Abstract
Therapeutic interventions for HIV-1 that successfully augment adaptive immunity to promote killing of infected cells may be a requisite component of strategies to reduce latent cellular reservoirs. Adoptive immunotherapies utilizing autologous monocyte-derived dendritic cells (DCs) that have been activated and antigen loaded ex vivo may serve to circumvent defects in DC function that are present during HIV infection in order to enhance adaptive immune responses. Here we detail the clinical preparation of DCs loaded with autologous Aldrithiol-2 (AT-2)-inactivated HIV that have been potently activated with the viral mimic, Polyinosinic-polycytidylic acid–poly-L-lysine carboxymethylcellulose (Poly-ICLC). HIV is first propagated from CD4+ T cells from HIV-infected donors and then rendered non-replicative by chemical inactivation with aldrithiol-2 (AT-2), purified, and quantified. Viral inactivation is confirmed through measurement of Tat-regulated β-galactosidase reporter gene expression following infection of TZM-bl cells. In-process testing for sterility, mycoplasma, LPS, adventitious agents, and removal of AT-2 is performed on viral preparations. Autologous DCs are generated and pulsed with autologous AT-2-inactivated virus and simultaneously stimulated with Poly-ICLC to constitute the final DC vaccine product. Phenotypic identity, maturation, and induction of HIV-specific adaptive immune responses are confirmed via flow cytometric analysis of DCs and cocultured autologous CD4+ and CD8+ T cells. Lot release criteria for the DC vaccine have been defined in accordance with Good Manufacturing Practice (GMP) guidelines. The demonstrated feasibility of this approach has resulted in approval by the FDA for investigational use in antiretroviral (ART) suppressed individuals. We discuss how this optimized DC formulation may enhance the quality of anti-HIV adaptive responses beyond what has been previously observed during DC immunotherapy trials for HIV infection.
Keywords: Dendritic cell, Therapeutic Vaccine, HIV-1, Poly-ICLC
Introduction
Long-term suppression of HIV-1 with antiretroviral therapy (ART) in infected individuals allows for viral persistence in cellular reservoirs, serving as a major barrier to eradication. Therapeutic vaccines for HIV-1 that successfully enhance cytotoxic T lymphocyte (CTL) function to promote killing of infected cells in combination with approaches to disrupt viral latency may result in reduction of cellular viral reservoirs that contain stably integrated, replication-competent virus [1]
As the most potent antigen presenting cells (APCs), myeloid dendritic cells (DCs) strongly stimulate the onset of CD4+ and CD8+ T cell responses, making them ideal targets for therapeutic vaccines. However, in the setting of chronic HIV infection, multiple DC functions are impaired including cytokine secretion and capacity to stimulate T cells and NK cells [2–4]. In order to bypass these defects, adoptive immunotherapies utilizing autologous monocyte-derived DCs that are activated and antigen loaded ex vivo have been explored [5–16]. These trials have demonstrated that DC immunization is safe and immunogenic during HIV infection, but viral control has been incomplete. Key steps in optimization of DC immunotherapies lie in maximizing the quality of DC preparations, through the provision of effective ex vivo maturation stimuli and HIV immunogens. Depending upon the maturation stimulus utilized, DCs upregulate co-stimulatory molecules and secrete a variable array of cytokines to modulate adaptive immune responses. In order to generate strong anti-viral immunity, selection of maturation stimuli that induce the secretion of Th1-skewing cytokines by DCs, including IL-12, is likely advantageous [17]
In terms of the HIV immunogen(s) utilized, the most successful DC immunotherapies to date have involved the use of autologous, inactivated HIV/SIV [9, 10, 16, 18]. Most notably, a recent randomized, controlled trial of autologous heat-inactivated HIV-pulsed DC resulted in a substantial decrease in viral load set point following ART interruption [10]. In contrast to conventional means of viral inactivation, including heat treatment, that result in damage to viral proteins, aldrithiol-2 (AT-2, 2,2′-dithiodipyridine) preserves the conformational and functional integrity of HIV envelope glycoproteins to allow for unaltered viral fusion, while rendering HIV non-infectious through covalent modification of key S-H- containing internal proteins [19]. Here we detail the clinical preparation of Poly-ICLC (Hiltonol, Oncovir, Inc) activated DCs that are loaded with autologous AT-2-inactivated HIV. This DC vaccine is now approved by the FDA for investigational use as a therapeutic immunotherapy in ART suppressed individuals (BB-IND-15382). The design of this optimized DC formulation aims to enhance the quality of anti-HIV adaptive immunity beyond what has been previously observed during DC immunotherapy trials.
METHODS
The schema in Figure 1 depicts an overview of the major steps involved in generation of the final DC vaccine product. Table 2 summarizes quality control measures including specified in process testing and lot release criteria as detailed throughout the methods.
FIGURE 1. Autologous DC Vaccine Production Schema.
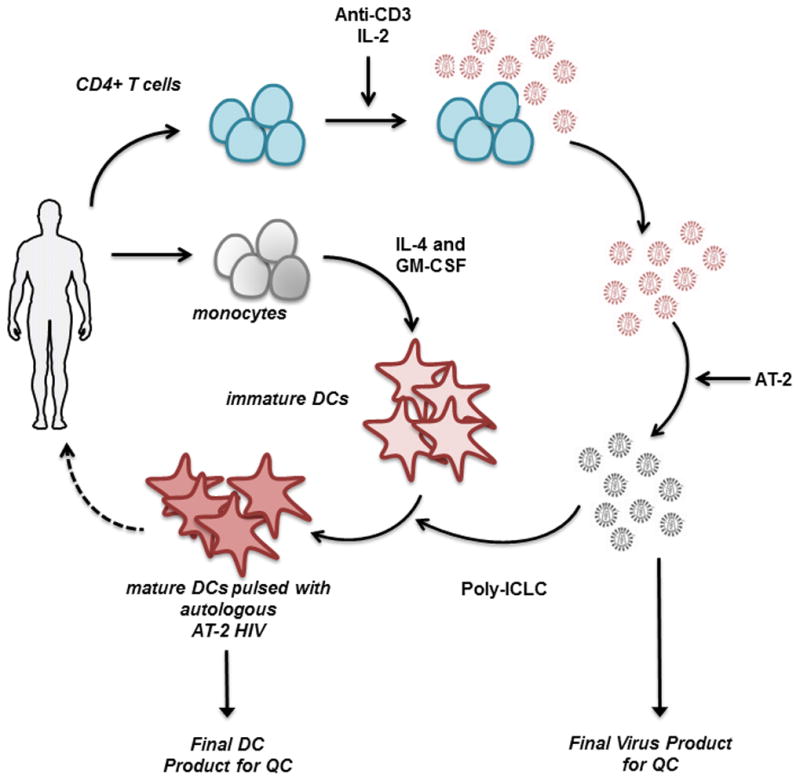
Following leukapheresis of viremic HIV-infected donors, virus is propagated from anti-CD3-activated CD4+ T cells in the presence of IL-2. Viral supernatants are inactivated with AT-2 and then purified. This autologous, inactivated HIV is then pulsed onto autologous monocyte-derived DCs in combination with Poly-ICLC. These mature, antigen-loaded moDCs may be injected back into the patient as a form of HIV immunotherapy.
Human Subjects
HIV-1-infected, anti-retroviral therapy (ART)-naïve subjects (N=18) (plasma HIV-1 RNA ranging from 496–1,860,000 copies HIV-1 RNA/mL, median 26,225 copies/ml and CD4+ cells from 221–1074 cells/mm3, median 622 cells/mm3) and ART-treated subjects (N=5) were recruited through NYU Langone Medical Center, Aaron Diamond AIDS Research Center (ADARC) and Center for HIV/AIDS Vaccine Immunology (CHAVI 012) and underwent informed consent and leukapheresis. This study was reviewed and approved by the Institutional Review Board of New York University School of Medicine, ADARC, and CHAVI.
Cell Lines
The TZM-bl cell line (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc.[20–23]) is a HeLa cell line that stably expresses CD4 and CCR5, with luciferase and β-galactosidase genes under control of the HIV-1 promoter. TZM-bl cells were sub-cultured in DMEM (90%), 10% FBS, 100 units of Penicillin and 0.1 mg/ml of Streptomycin. The Sup-T1.CCR5 cell line was obtained from Dr. Jim Hoxie [24].
Reagents and antibodies
AIM-V media and CD4+ and CD8+ Dynal beads were purchased from Invitrogen. RPMI-1640 media and X-Vivo 15 media (BioWhittaker™) were from Lonza Biologics, Inc. Pooled Human AB Serum (PHS) was from Valley Biomedical. Ficoll-Paque Premium was from GE Healthcare Sciences. Aldrithiol-2 (AT-2) and reduced L-Glutathione were from Sigma-Aldrich. Good manufacturing practice-certified (GMP-certified) Interleukin-2 (IL-2), GMP-certified Interleukin-4 (IL-4), Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF), GMP-certified Anti-CD3 antibody, CliniMACS CD4 and CD14 microbeads, CliniMACS LS Tubing sets, CryoMACS DMSO and CliniMACS PBS/EDTA buffer were from Miltenyi Biotec, Inc. Polyinosinic-polycytidylic acid – poly-L-lysine carboxymethylcellulose (Poly-ICLC) was from Oncovir, Inc. Fluorescent antibodies for IL-2, -IFNγ, -TNFα, -MIP-1β, -CD3, -CD4, and -CD8 were purchased from BD biosciences. Antibodies for CD86, -CD14, -CD11c, and -HLA-DR were purchased from Biolegend. Recombinant human IL-2 and IL-7 were from R&D Systems. The following antibodies were manufactured in-house at the AIDS and Cancer Virus Program, Leidos Biomedical Research, Inc., Frederick National Laboratory: anti-p24CA [94–0001], anti-p17MA [R193244], anti-gp120HIV [P3F5-D5-F8]. Anti-gp41TM(4E10) was obtained from Polymun [AB004].
Autologous virus production
CD4+ T cells from viremic donors were isolated from fresh leukaphereses via CD14-CD4+ selection using CD14+ and CD4+ magnetic beads and a CliniMACS Separation System. The CliniMACS device is a closed GMP system used for cell isolation. Isolated CD4+ T cells were activated upon culture with plate-bound anti-CD-3 antibody (5 μg/mL), and maintained in the presence of 100 IU/mL IL-2 in AIM-V media supplemented with 10% PHS, 1% HEPES buffer and gentamicin (24μg/ml)(BioWhittaker™). Supernatants containing HIV were collected and frozen with each IL-2-supplemented media change (every 3 ± 1 days). Supernatants were monitored for virus content at each media change using HIV-1 p24 antigen capture immunoassay (AIDS and Cancer Virus Program, NCI-Frederick). Cultures that produced < 2 ηg/mL at 3 consecutive time points were terminated. Frozen CD4+ culture supernatants were maintained at −80°C for 1–2 months until viral inactivation and purification.
Inactivation of virus
Supernatants from CD4+ T cell culture were thawed, pooled by donor, clarified through low speed centrifugation using a SX4750 rotor (4,000 × g × 15 mins)(Beckman Coulter), and filtered through a 0.22 μm filter. AT-2 (0.5 M) in DMSO (CryoMACS) was added to the clarified supernatant to a final concentration of 1 mM and incubated overnight for 12–18 hours at 4°C with gentle stirring. A portion of supernatant was incubated overnight under similar conditions without AT-2 for comparative use in residual infectivity assays.
Virus purification and removal of AT-2
The AT-2 inactivated virus (AT-2 HIV) from each batch of supernatant was purified and concentrated by sequential ultracentrifugation. The virus was first pelleted by centrifuging at 4°C using a SW32 rotor (100,000 × g × 60 mins) (Beckman Coulter). The resultant pellet was resuspended with 1mL of 10 mM reduced L-Glutathione to react with any residual AT-2 present and centrifuged at 4°C using a Type 50.4 Ti rotor (198,000 × g × 6 mins)(Beckman Coulter). The L-Glutathione was decanted and the pellet resuspended in 300μl PBS. The purified AT-2-inactivated virus was aliquoted and stored in liquid nitrogen.
Quantification of purified virus
The total amount of purified virus was quantified by SDS-PAGE with Coomassie staining using p24 to calibrate the gel [25].
Confirmation of purified virus identity
The purity and biochemical composition of the purified virus was evaluated by SDS-PAGE followed by immunoblotting for HIV p24, gp120, and gp41.
Confirmation of Virus Inactivation
Lack of residual infectivity of AT-2-inactivated HIV-1 was measured in a TZM-bl assay as a function of Tat-regulated β-galactosidase reporter gene expression (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc.[20–23]). Infectious virus was quantified as a function of β-galactosidase activity expressed as relative spot-forming units (SFU) before and after AT-2 treatment, in donor CD4+ T cell supernatants and purified virus samples. Viral controls of infectious and AT-2 inactivated HIV-1 BaL supernatant (AIDS and Cancer Virus Program, Leidos Biomedical Research, Inc., National Laboratory for Cancer Research) of known infectivity were included in each assay. TZM-bl plate images were captured with a Cellular Technology, Ltd. CTL-ImmunoSpot® S6 Micro Analyzer ELISPOT Reader and the number of SFUs was analyzed with ImmunoSpot® Professional Software’s Immuno Capture, v6.3.
Confirmation of AT-2 removal from virus preparations
Removal of unreacted AT-2 in the purified virus preparation (lysed in 8M Guanidine-HCl) was confirmed via HPLC with a threshold sensitivity of 0.5 ng/ul of purified virus.
Preparation of Monocyte-derived Dendritic Cells and Final DC Vaccine Product
PBMCs from leukaphereses were frozen using a controlled rate freezer and later thawed to generate monocyte derived dendritic cells (DCs). Monocytes were enriched from frozen PBMCs by plastic adherence and cultured in RPMI 1640 media supplemented with 5% PHS, 1% HEPES, and Gentamicin (24ug/ml) in the presence of 100 IU/mL GM-CSF (Leukine) and 400 IU/mL IL-4 for 6 days. On day 6, immature DCs (iDCs) were pulsed overnight with autologous, purified AT-2 HIV at a concentration of 100–300 ηg/mL of p24 equivalents [26–29]. These DCs were matured with 5μg/mL Poly-ICLC to constitute the final DC vaccine product. The final DC vaccine product was frozen in PHS/10% DMSO.
Detection of Bacteria, Mycobacteria, and Fungus
BacT/Alert culture bottles (aerobic, anaerobic, and mycobacteria) were inoculated with culture supernatant and incubated for 2 weeks to detect bacterial and fungal contamination, and 8 weeks to detect mycobacterial at the beginning and end of CD4+ T cell culture, on day 5 of DC preparation, and on the final DC vaccine product. Throughout CD4+ T cell culture, supernatants were added to a TSA (Trypticase Soy Agar) 5% Sheep’s Blood/MacConkey Agar Biplate weekly and incubated for 7 days to detect the presence of bacteria.
Detection of Mycoplasma
Culture supernatants were sent to Clongen Laboratories LLC to evaluate for the presence of mycoplasma at the beginning and end of CD4+ T cell culture, on day 5 of DC preparation, and on the final DC vaccine product. Cultures are maintained for 14 days and DNA fluorochrome staining performed after 5 days.
Detection of Endotoxin
The level of Gram negative bacterial endotoxin was measured in culture supernatants using a Kinetic Chromogenic LAL Kit (Associates of Cape Cod, Inc., range 0.005 EU/ml – 50.0 EU/ml) per manufacturer’s protocol, at the beginning and end of CD4+ T cell culture, on day 5 of DC preparation, and on the final DC vaccine product.
Detection of Adventitious Viruses
The presence of adventitious virus was evaluated at the end of each CD4+ T cell culture via in vitro virus testing (Clongen Laboratories LLC). Three indicator cells lines (MRC-5, Vero, and SC-1) were inoculated with culture supernatants and observed for 14 days for development of cytopathic effects (CPE) per Clongen’s protocol.
Viability, Identity, and Maturation of the DC vaccine
The identity of DCs after pulsing with AT-2 HIV and maturation with Poly-ICLC was evaluated by flow cytometry. Frozen DC were thawed and stained with antibodies against CD3, HLA-DR, CD11c, CD14, CD86, and CD40. The viability of each DC vaccine product was determined by Live/Dead staining with Guava Viacount Reagent (Millipore, Billerica, MA).
Additional experiments utilizing DC derived from ART- suppressed HIV-infected individuals were performed under non-GMP conditions to compare maturation with Poly-ICLC to the widely used standard cytokine cocktail Monocyte Conditioned Medium (MCM) MIMIC (1 ug/mL PGE2, 5 ng/mL TNFα and IL-1β, 150 ng/mL IL-6). Up regulation of CD86 and CD40, and secretion of cytokines, including the pro-Th1 cytokine, IL-12, was measured after pulsing DCs with AT-2 HIV and maturation with Poly-ICLC (5 ug/mL) or MCM MIMIC. Cytokines were measured by Human Inflammatory Cytokine Bead Array (BD Biosciences).
Immunogenicity of the DC Vaccine
Immunogenicity was evaluated as a function of activation of autologous HIV-specific CD4+ and CD8+ T cell responses. DCs were derived as described and matured with Poly-ICLC +/− pulsed with AT-2 HIV (100 ng/mL-300 ng/mL) on d6 of growth and co-cultured with magnetic bead sorted autologous CD4+ and CD8+ T cells at a ratio of 1:5 (DC:T cell). Cultures were maintained for 10 days in RPMI-1640 supplemented with 1mM HEPES, 2mM L-glutamine and 10% PHS in the presence of rhIL-2 (10 IU/mL) and rhIL-7 (5ng/mL). To evaluate for the expansion of HIV-specific T cell responses following co-culture, T cells were restimulated with autologous DC +/− pulsed with autologous AT-2 HIV at a ratio of 1:5 (DC:T cell) for 6 hours followed by fixation, permeabilization, and fluorescent staining for IFNγ, TNFα, MIP1β, and CD107a. Data were acquired with an LSR II cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar).
RESULTS
HIV Production, Purification and Quantification
Donor CD4+ T cells cultured under GMP conditions (N=4) remained viable and contained ≥2 ng/mL p24 by ELISA for 36-55 days (Fig. 2A). Following purification via ultracentrifugation, quantification of the AT-2 inactivated virus batches yielded an average of 200,071 ng p24CA [range 27,904 – 489,224 ng] (Fig. 2B). To further establish feasibility of virus production, CD4+ T cells from 14 additional untreated HIV-infected donors were cultured, and virus containing supernatants were inactivated and purified under non-GMP conditions with similar culture duration and viral yield (Supplemental Figure 1). No correlation was found between viral yield and donor plasma HIV-1 RNA or CD4+ T cell count (not shown).
FIGURE 2. HIV Production, Purification and Quantification.
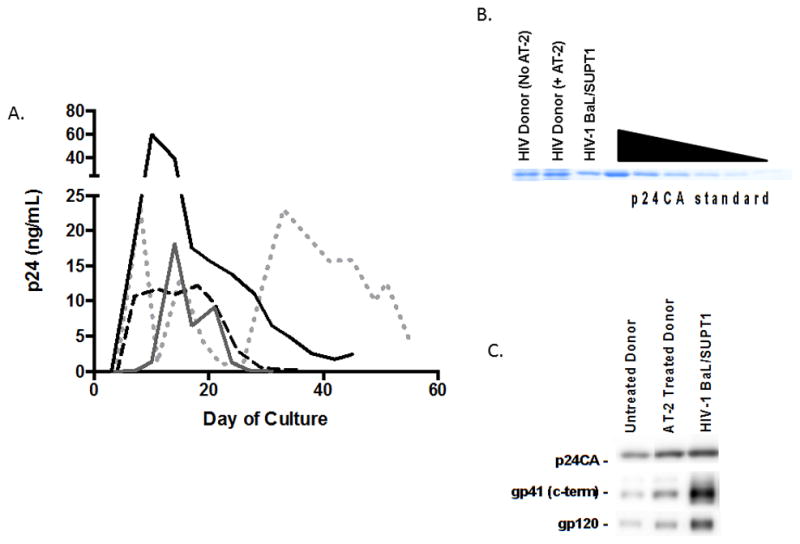
(A) p24 content of CD4+ T cell culture supernatants from HIV-infected donors (N=4) under GMP conditions was quantified by ELISA. To further establish feasibility, 14 additional donors were cultured under non-GMP conditions demonstrating similar concentration of p24 (Supplemental Figure 1).
(B) Coomassie staining of purified, inactivated virus from a representative HIV-infected donor before and after treatment with AT-2. p24CA standard ranges from 500 to 15.1625 ng. This was performed on all purified virus samples cultured under GMP conditions (N=4), with an average yield of 200,071 ng p24CA [range 27,904 –489,224 ng]. Viruses from 14 additional untreated HIV-infected donors were cultured, inactivated, and purified under non-GMP conditions with similar yield of p24CA (data not shown).
(C) Immunoblots for the viral proteins p24CA, gp41TM (c-terminus), and gp120 were performed on purified, inactivated virus preparations (N=4, figure displays representative donor). This was performed to confirm the presence of whole virions rather than free p24CA in the virus preparations.
Immunoblots confirmed the presence of whole virions in the purified virus preparations in all donors (N=4) (Fig. 2C).
Confirmation of viral inactivation and removal of AT-2 from virus preparation
Virus inactivation was confirmed in CD4+ T cell culture supernatants before and after AT-2 treatment (Fig. 3A) and also following purification and concentration of viral supernatants (data not shown) for each donor via TZM-bl assays. Following AT-2 treatment, there was no detectable infectivity present in all culture supernatants and purified viruses tested. The removal of residual AT-2 was confirmed in all virus preparations by HPLC (N=4) (Fig. 3B).
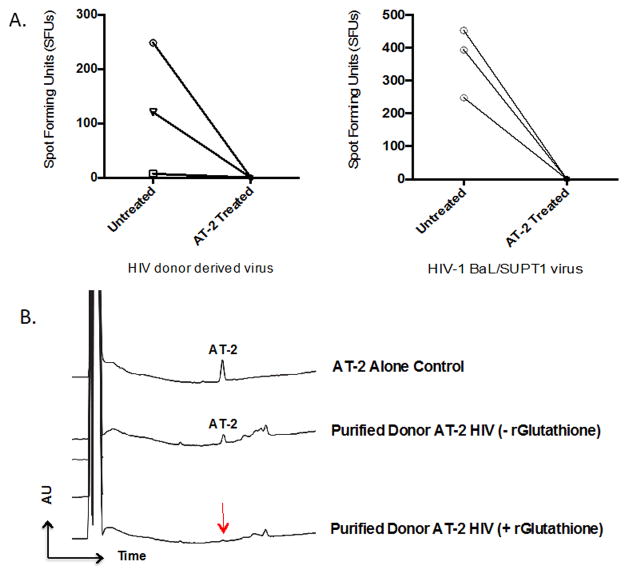
FIGURE 3. Confirmation of viral inactivation and removal of AT-2 from viral preparation.
(A) Evaluation for residual infectivity was performed via TZM-bl reporter assays. Infectious virus (quantified as spot forming units (SFU)) is shown before and after AT-2 treatment in donor CD4+ T cell supernatants (N=3) (left) and HIV-1 BaL/SUPT1 supernatants (right). Viral inactivation was also confirmed following purification and concentration of viral supernatants for each donor with no detectable infectivity following AT-2 treatment (data not shown).
(B) HPLC analysis of 0.5M AT-2 solution (AT-2 alone control) and purified AT-2-inactivated HIV from a representative donor with and without the addition of 10 mmol r-Glutathione. HPLC was performed at a flow rate of 300 mL/min on 3 m, 2 × 100 mm Luna C18(2), (Phenomenex), using aqueous acetonitrile/trifluoroacetic/acid solvents on a Shimadzu HPLC system. Peaks were detected by UV absorption at 206 and 280 nm.
In Process Testing and Lot Release Criteria
In process and lot release criteria testing was performed on select samples depending on availability of specimens manufactured under GMP conditions as detailed in Table 1. All CD4+ T cell culture supernatants evaluated weekly for gross contamination with bacteria tested negative for each culture (N=4; ranging from 4–6 time points per culture). Supernatants did not contain bacteria, fungus, mycobacteria, or Mycoplasma at the start or end of culture (N=4). Supernatants did not contain adventitious virus at the end of culture (N=4). All samples evaluated for endotoxin levels met the criteria for in process testing and lot release of <50 EU/mL.
TABLE 1.
In Process Testing and Lot Release Criteria.
| Virus Production Tests (In process tests) | Method | Criteria |
|---|---|---|
| Virus Quantification | HIV p24 ELISA | Culture terminated when p24 ≤ 2 ng/mL at 3 consecutive time points |
| Sterility – Bacteria, Mycobacteria, and Fungi | Detection of aerobic and anaerobic bacteria and fungi by gram stain (7 days) and direct culture using BacTAlert (2 weeks for bacteria and fungi and 8weeks for mycobacteria) | No growth |
| Mycoplasma | Direct Culture on mycoplasma selective agar (14 days) and DNA Fluorochrome Staining Assay with Vero Indicator Cell Line (5 days) | Negative |
| Endotoxin | Kinetic Chromogenic LAL Assay | < 50 EU/mL |
| Adventitious Virus Testing | In vitro culture using 3 cell lines (14 days) | Negative |
| Purity – Residual AT-2 | HPLC for AT-2 | < 0.5 ng of AT-2 detected in analyte |
| Identity – Viral Proteins | Western blot to confirm presence of full virion | Presence of HIV p24CA, gp41TM, and gp120 |
| Virus Quantification | Quantification of p24CA by Coomassie staining and densitometry analysis | Density of p24CA compared to p24CA standard |
| Infectious Titre | Residual infectivity by TZM-bl reporter assay | ≤SFUs of media control (≤1.48 × 10−4 to 1.65 × 10−5 ng p24 HIV) |
| DC vaccine Tests (Lot Release Criteria) | Method | Criteria |
|---|---|---|
| Cell Viability | Viability staining after storage in LN2 vapor phase | > 70% viable |
| Identity | Flow Cytometry - % CD11c+ Flow Cytometry - % CD11c+CD14+ Flow Cytometry - % CD11c+CD86+ |
> 50% < 30% > 50% |
| Sterility – Bacteria, Mycobacteria, and Fungi | Direct Culture for aerobic and anaerobic bacteria, mycobacteria and fungi (2 weeks) | No growth |
| Mycoplasma | Direct Culture on mycoplasma selective agar (14 days) and DNA Fluorochrome Staining Assay with Vero Indicator Cell Line (5 days) | Negative |
| Endotoxin | Kinetic Chromogenic LAL Assay | < 50 EU/mL |
Viability, Identity and Maturation of DC Vaccine
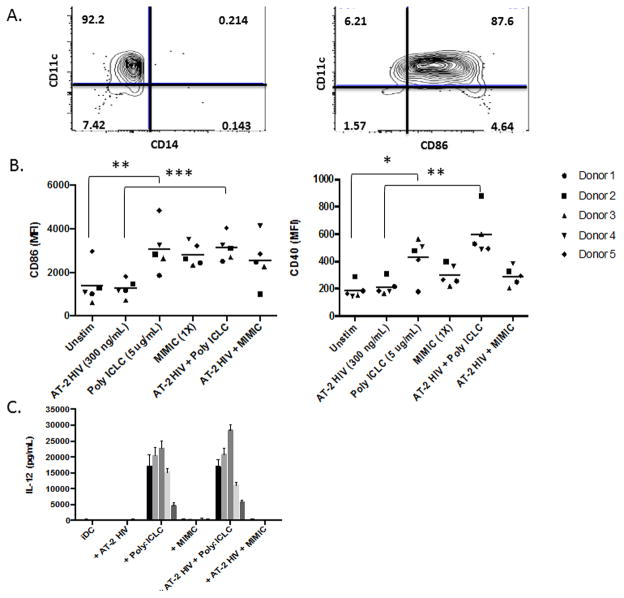
The DC vaccine was stored in the vapor phase of liquid nitrogen for 5 months and evaluated for viability, phenotype, and sterility after thawing. Lot release criteria were met for viability (>70%) and identity (>50% CD11c+, <30+ CD11c+/CD14+, >50% CD11c+CD86+). Viability was >90% after thawing and DCs were >75% CD11c+, <5% CD11c+/CD14+, and >70% CD11c+CD86+ (N=2) (Fig 4A). Given the limited number of GMP samples, these criteria along with additional parameters were also evaluated under non-GMP conditions to further confirm identity and maturation (N=5). Poly-ICLC resulted in significant up regulation of CD86 and CD40 compared with unstimulated DCs (both nonpulsed and AT-2 HIV pulsed conditions) (Fig. 4B). When compared with the standard clinical DC maturation stimulus, MCM MIMIC, DCs matured with Poly-ICLC produced robust levels of the pro-Th1 cytokine, IL-12 (Fig. 4C). Additionally, Poly-ICLC induced strong secretion of TNFα, IL-1β, and IL-6 from moDCs (both nonpulsed and AT-2 HIV pulsed conditions) (Supplemental Figure 2).
FIGURE 4. Identity, Maturation, and IL-12 Secretion.
(A) To ascertain fulfillment of identity and maturation lot release criteria, thawed final DC vaccine products were stained for CD11c, CD14, and CD86. Representative donor is displayed. (B) DCs from ART-suppressed donors (N=5) were pulsed with patient-propagated AT-2 HIV and stimulated with Poly-ICLC or the standard cytokine cocktail, MCM MIMIC. Poly-ICLC resulted in upregulation of CD86 and CD40 compared with unstimulated DCs (both nonpulsed and AT-2 pulsed conditions). (C) IL-12 secretion by DCs from these same donors and conditions was evaluated. In contrast to unstimulated and MCM MIMIC DCs, Poly ICLC induced robust secretion of IL-12 by DCs.
Immunogenicity of DC Vaccine
The DC vaccine stimulated polyfunctional autologous HIV specific CD4+ and CD8+ T cells in all donors tested as evidenced by secretion of IFNγ, TNFα, MIP1β, and/or CD107a (N=3) (Fig. 5). For all donors, cytokine levels secreted by CD4+ T cells following expansion and restimulation with AT-2 HIV-pulsed (either 100ng/ml or 300ng/ml) DCs were >3X higher in at least 2 cytokines and >2X higher in at least 3 cytokines when compared with control (background) conditions (T cells expanded and restimulated with nonpulsed Poly-ICLC matured DCs). Overall, CD8+ T cell responses were generally lower in magnitude compared with CD4+ T cell responses, but remained >3X higher in at least 1 cytokine and >2X higher in at least 2 cytokines, in all donors compared with background. There was no consistent dose response when comparing DCs that were pulsed with 100ng versus 300ng of AT-2 HIV. This pattern of induction of CD4+≫CD8+ T cells has been observed in previous in vitro studies of AT-2 HIV pulsed DCs and also in a clinical trial in untreated individuals [16, 27]. AT-2 HIV has been found to stimulate HIV-specific CD8+ T cells via the classical endogenous pathway following CD4+ binding and entry [28].
FIGURE 5. Immunogenicity of DC Vaccine.
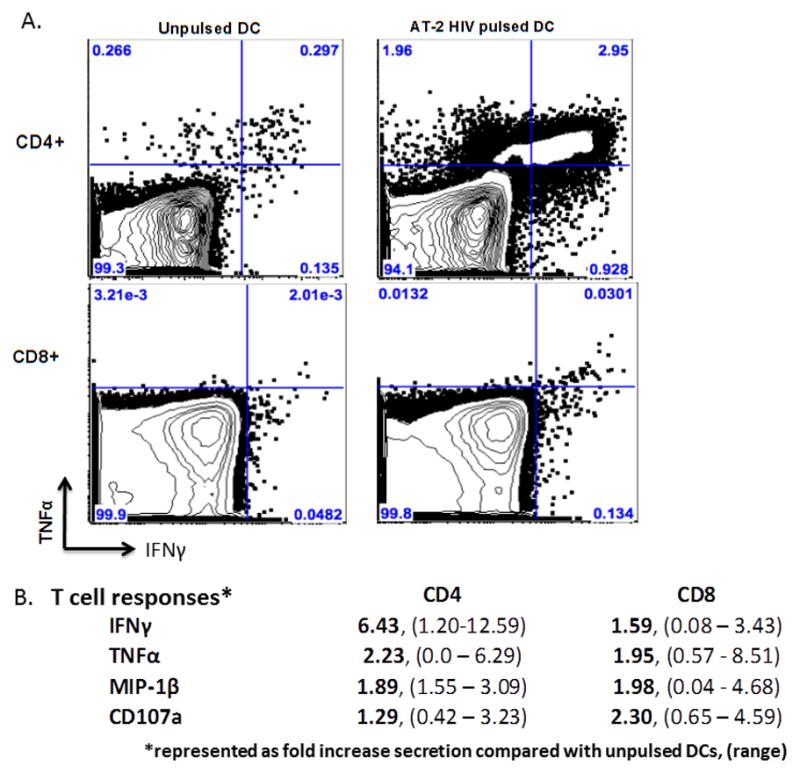
To evaluate potency/immunogenicity, the autologous DC vaccine (Poly-ICLC matured DCs pulsed with AT-2 HIV (100ng/ml and/or 300ng/ml) or nonpulsed autologous DCs were co-cultured with autologous CD4+ (top) and CD8+ (bottom) T cells at a ratio of 1:5 (DC:T cell). Following 10 days of co-culture, expansion of HIV-specific T cell responses was measured via flow cytometry as a function of intracellular cytokine secretion in response to restimulation with corresponding autologous Poly-ICLC matured DCs ± AT-2 HIV. A) Results are displayed from representative donors for IFNγ and TNFα. Top panel shows expansion of CD4+ T cells upon coculture with autologous DCs pulsed with AT-2 HIV (100ng/ml). Bottom panel shows expansion of CD8+ T cells upon coculture with autologous DCs AT-2 HIV (300ng/ml). B) Summary of intracellular cytokines secreted by CD4+ and CD8+ T cells following expansion with autologous DCs pulsed with AT-2 HIV. Data are represented as fold change in cytokine secretion of T cells expanded and restimulated with autologous AT-HIV pulsed DCs compared with control (nonpulsed) conditions (mean, (range)).
Discussion
We detail GMP methods for the generation and subsequent AT-2 inactivation, purification, quantification and quality control of sufficient quantities of autologous HIV from donor CD4+ T cells to allow for its use as an immunogen in DC immunotherapies. These methods can be readily reproduced in GMP laboratories without the need for highly specialized equipment. Upon pulsing autologous DCs with purified autologous AT-2 HIV and maturation with Poly-ICLC, we demonstrate the in vitro stimulation of autologous, polyfunctional HIV-specific adaptive immune responses. This is consistent with previous in vitro and in vivo studies by our group and others, revealing that AT-2 HIV/SIV pulsed, mature DCs effectively prime and expand polyfunctional HIV/SIV-specific CD4+ and CD8+ T cell responses, resulting in enhanced viral control during untreated HIV/SIV-infection [16],[18],[27, 28, 30, 31]. In contrast with autologous heat-inactivated HIV [10], AT-2 HIV DC preparations have not been studied in ART suppressed individuals, in part because they have not been previously approved for investigational use in North America or Europe. The use of AT-2 HIV may enhance the immunogenicity of DC therapeutic vaccines beyond what has been seen with heat-inactivated HIV, given it minimizes conformational damage to viral proteins. One potential limitation of the use of autologous virus-containing vaccines is that cells from aviremic individuals are less optimal for generating large quantities of virus. This requires that the initial leukapheresis for virus generation be performed prior to the initiation of ART or, alternatively, following a closely monitored structured treatment interruption in select patient populations. Notably, in the aforementioned in vivo study using DCs pulsed with heat-inactivated HIV, autologous HIV was generated using cells from ART-suppressed patients without treatment interruption [10]. Therefore, significantly lower amounts of immunogen may be sufficient for generating an effective vaccine, and early phase studies that evaluate dose titrations of inactivated virus will be useful.
Furthermore, we describe a means to augment the quality of DC immunotherapies for HIV-infected individuals through the ex vivo activation of DCs with Poly-ICLC, a synthetic dsRNA that is recognized by TLR3 in endosomes and cytoplasmic sensors, including MDA5 and DHX/DDX RNA helicases [32–34]. Consistent with prior studies in seronegative donors [35, 36], we demonstrate that Poly-ICLC matures DCs from HIV-infected donors, as evidenced by upregulation of costimulatory molecules and secretion of cytokines. Notably, Poly-ICLC stimulated robust secretion of the pro-Th1 cytokine, IL-12, by DCs from donors on suppressive ART, compared with standard cytokine cocktails that have been previously employed in clinical trials, which has likely limited their anti-viral potency [9, 10, 16]. Powerful anti-viral effects were observed by Poly-ICLC and AT-2-inactivated SIV in a therapeutic tonsillar vaccine study in non-human primates [37]. Here viral loads following treatment interruption were 1–2 logs lower in vaccinated animals compared with unvaccinated controls or when Cytosine-phosphate-guanine class C (TLR 9 agonist) was used as an adjuvant. Our finding of increased IL-12 secretion by DCs from ART-treated HIV donors aligns with our previous work revealing that Poly-ICLC significantly rescues DC cytokine secretion in the presence of plasma from ART-suppressed individuals, when compared with plasma from untreated HIV donors, though some defect in cytokine production still remains, compared with plasma from uninfected donors [4]. Given the suppressive role that HIV donor plasma has been shown to have on DCs, an important factor in our culture system is the use of clinical grade plasma from seronegative donors, during the derivation and activation of DCs, rather than autologous plasma which is commonly utilized and may diminish DC cytokine secretion upon ex vivo stimulation [3, 4].
Taken together, these modifications may improve the success of DC immunotherapies beyond what has been observed in previous trials when administered to ART suppressed individuals. Unfortunately, due to practical limitations and high cost of DC immunotherapies, these strategies are unlikely to be feasible on a large-scale for a wide population of infected individuals or as a measure to prevent the acquisition of HIV infection in at-risk individuals. However, given that DCs have been shown to be dysfunctional in situ during HIV infection, successful DC immunotherapy would provide an important proof of concept, that augmentation of DC function is vital for generating more effective HIV-specific adaptive immune responses to ultimately lower barriers to functional cure and/or eradication.
Supplementary Material
Highlights.
Autologous AT-2 inactivated HIV can be derived from donor CD4+ T cells for use a DC vaccine immunogen.
This immunogen can be generated in GMP laboratories without highly specialized equipment.
AT-2 HIV-pulsed DCs matured with Poly-ICLC secrete IL-12 and induce HIV-specific immunity.
This formulation is now approved for investigational use in ART-suppressed individuals.
This formulation may enhance anti-viral immunity beyond previous therapeutic DC vaccine trials.
Acknowledgments
Acknowledgements/Funding
This work has been supported by National Institutes of Health [K08 AI84578 to E.A.M., R37 AI044628 to N.B., and U01 A1067854]; National Institutes of Health, National Cancer Institute under contract HHSN261200800001E; Bill and Melinda Gates Foundation [Collaboration for AIDS Vaccine Discovery Grant ID: 38645]; Center for AIDS Research [P01AI057127]; and the New York University Langone Medical Center Grunebaum AIDS Research Fund and Saul Farber Scholarship.
Footnotes
Conflicts of Interest
Nina Bhardwaj reports a potential conflict as a co-inventor on patents related to DC function and differentiation. Andres Salazar is the CEO of Oncovir, Inc which manufactures Poly-ICLC. The remaining authors report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buisson S, et al. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. The Journal of infectious diseases. 2009;199(12):1862–71. doi: 10.1086/599122. [DOI] [PubMed] [Google Scholar]
- 3.Frleta D, et al. HIV-1 infection-induced apoptotic microparticles inhibit human DCs via CD44. J Clin Invest. 2012;122(12):4685–97. doi: 10.1172/JCI64439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller EA, et al. Plasma factors during chronic HIV-1 infection impair IL-12 secretion by myeloid dendritic cells via a virus-independent pathway. J Acquir Immune Defic Syndr. 2012;61(5):535–44. doi: 10.1097/QAI.0b013e31826afbce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi RT, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27(43):6088–94. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia F, et al. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J Infect Dis. 2005;191(10):1680–5. doi: 10.1086/429340. [DOI] [PubMed] [Google Scholar]
- 7.Ide F, et al. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J Med Virol. 2006;78(6):711–8. doi: 10.1002/jmv.20612. [DOI] [PubMed] [Google Scholar]
- 8.Garcia F, Routy JP. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine. 2011;29(38):6454–63. doi: 10.1016/j.vaccine.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 9.Garcia F, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis. 2011;203(4):473–8. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia F, et al. A Dendritic Cell-Based Vaccine Elicits T Cell Responses Associated with Control of HIV-1 Replication. Sci Transl Med. 2013;5(166):166ra2. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- 11.Allard SD, et al. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin Immunol. 2012;142(3):252–68. doi: 10.1016/j.clim.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Van Gulck E, et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS. 2012;26(4):F1–12. doi: 10.1097/QAD.0b013e32834f33e8. [DOI] [PubMed] [Google Scholar]
- 13.Routy JP, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134(2):140–7. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly NC, et al. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clinical and vaccine immunology : CVI. 2008;15(2):284–92. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Routy JP, Nicolette C. Arcelis AGS-004 dendritic cell-based immunotherapy for HIV infection. Immunotherapy. 2010;2(4):467–76. doi: 10.2217/imt.10.28. [DOI] [PubMed] [Google Scholar]
- 16.Lu W, et al. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10(12):1359–65. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 17.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, et al. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9(1):27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- 19.Rossio JL, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72(10):7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derdeyn CA, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74(18):8358–67. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platt EJ, et al. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J Virol. 2009;83(16):8289–92. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt EJ, et al. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Means RE, et al. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J Virol. 2001;75(8):3903–15. doi: 10.1128/JVI.75.8.3903-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Prete GQ, et al. Restricted replication of xenotropic murine leukemia virus-related virus in pigtailed macaques. J Virol. 2012;86(6):3152–66. doi: 10.1128/JVI.06886-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Che KF, et al. HIV-1 impairs in vitro priming of naive T cells and gives rise to contact-dependent suppressor T cells. Eur J Immunol. 2010;40(8):2248–58. doi: 10.1002/eji.201040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubong Sabado R, et al. In vitro priming recapitulates in vivo HIV-1 specific T cell responses, revealing rapid loss of virus reactive CD4 T cells in acute HIV-1 infection. PLoS One. 2009;4(1):e4256. doi: 10.1371/journal.pone.0004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabado RL, et al. Pathways utilized by dendritic cells for binding, uptake, processing and presentation of antigens derived from HIV-1. Eur J Immunol. 2007;37(7):1752–63. doi: 10.1002/eji.200636981. [DOI] [PubMed] [Google Scholar]
- 29.Sabado RL, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116(19):3839–52. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson M, et al. Activation of HIV-1 specific CD4 and CD8 T cells by human dendritic cells: roles for cross-presentation and non-infectious HIV-1 virus. AIDS. 2002;16(10):1319–29. doi: 10.1097/00002030-200207050-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lu W, Andrieu JM. In vitro human immunodeficiency virus eradication by autologous CD8(+) T cells expanded with inactivated-virus-pulsed dendritic cells. Journal of Virology. 2001;75(19):8949–56. doi: 10.1128/JVI.75.19.8949-8956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wille-Reece U, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203(5):1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, et al. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34(6):866–78. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, et al. DHX9 pairs with IPS-1 to sense double-stranded RNA in myeloid dendritic cells. J Immunol. 2011;187(9):4501–8. doi: 10.4049/jimmunol.1101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zobywalski A, et al. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. Journal of translational medicine. 2007;5:18. doi: 10.1186/1479-5876-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogunovic D, et al. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Res. 2011;71(16):5467–76. doi: 10.1158/0008-5472.CAN-10-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagenas P, et al. A tonsillar PolyICLC/AT-2 SIV therapeutic vaccine maintains low viremia following antiretroviral therapy cessation. PLoS One. 2010;5(9):e12891. doi: 10.1371/journal.pone.0012891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.