Abstract
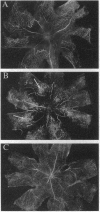
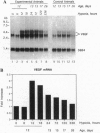
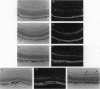
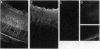
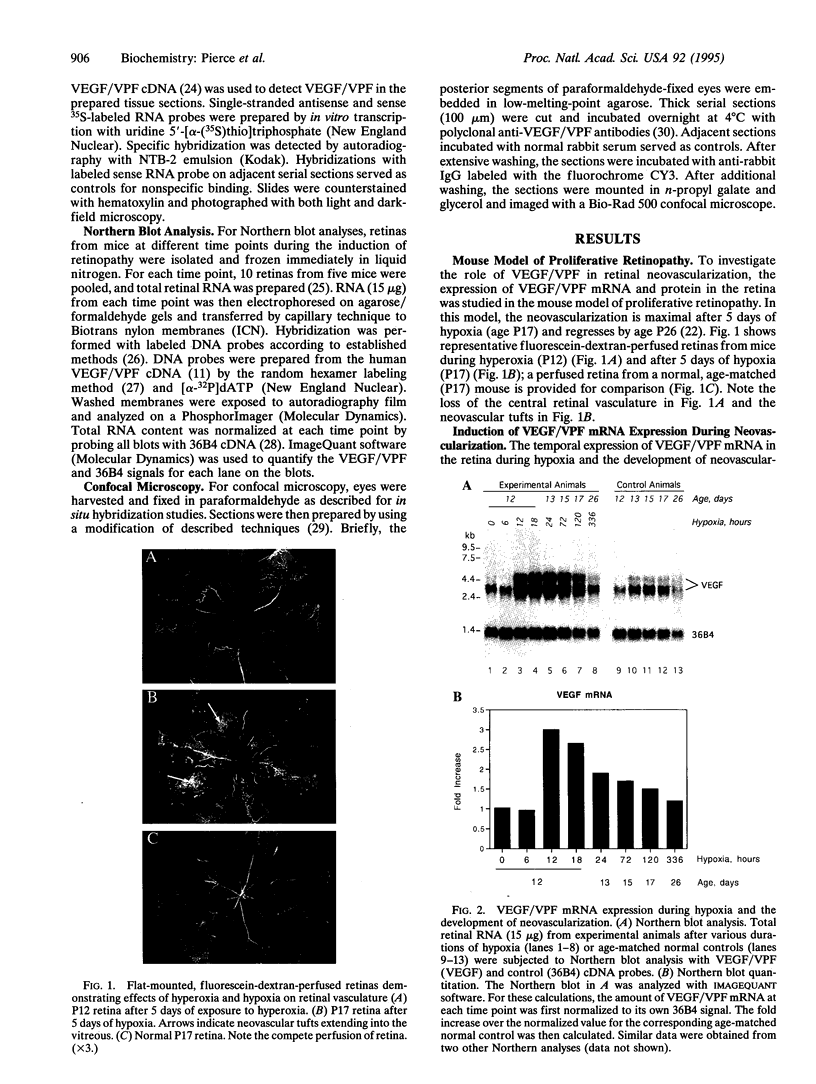
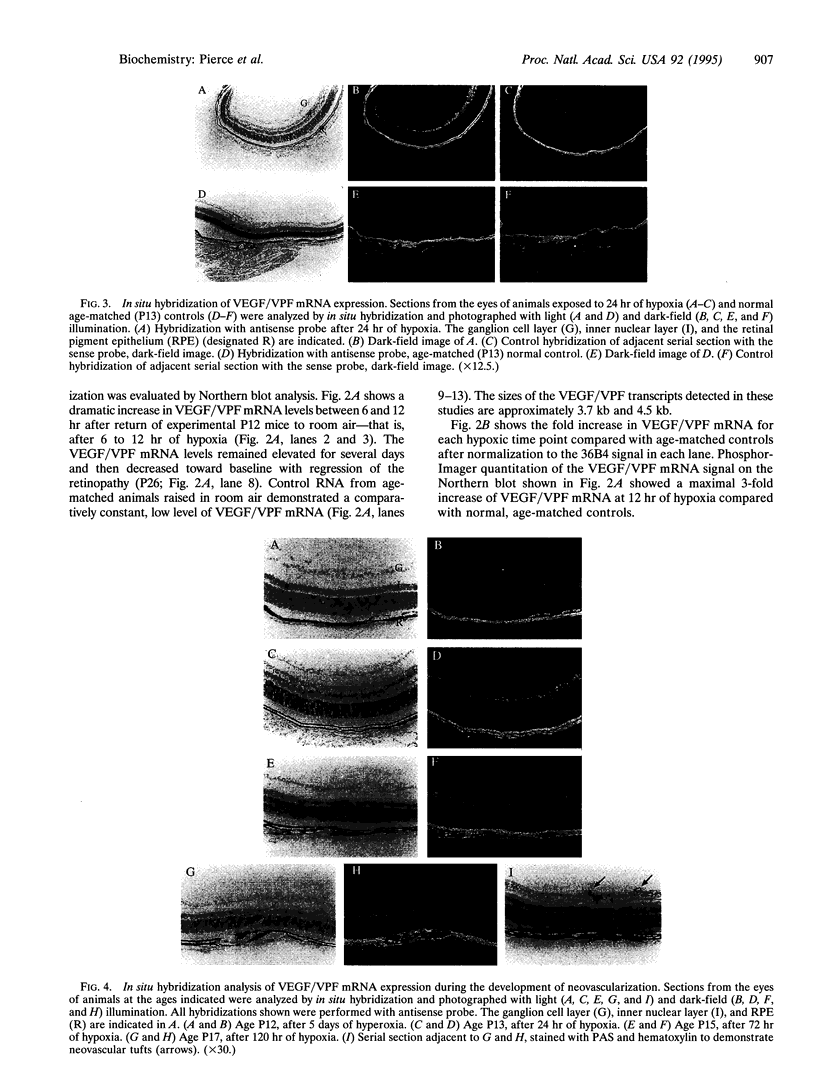
Neovascular diseases of the retina are a major cause of blindness worldwide. Hypoxia is thought to be a common precursor to neovascularization in many retinal diseases, but the factors involved in the hypoxic neovascular response have not been fully identified. To investigate the role of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in retinal neovascularization, the expression of VEGF/VPF mRNA and protein were studied in a mouse model of proliferative retinopathy. RNA (Northern) blot analysis revealed that retinal VEGF/VPF mRNA expression increased 3-fold between 6 and 12 hr of relative retinal hypoxia and remained elevated during the development of neovascularization. In situ hybridization localized VEGF/VPF mRNA to cells bodies in the inner nuclear layer of the retina. Immunohistochemical confocal microscopy demonstrated that VEGF/VPF protein levels increase with a time course similar to that of the mRNA. The cells in the inner nuclear layer of the retina that produce VEGF/VPF were identified morphologically as Müller cells. These data suggest that VEGF/VPF expression in the retina plays a central role in the development of retinal ischemia-induced ocular neovascularization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N., WARD B., SERPELL G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954 Jul;38(7):397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamis A. P., Shima D. T., Yeo K. T., Yeo T. K., Brown L. F., Berse B., D'Amore P. A., Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993 Jun 15;193(2):631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Avery R. L., Arrigg P. G., Keyt B. A., Jampel H. D., Shah S. T., Pasquale L. R., Thieme H., Iwamoto M. A., Park J. E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Breier G., Albrecht U., Sterrer S., Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992 Feb;114(2):521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Yeo K. T., Berse B., Yeo T. K., Senger D. R., Dvorak H. F., van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992 Nov 1;176(5):1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claffey K. P., Wilkison W. O., Spiegelman B. M. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992 Aug 15;267(23):16317–16322. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Henzel W. J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991 Dec;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Leung D. W., Rowland A. M., Winer J., Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992 Dec 25;267(36):26031–26037. [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Scheuenstuhl H., Halliday B. J., Werb Z., Banda M. J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983 Sep 23;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991 Jul 25;19(14):3998–3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Miller J. W., Adamis A. P., Shima D. T., D'Amore P. A., Moulton R. S., O'Reilly M. S., Folkman J., Dvorak H. F., Brown L. F., Berse B. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994 Sep;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- Patz A. Clinical and experimental studies on retinal neovascularization. XXXIX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1982 Dec;94(6):715–743. doi: 10.1016/0002-9394(82)90297-5. [DOI] [PubMed] [Google Scholar]
- Penn J. S., Tolman B. L., Lowery L. A. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993 Mar;34(3):576–585. [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Pournaras C. J., Tsacopoulos M., Strommer K., Gilodi N., Leuenberger P. M. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990 Oct;97(10):1321–1328. doi: 10.1016/s0161-6420(90)32415-6. [DOI] [PubMed] [Google Scholar]
- Pournaras C. J., Tsacopoulos M., Strommer K., Gilodi N., Leuenberger P. M. Scatter photocoagulation restores tissue hypoxia in experimental vasoproliferative microangiopathy in miniature pigs. Ophthalmology. 1990 Oct;97(10):1329–1333. doi: 10.1016/s0161-6420(90)32414-4. [DOI] [PubMed] [Google Scholar]
- Sassoon D., Rosenthal N. Detection of messenger RNA by in situ hybridization. Methods Enzymol. 1993;225:384–404. doi: 10.1016/0076-6879(93)25027-y. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Simorre-Pinatel V., Guerrin M., Chollet P., Penary M., Clamens S., Malecaze F., Plouet J. Vasculotropin-VEGF stimulates retinal capillary endothelial cells through an autocrine pathway. Invest Ophthalmol Vis Sci. 1994 Aug;35(9):3393–3400. [PubMed] [Google Scholar]
- Smith L. E., Wesolowski E., McLellan A., Kostyk S. K., D'Amato R., Sullivan R., D'Amore P. A. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):101–111. [PubMed] [Google Scholar]
- Tischer E., Mitchell R., Hartman T., Silva M., Gospodarowicz D., Fiddes J. C., Abraham J. A. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991 Jun 25;266(18):11947–11954. [PubMed] [Google Scholar]