. The results between Caucasians vs. other racial/ethnic groups are shown in Figure 1. In general there was an increased difference between Caucasians and other donors in CD34+ cells/L collected as the BMI increased in males. In female donors, differences in CD34+ cells collected were variable. Hispanic donors had a significantly higher CD34+ cells/L compared to Caucasians only among obese male donors. AA, however, had significantly higher CD34+ cells/L compared to Caucasians among the overweight and obese populations in both genders. API donors had higher CD34+ cells/L compared to Caucasians only in overweight and obese males. No differences in CD34+ cells/L were seen between NA and Caucasian donors. In comparisons among the four minority race groups, AA donors had better collection compared to Hispanic donors (primarily among overweight and obese males), API donors (primarily among obese males), and NA donors (primarily among overweight and obese males). A multivariate model, which included CVC placement as a covariate in addition to BMI, age, gender, and year of apheresis, found statistically significant effect of CVC placement on collection yields. The placement of CVC was associated with a mean reduction in CD34+ cell yields of 1.91 × 106/L (p=0.003). No significant interactions were seen between CVC placement and race/ethnicity, which implies that the effect of CVC placement was similar among all racial/ethnic groups.
Figure 1. Pairwise analysis of race by gender and BMI on CD34+ cells/L blood processed using Caucasian as baseline.
Model adjusted for gender, age, BMI, and year of apheresis. Box indicates mean difference (x106/L) with 95% confidence interval.
The analysis of MNC collected/L of blood processed found a significant interaction between the main effect of race and gender. The comparisons between Caucasians vs. other race groups are summarized in Figure 2. AA male donors had better MNC/L collection compared to Caucasian male donors. No significant differences in MNC/L were found between Caucasians and any other race groups.
Figure 2. Pairwise analysis of race by gender on MNC/L blood processed using Caucasian as baseline.
Model adjusted for gender, age, and BMI. Box indicates mean difference (x109/L) with 95% confidence interval.
For product CD34+ cells/MNC ratio, there was no significant difference between Caucasian donors and NA donors, while Hispanic, AA, and API donors all had greater CD34+/MNC ratios compared to Caucasian donors. No significant differences in CD34+/MNC were found between the four minority race groups (data not shown).
A linear mixed model with a random effect for apheresis center was used to assess the effect of donor race/ethnicity on the changes in peripheral blood cell counts from baseline to pre-apheresis after adjusting for significant effects among donor gender, age, BMI, baseline CBC, and year of donation. Caucasian donors had lower changes in WBC and neutrophil counts compared to African American, Hispanic, and API donors. Caucasian donors also had lower changes in MNC compared to African American donors and API donors (primarily among males). However, Caucasian donors had greater changes in platelet counts compared to Hispanic and API donors. No significant differences in the changes in peripheral blood cell counts were found between Caucasian donors and Native American donors. For comparisons between the four minority race groups, African American donors were found to have greater changes in WBC, neutrophil and MNC counts compared to Hispanic donors, API donors, and Native American donors (primarily among males for MNC counts). African American donors also had greater change in platelet counts compared to Hispanic and API donors. API donors had greater changes in WBC and neutrophil counts but lower change in platelet counts compared to Hispanic donors. API donors also had lower change in platelet counts compared to Native American donors (data not shown).
Discussion
This is the largest study investigating PBSC collection outcomes in unrelated donors. Prior studies on factors influencing PBSC collection in volunteer donors have primarily focused on gender, age, and G-CSF dose/timing.4-7 Several studies have been published on the effect of race/ethnicity in related donors.9-11 However, most are limited by the number of patients, especially in the non-Caucasian cohorts.
We found statistically significant differences in baseline counts among different ethnic groups, with AA having the lowest baseline counts while Hispanic and NA have the highest baselines. As far as mobilization response to G-CSF, AA had the highest response, both in terms of CD34+ cells collected/L blood processed and CD34+ cells/MNC. This translates into a median CD34+ cell dose of 8.0 × 106 CD34+ cells/kg in Caucasians versus 9.9×106 CD34+ cells/kg in AA (using median apheresis volumes and CD34+ cells collected/L and a typical donor weight of 80 kg). This implies the presence of a low baseline count in a volunteer donor does not necessarily predict for failure to collect adequate amounts of CD34+ cells.
We found the use of a CVC catheter had a negative effect on the collection of CD34+ cells and was consistent among all racial/ethnic groups. The reasons for this is not clear, but, hematopoietic progenitor cells are known to adhere to both plastic and stromal cell layers in culture13 and may contribute to this observation. Timing of CVC catheter placement may also have an effect on CD34+ cell collections. Peak concentration of CD34+ cells occurs approximately 4-6 days after start of G-CSF administration.6 CVC catheters for volunteer donors are typically placed just prior to the start of the first day of apheresis. This delays the start of apheresis by several hours and may miss the peak concentration of circulating CD34+ cells resulting in a lower CD34+ collection yield.
Interestingly, differences in mobilization yields among the ethnic groups appeared to be most prominent in males in the higher BMI categories. However, this effect does not appear to be related to total dose of G-CSF/kg. Even though there were a higher percentage of obese AA donors compared to Caucasian donors (46% vs. 28%), the median weight of Caucasian donors was higher than AA donors in each BMI category (Table 2). A multivariate model, which included total dose of G-CSF/kg as a covariate in addition to BMI, age, gender, and year of apheresis, found no statistically significant effect of G-CSF dose on collection yields (data not shown). These observations imply that the difference in CD34+ cell collection yields is not due to G-CSF dose.
These findings are consistent with information published in related PBSC donors. Carilli et al. reported in a small case control study of 22 AA and 12 Hispanic related donors significantly lower baseline WBC and neutrophil counts in AA donors compared to matched Caucasian donors.10 Despite these differences, they found no significant difference in product cell counts after G-CSF mobilization. Richa et al. reported in a cohort of 195 consecutive sibling PBSC donors (including 16 AA donors) a mean CD34+ count of 102.5 cells/μL in AA compared to 84.7 cells/μL in Caucasians which was not statistically significant (P = 0.12).11 A larger trial of 639 allogeneic PBSC donors by Vasu, et al. (including 75 AA donors), had similar findings.9 In their multivariate analysis, G-CSF dose was most strongly associated with day 5 CD34+ cell counts. However, donor ethnicity was also found to be a statistically significant risk factor with Caucasians associated with significantly lower post-G-CSF CD34+ cell counts.
The differences in baseline counts and response to G-CSF suggest a potential impact of racially related genetic polymorphisms in the G-CSF receptor and other proteins involved with mobilization. The mechanism of action of G-CSF mobilization has been previously reviewed.14 G-CSF is thought to produce alterations in the HPC niches, which includes down-regulation of adhesion proteins and chemokines such as VCAM-1, SDF-1, and SCF.15,16 Additionally, activation of the complement and thrombolytic pathways by release of neutrophil proteases may also contribute to mobilization by causing the release of erythrocyte sphingosine-1-phosphate (a chemo-attractant) into the plasma.17 Genetic linkage analyses in mice have identified several loci associated with poor mobilization.18,19 In humans, polymorphisms in the G-CSF receptor have been identified which result in inferior mobilization responses and survivals in the autologous setting.20 Polymorphisms in SDF-1, VCAM-1 and CD44 have also been identified.21,22 A recent report, however, found no significant effect of SCF-1 polymorphism on the mobilization efficiency in 467 healthy volunteer donors.23 Currently, there are no published systematic investigations of genetic differences of these molecules among ethnic lines and further research in this area is warranted.
Alterations in the HPC microenvironment can also result in alteration in the ability to mobilize cells. Aging has been associated with decreased osteoblastic activity and may result in decreased niches for development of HPCs.24 Parathyroid hormone has been shown to alter the conditions of the stem cell niche through activation osteoblasts and can enhance stem cell collection.25 This may partially explain differences in mobilization responses as increased PTH levels have been seen in African American males26 and in obese patients.27
There are several limitations to this study. Classification of donors on race/ethnicity is self-reported, which can cause misclassification of donors into the appropriate racial/ethnic group if the donor's recollection of their genealogy is imperfect or erroneous. Although all donors underwent rigorous screening prior to apheresis, information concerning concomitant medications was not captured in this study. Beta-adrenergic blocking agents have been shown to reduce the ability to mobilize stem cells in a mouse model.28 The same report also showed use of a beta agonist increased the number of circulating CD34+ cells. However, the significance of this finding in humans is not known.
Time of day of apheresis was not analyzed in our study. There is a circadian rhythm in the production of SDF-1 in HPC niche cells and CXCR4 expression in HPCs, which is controlled by beta-adrenergic sympathetic nerves.29 In a report of 85 healthy donors by Lucas, et al., for donors who were apheresed in the afternoon, when the level of circulating catecholamines are at their lowest, there was a statistically significant higher average mobilization yield (0.55±0.05 CD34+cells/ml/kg) compared to donors apheresed in the morning (0.35±0.02 CD34+cells/ml/kg). The effect was persistent, even when accounting for age and gender of the donors.30
Concomitant medical conditions in the donor may also have an effect in the ability to mobilize stem cells. In a mouse model, high cholesterol levels have been implicated to enhance mobilization by disrupting the SDF1/CXCR4 axis.31 A study by Crysandt, et al., revealed improved mobilization in patients with hypercholesterolemia in a cohort of 104 patients undergoing autologous apheresis in a mixed disease population.32 However, in the general population, hypercholesterolemia is more prevalent in the Caucasian groups compared to AA or Hispanic ethnicities.33
In conclusion, these results show there are significant differences in both baseline CBC and in G-CSF response and mobilization yields among different ethnicities and should be taken into account when implementing standard mobilization protocols. It is unclear why these differences exist, however, it has not prevented the ability to mobilize and collect adequate quantities of stem cells for transplantation from all groups. Understanding the reasons for these differences may improve our ability to collect PBSC in volunteer donors and develop alternative mobilization regimens for potentially difficult mobilizers.
Highlights.
There are racial/ethnic differences in baseline blood counts and response to G-CSF.
Variations are seen in CD34+ cell collection efficiency among races/ethnicities.
Differences in CD34+ cell collection are most pronounced in high BMI donors.
Racial/ethnic differences in CD34+ cell collection is independent of donor weight.
Acknowledgments
This project has been supported by funding from the National Marrow Donor Program and the Health Resources and Services Administration Contract Nos. HHSH234200637020C and HHSH250201200024C to the National Marrow Donor Program. The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration or the National Marrow Donor Program.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Actinium Pharmaceuticals; Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; University of Minnesota; University of Utah; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Conflict of Interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344(3):175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 2.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2013 Available at: http://www.cibmtr.org.
- 3.Takeyama K, Ohto H. PBSC mobilization. Transfus Apher Sci Off J World Apher Assoc Off J Eur Soc Haemapheresis. 2004;31(3):233–243. doi: 10.1016/j.transci.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Ings SJ, Balsa C, Leverett D, Mackinnon S, Linch DC, Watts MJ. Peripheral blood stem cell yield in 400 normal donors mobilised with granulocyte colony-stimulating factor (G-CSF): impact of age, sex, donor weight and type of G-CSF used. Br J Haematol. 2006;134(5):517–525. doi: 10.1111/j.1365-2141.2006.06223.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderlini P, Przepiorka D, Seong C, et al. Factors affecting mobilization of CD34+ cells in normal donors treated with filgrastim. Transfusion (Paris) 1997;37(5):507–512. doi: 10.1046/j.1537-2995.1997.37597293882.x. [DOI] [PubMed] [Google Scholar]
- 6.Grigg AP, Roberts AW, Raunow H, et al. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 1995;86(12):4437–4445. [PubMed] [Google Scholar]
- 7.Miflin G, Charley C, Stainer C, Anderson S, Hunter A, Russell N. Stem cell mobilization in normal donors for allogeneic transplantation: analysis of safety and factors affecting efficacy. Br J Haematol. 1996;95(2):345–348. doi: 10.1046/j.1365-2141.1996.d01-1897.x. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh MM, Tisdale JF, Rodgers GP, Young NS, Trimble EL, Little RF. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy? J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(10):1633–1637. doi: 10.1200/JCO.2009.24.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasu S, Leitman SF, Tisdale JF, et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112(5):2092–2100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carilli AR, Sugrue MW, Rosenau EH, et al. African American adult apheresis donors respond to granulocyte-colony-stimulating factor with neutrophil and progenitor cell yields comparable to those of Caucasian and Hispanic donors. Transfusion (Paris) 2012;52(1):166–172. doi: 10.1111/j.1537-2995.2011.03253.x. [DOI] [PubMed] [Google Scholar]
- 11.Richa E, Papari M, Allen J, et al. Older age but not donor health impairs allogeneic granulocyte colony-stimulating factor (G-CSF) peripheral blood stem cell mobilization. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2009;15(11):1394–1399. doi: 10.1016/j.bbmt.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42(Suppl 1):S1–S2. doi: 10.1038/bmt.2008.101. [DOI] [PubMed] [Google Scholar]
- 13.Gordon MY, Bearpark AD, Clarke D, Dowding CR. Haemopoietic stem cell subpopulations in mouse and man: discrimination by differential adherence and marrow repopulating ability. Bone Marrow Transplant. 1990;5(Suppl 1):6–8. [PubMed] [Google Scholar]
- 14.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25(2):211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 15.Lévesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98(5):1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 16.Lévesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31(2):109–117. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- 17.Ratajczak MZ, Lee H, Wysoczynski M, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24(5):976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts AW, Foote S, Alexander WS, Scott C, Robb L, Metcalf D. Genetic influences determining progenitor cell mobilization and leukocytosis induced by granulocyte colony-stimulating factor. Blood. 1997;89(8):2736–2744. [PubMed] [Google Scholar]
- 19.Geiger H, Szilvassy SJ, Ragland P, Van Zant G. Genetic analysis of progenitor cell mobilization by granulocyte colony-stimulating factor: verification and mechanisms for loci on murine chromosomes 2 and 11. Exp Hematol. 2004;32(1):60–67. doi: 10.1016/j.exphem.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Bogunia-Kubik K, Gieryng A, Gebura K, Lange A. Genetic variant of the G-CSF receptor gene is associated with lower mobilization potential and slower recovery of granulocytes after transplantation of autologous peripheral blood progenitor cells. Cytokine. 2012;60(2):463–467. doi: 10.1016/j.cyto.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Bogunia-Kubik K, Gieryng A, Dlubek D, Lange A. The CXCL12-3'A allele is associated with a higher mobilization yield of CD34 progenitors to the peripheral blood of healthy donors for allogeneic transplantation. Bone Marrow Transplant. 2009;44(5):273–278. doi: 10.1038/bmt.2009.30. [DOI] [PubMed] [Google Scholar]
- 22.Martín-Antonio B, Carmona M, Falantes J, et al. Impact of constitutional polymorphisms in VCAM1 and CD44 on CD34+ cell collection yield after administration of granulocyte colony-stimulating factor to healthy donors. Haemat ologica. 2011;96(1):102–109. doi: 10.3324/haematol.2010.026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenk J, Bornhauser M, Kramer M, et al. Sex and body mass index but not CXCL12 801 G/A polymorphism determine the efficacy of hematopoietic cell mobilization: a study in healthy volunteer donors. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2013;19(10):1517–1521. doi: 10.1016/j.bbmt.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Köhler A, Schmithorst V, Filippi MD, et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. 2009;114(2):290–298. doi: 10.1182/blood-2008-12-195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams GB, Martin RP, Alley IR, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25(2):238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 26.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76(2):470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 30.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3(4):364–366. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes AL, Carvalho T, Serpa J, Torre C, Dias S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood. 2010;115(19):3886–3894. doi: 10.1182/blood-2009-08-240580. [DOI] [PubMed] [Google Scholar]
- 32.Crysandt M, Hilgers RD, von Hobe S, et al. Hypercholesterolemia and its association with enhanced stem cell mobilization and harvest after high-dose cyclophosphamide+G-CSF. Bone Marrow Transplant. 2011;46(11):1426–1429. doi: 10.1038/bmt.2010.327. [DOI] [PubMed] [Google Scholar]
- 33.Meadows TA, Bhatt DL, Hirsch AT, et al. Ethnic differences in the prevalence and treatment of cardiovascular risk factors in US outpatients with peripheral arterial disease: insights from the reduction of atherothrombosis for continued health (REACH) registry. Am Heart J. 2009;158(6):1038–1045. doi: 10.1016/j.ahj.2009.09.014. [DOI] [PubMed] [Google Scholar]