Abstract
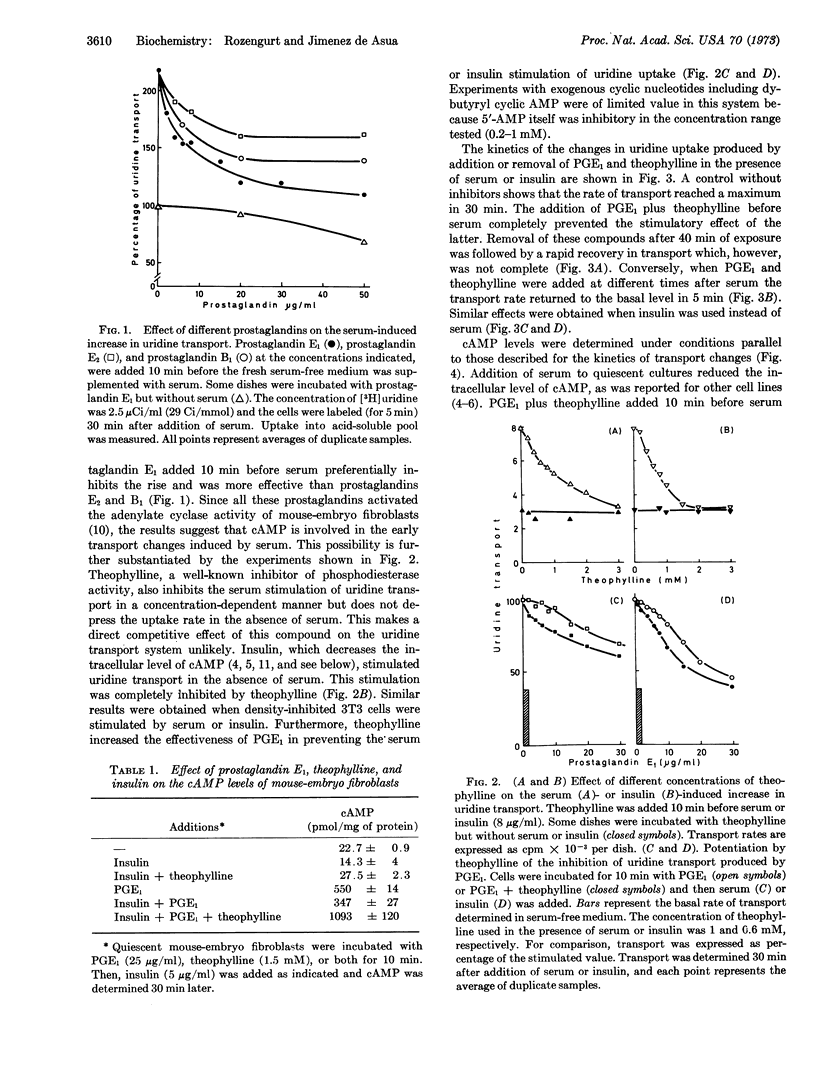
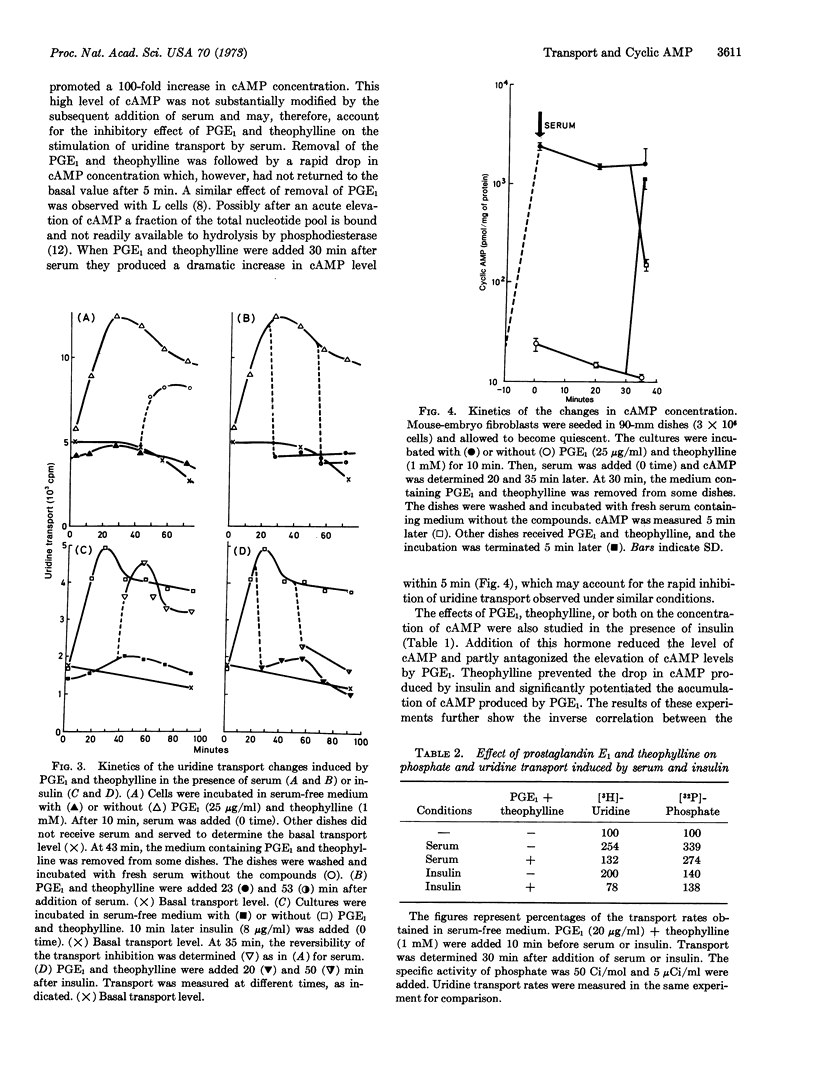
Serum or insulin added to quiescent mouse-embryo fibroblasts produced rapid increases in the rate of transport of uridine and phosphate and a decrease of the intracellular concentration of cyclic AMP. Incubation of the cells with prostaglandin E1, theophylline, or both prevented the increase in uridine transport produced by serum or insulin. Prostaglandin E1 was more effective than prostaglandins E2 and B1. Kinetic experiments showed that the addition of prostaglandin E1 and theophylline causes the uridine transport rate to return to the basal level within 5 min; the rate rose rapidly after their removal. Similar effects were obtained when insulin was used instead of serum. Associated with these transport changes were significant variations in the levels of cyclic AMP. An inverse correlation between the changes in uridine transport and those in cyclic AMP concentration was shown under various experimental conditions. In contrast to the effects observed with uridine transport, phosphate uptake was only slightly affected by changes in the endogenous level of cyclic AMP. We propose that at least two different sorts of membrane changes are rapidly initiated by serum, one under cyclic AMP control and the other not related to this nucleotide.
Keywords: uridine, phosphate, prostaglandins, tissue culture
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Cyclic AMP and growth of fibroblasts: effect of environmental pH. Nat New Biol. 1973 Mar 21;242(116):78–80. doi: 10.1038/newbio242078a0. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Walton K. G., Curran P. F., Greengard P. Regulation of phosphorylation of a specific protein in toad-bladder membrane by antidiuretic hormone and cyclic AMP, and its possible relationship to membrane permeability changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):880–884. doi: 10.1073/pnas.70.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J., Schroeder J. L. Dibutyryl cyclic adenosine 3'5' monophosphate, sugar transport, and regulatory control of cell division in normal and transformed cells. J Cell Biol. 1973 Feb;56(2):487–491. doi: 10.1083/jcb.56.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez de Asúa L., Surian E. S., Flawia M. M., Torres H. N. Effect of insulin on the growth pattern and adenylate cyclase activity of BHK fibroblasts. Proc Natl Acad Sci U S A. 1973 May;70(5):1388–1392. doi: 10.1073/pnas.70.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Mamont P., Tomkins G. M. Pleiotypic control by adenosine 3':5'-cyclic monophosphate: a model for growth control in animal cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1432–1436. doi: 10.1073/pnas.70.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maganiello V., Vaughan M. Prostaglandin E 1 effects on adenosine 3':5'-cyclic monophosphate concentration and phosphodiesterase activity in fibroblasts (mouse L cells-tissue culture-enzyme kinetics-prostaglandin homologues). Proc Natl Acad Sci U S A. 1972 Jan;69(1):269–273. doi: 10.1073/pnas.69.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dea R. F., Haddox M. K., Goldberg N. D. Interaction with phosphodiesterase of free and kinase-complexed cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1971 Oct 25;246(20):6183–6190. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Peery C. V., Johnson G. S., Pastan I. Adenyl cyclase in normal and transformed fibroblasts in tissue culture. Activation by prostaglandins. J Biol Chem. 1971 Sep 25;246(18):5785–5790. [PubMed] [Google Scholar]
- Rozengurt E., Pardee A. B. Opposite effects of dibutyryl adenosine 3':5' cyclic monophosphate and serum on growth of Chinese hamster cells. J Cell Physiol. 1972 Oct;80(2):273–279. doi: 10.1002/jcp.1040800215. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. The role of cyclic AMP in the phosphorylation of proteins in human erythrocyte membranes. Biochem Biophys Res Commun. 1973 Jan 23;50(2):421–429. doi: 10.1016/0006-291x(73)90857-7. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Rubin H. Uridine transport and RNA synthesis in growing and in density-inhibited animal cells. J Cell Physiol. 1971 Apr;77(2):157–168. doi: 10.1002/jcp.1040770205. [DOI] [PubMed] [Google Scholar]



