Abstract
The full potential of vaccines relies on development of effective delivery systems and adjuvants and is critical for development of successful vaccine candidates. We have shown that recombinant vaults engineered to encapsulate microbial epitopes are highly stable structures and are an ideal vaccine vehicle for epitope delivery which does not require the inclusion of an adjuvant. We studied the ability of vaults which were engineered for use as a vaccine containing an immunogenic epitope of C. trachomatis, polymorphic membrane protein G (PmpG), to be internalized into human monocytes and behave as a “natural adjuvant”. We here show that incubation of monocytes with the PmpG-1-vaults activates caspase-1 and stimulates IL-1β secretion through a process requiring the NLRP3 inflammasome and that cathepsin B and Syk are involved in the inflammasome activation. We also observed that the PmpG-1-vaults are internalized through a pathway that is transiently acidic and leads to destabilization of lysosomes. In addition, immunization of mice with PmpG-1-vaults induced PmpG-1 responsive CD4+ cells upon re-stimulation with PmpG peptide in vitro, suggesting that vault vaccines can be engineered for specific adaptive immune responses. We conclude that PmpG-1-vault vaccines can stimulate NLRP3 inflammasomes and induce PmpG-specific T cell responses.
Keywords: Vaults, inflammasomes, Chlamydia, lysosomes
1. INTRODUCTION
Chlamydia trachomatis is the most prevalent bacterial sexually transmitted disease (STD) in the United States. Chlamydial infections in women can cause pelvic inflammatory disease (PID) and result in infertility, ectopic pregnancy, and chronic pelvic pain [1–3]. Most Chlamydia infections are asymptomatic, increasing the risk of transmission of Chlamydia to unsuspecting females and result in PID [4–6]. Identification of protective responses is a key component of vaccine development. Intensive studies have been done in order to dissect immunity towards to resolution of primary chlamydial infection, and immunity to reinfection in mouse genital infection model. CD4+ T cells play major role in resolving primary genital infection [7], particularly IFN-γ secreting CD4+ T cells (Th1 cells) [8], with or without CD8+ T cells or antibody [9, 10]. CD4+ T cells and/or antibody are also essential for resistance to reinfection. However, CD8+ T cells appear to be unnecessary against reinfection [10]. Development of a protective vaccine for prevention of Chlamydia PID is challenging due to difficulties in identifying and delivering relevant T cell antigens and developing a safe adjuvant that does not produce excessive inflammatory responses which can diminish the likelihood of public acceptance [11–13].
The full potential of vaccines relies on development of effective delivery systems and adjuvants and is critical for development of successful vaccine candidates. Vaults are large cytoplasmic ribonucleoprotein (RNP) particles consisting of three proteins and a small untranslated RNA [14, 15]. Their function within cells has not been identified but reports have suggested their involvement with multidrug resistance, cell signaling and innate immunity [16–24]. In vitro expression of MVP in insect cell can form hollow vault-like particles identical to native vaults [25]. An MVP interaction domain (INT) associates non-covalently with MVP binding site and can be used to internally package other proteins of interests. We have shown that vaults can be engineered in vitro as a vaccine which effectively delivers antigen for generation of a protective immune response. However, we and others [26–28] also discovered that recombinant vaults can interact with host immune cells and display self-adjuvanting properties, distinguishing them from other vaccine preparations. Moreover, we reported that vaults engineered to contain a recombinant Chlamydia protein (MOMP-vault vaccine) induced strong protective anti-chlamydial immune responses without eliciting excessive inflammation as measured by TNF-α production [29]. We hypothesized that vaults vaccines act as “smart adjuvants” and can be engineered to produce a tailored immune response against specific antigens by housing proteins in the central cavity of the recombinant vault that is hollow and large enough to accommodate multiple copies of foreign epitopes [26, 29]. Our data further suggested that the vault vaccine induced inflammasomes, an innate immune response that could possibly account for the self-adjuvanting property of vault-vaccines upon phagocytosis.
Inflammasomes serve as the first line of immune defense against inducers of cellular stress [30]. Following detection of stress inducers such as infection, inflammasomes promote maturation and secretion of IL-1β [31]. The inflammasome containing the Nod-like receptor (NLR) family member, NLRP3, is one of the best studied inflammasomes and can be activated by a wide range of stimuli, including membrane-damaging toxins, pathogen associated molecular patterns (PAMPs), and danger associated molecular patterns (DAMPs) [32–35]. The NLRP3 inflammasome can also be stimulated by large particles such as monosodium urate (MSU) crystals, silica, nanoparticles, and the adjuvant, alum, which can lead to lysosomal damage after engulfment by phagocytes and the release of lysosomal proteases such as cathepsin B [36–38]. When these stimuli are detected, NLRP3 interacts with the adaptor, ASC (Apoptosis-associated speck-like protein containing a CARD), which in turn recruits the protease, pro-caspase-1. When pro-caspase-1 is assembled into the inflammasome, it becomes auto-activated and cleaved into a 20 kD fragment and induces caspase-1-dependent maturation and secretion of proinflammatory cytokines such as IL-1β [35, 39–44]. Upon activation of the NLRP3 inflammasome, the mature IL-1β is secreted out of the cell. In many cells such as monocytes and macrophages, the activated 20 kD form of caspase-1 is also secreted.
In this report, we have used a different chlamydial protein, PmpG-1, and convincingly show that PmpG-1-vault vaccines induce NLRP3 inflammasome activation that differs from other particulate induces following phagocytosis in vitro. PmpG-1-vault vaccines also induce a T cell response against a PmpG-1 peptide demonstrating that vault-vaccines can be engineered for a tailored immune response.
2. MATERIALS AND METHODS
2.1 Assembly of PmpG-1-vaults vaults
Recombinant baculoviruses were generated using the Bac-to-Bac protocol (Invitrogen, Carlsbad, CA). The 17 amino acid coding region of PmpG-1 (ASPIYVDPAAAGGQPPA) was fused to the N-terminus of the INT domain derived from VPARP (amino acids 1563–1724) by PCR using the following primers: PmpG-1-INT Forward BamHI-5′GGGATCCATGGCAAGCCCAATTTATGTCGACCCAGCAGCAGCAGGTGGTCAACCACCAGCATGCACACAACACTGGCAGGA-3′ and INT Reverse XhoI-5′-GCTCGAGTTAGCCTTGACTGTAATGGAGGA-3′ using INT in pET28 as the template. The resultant PCR product containing the fused PmpG-1-mINT was purified on a Qiagen column (Qiagen, Germantown, MD), digested with BamHI and XhoI, gel purified, and ligated to pFASTBAC to form PmpG-1-mINT pFASTBAC. Construction of cp-MVP-z in pFASTBAC has been described previously [25]. All primers used in this study were purchased from Invitrogen (Carlsbad, CA). Sf9 cells were infected with cp-MVP-z, and PmpG-1-INT recombinant baculoviruses at a multiplicity of incubation (MOI) of 0.01 for approximately 72 h and then pelleted and stored at −80°C until needed. PmpG-1-INT and cp-MVP-z pellets were lysed on ice in buffer A [50 mM Tris-HCl (pH 7.4), 75 mM NaCl, and 0.5 mM MgCl2] with 1% Triton X-100, 1 mM dithiothreitol, 0.5 mM chymostatin, 5 μM leupeptin, 5 μM pepstatin) (Sigma, St. Louis, MO). Lysates containing PmpG-1-vaults were mixed with lysates containing PmpG-1-INT and incubated on ice for 30 min to allow the INT fusion proteins to package inside of vaults. As a control, another lysate of cp-MVP-z pellets was prepared without PmpG-1-INT. Recombinant vaults were purified as previously described and resuspended in sterile RPMI media [25, 45, 46]. The protein concentration was determined using the BCA assay (Pierce) and sample integrity was analyzed by negative stain electron microscopy and SDS-PAGE with Coomassie staining.
The PmpG-1 was cloned in frame with the INT (interaction domain amino acids 1563–1724 of VPARP ref) protein by PCR ligation, resulting in a ~20 kD fusion protein. Addition of this fusion protein to vaults results in packaging inside the particle [47]. An IgG binding domain (the Z domain) was engineered to the C-terminus of MVP to enhance immunity [29] and a cysteine-rich peptide was added to the N-terminus of MVP to enhance particle stability [47]. These vaults are referred to as cp-MVP-z and following packaging of the PmpG-1-INT fusion protein they are designated cp-MVP-z/ PmpG-1-INT (and abbreviated PmpG-1-vaults).
2.2 Cell culture and inhibitor treatment
THP-1 cells were grown in RPMI 1640 (Sigma-Aldrich) with 10% FBS (Invitrogen) and 10 μg/ml gentamicin. A total of 1×106 cells per well in a 6-well plate were differentiated with 500 nM PMA for 3 hrs. Differentiated THP-1 cells were washed with 1XPBS 3 times and incubated for 24 hrs at 37°C with 5% CO2. Z-WEHD (100 nM) and CA-074 Me (10 μM) were used 1.5 hrs before treatment with vaults. Syk-inhibitor (10 μM) was used 30 minutes prior to addition of vaults. PmpG-1-vaults (250 nM) were incubated with cells, and after 6 hrs post-incubation, we collected the supernatant from the treated cells.
2.3 Gene product depletion by RNA interference
THP-1 stably expressing shRNA against NRLP3, ASC, Syk and caspase-1 were obtained by transducing THP-1 cells with lentiviral particles containing shRNAs. The sequences 5′-CCGGGCGTTAGAAACACTTCAGAACTCGAGTTCTTGAAGTGTTTCTAACGCTTTTTG-3′ for human NLRP3 (Sigma; catalog number NM_004895), 5′CCGGCGGAAGCTCTTCAGTTTCACACTCGAGTGTGAAACTGAAGATTCCGTTTTTG-3′ for human ASC (Sigma; catalog number NM_013248), 5′-CCGGGCAGGCCATCATCAGTCAGAACTCGAGTTCTGACTGATGATGGCCTGCTTTTT-3′ for human spleen tyrosine kinase (Syk) (Sigma; catalog number NM-003177), and five sequences for caspase-1 (Sigma; catalog number NM-001223): 5′CCGGGAAGAGTTTGAGGATGATGCTCTCGAGAGCATCATCCTCAAACTCTTCTTTTT-3′,5′CCGGTGTATGAATGTCTGCTGGGCACTCGAGTGCCCAGCAGACATTCATACATTTTT3′,5′CCGGCACACGTCTTGCTCTCATTATCTCGAGATAATGAGAGCAAGACGTGTGTTTTT3′,5′CCGGCTACAACTCAATGCAATCTTTCTCGAGAAAGATTGCATTGAGTTGTAGTTTTT3′,5′CCGGCCAGATATACTACAACTCAATCTCGAGATTGAGTTGTAGTATATCTGGTTTTT-3′ were used separately to silence gene expression following the manufacturer’s instructions. Nontarget shRNA control cells were also generated using an irrelevant sequence (Sigma; catalog number SHC002 V). Cells (3 × 105) were plated at 35% confluency 24 h prior to transduction and then the corresponding lentiviral transduction particles were added at an moi of 3 overnight. Fresh media were added the next day, and transduced cells were selected by addition of media containing 2 μg/ml puromycin (Sigma). The knockdown (KD) efficiency was tested by qPCR. mRNA was isolated from cells after indicated treatments or incubations using the Qiagen RNeasy Kit (Qiagen, Valencia, VA) following the manufacturer’s instruction.
2.4 IL-1β & TNF-α ELISA assay
Supernatant from vaults-treated cells was collected after 6 hrs post-incubation and stored at −80°C until ready for use in the assay. Measurement of IL-1β was carried out using human IL-1β ELISA kit (eBioscience, San Diego, CA), following manufacturer’s instructions.
2.5 Western blotting
Supernatants from vaults-treated cells were collected and precipitated with TCA. Samples were lysed using 1× RIPA Lysis Buffer (Millipore) with 1× protease inhibitor cocktail (Biovision) and loaded onto a 12% SDS-polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane (Millipore). For detection of the active caspase-1 subunit (p20), the blot was probed with 1 mg/ml rabbit anti-human caspase-1 antibody (Millipore), and then incubated again with conjugated 1:10000 dilution of anti-rabbit IgG horseradish peroxidase (Millipore). To detect mature IL-1β, the blot was probed with IL-1β antibody (Cell Signaling) at a 1:1000 dilution, and then incubated again with 1:10000 dilution of anti-mouse secondary antibody (Santa Cruz Biotechnology). Western blotting detection reagents (Amersham Biosciences) were used following manufacturer’s instructions and chemiluminescence was detected using a gel doc system (Bio-Rad).
2.6 Fluorescence-activated cell sorting (FACS)
THP-1 cells (2 × 106/well) were plated in 6-well plates and primed for 3 hr with 0.5 μM Phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO). Recombinant PmpG-1-vaults were dual-labeled with the fluorescent dyes FITC and TRITC by primary amine reaction following manufacturer’s instructions (Pierce, Thermo Scientific, Rockford, IL). Unconjugated dye was removed by filtration on a PD-10 column (GE Healthcare, Piscataway, NJ). Primed THP-1 cells were incubated in duplicate with FITC-TRITC dual-labeled vaults for 6, 18, 24 or 48 h. Half of the treatments were incubated with bafilomycin (Sigma-Aldrich, St. Louis, MO), an ATPase inhibitor, for 30 min to neutralize all subcellular compartments. Cells were collected by trypsinization, washed and immediately analyzed by flow cytometry using a BD FACSCalibur (BD Biosciences, Franklin Lakes, New Jersey) and data was analyzed using Flowjo software (Tree Star, Inc., Ashland, OR). A total of 105 cells were analyzed.
For FACS analysis of lymphocytes, the spleen was harvested from individual mice, and single cell suspensions were prepared by dissociating the lymphocytes through a 40 μm cell strainer (BD Falcon). Individual cells were washed with 1% PBS followed by red blood cell lysis treatment. Lymphocytes were re-suspended in RPMI 1640 at 4°C until used. For intracellular cytokine staining, lymphocytes isolated from spleen were incubated in RPMI 1640 in the presence of PmpG-1303–311 peptide for 6–8 hrs. Brefeldin A (Sigma) was added 4 hrs before the end of culture. Cells were directly stained with fluorochrome-labeled antibodies against CD3 (clone 145–2C11) or CD4 (clone GK1.5). After washing, the cells were incubated with Cytofix/Cytoperm (BD Biosciences) for 1 h and stained with fluorochrome-conjugated anti-IFN-γ (clone XMG1.2), washed again, re-suspended in Cell Fix solution, and analyzed on a SORP BD LSR II (Beckman Dickinson, Franklin Lakes, NJ). FACS data were analyzed by Flowjo (Tree Star, Oregon).
2.7 Chlamydiae, immunization and challenge of mice
Chlamydia muridarum (MoPn) was grown on confluent McCoy cell monolayers, purified on Renograffin gradients and stored at −80°C in SPG buffer (sucrose-phosphate-glutamine) as previously described [48]. Female C57BL/6 mice, 5–6 weeks old were housed according to American Association of Accreditation of Laboratory Animal Care guidelines [48]. Mice receiving vaults were anesthetized with a mixture of 10% ketamine plus 10% xylazine and immunized i.n. with 100 μg PmpG-1-vaults in 20 μl saline for a total of 3 times every two weeks. Mice were hormonally synchronized by subcutaneous injection with 2.5 mg of medroxyprogesterone acetate (Depo Provera, Upjohn, Kalamazoo, MI) in 100 μl saline 7 days prior to a vaginal challenge with 1.5×105 IFU of C. muridarum and infection was monitored by measuring infection forming units (IFU) from cervical-vaginal swabs (Dacroswab Type 1, Spectrum Labs, Rancho Dominguez, CA) as previously described [48].
2.8 Colocalization studies
The following antibodies were used for immunofluorescence at the indicated dilutions: anti-early endosome antigen 1 (EEA1, G-4; 1:100; Santa Cruz Biotechnology, Dallas, TX), anti-lysosomal-associated membrane protein1 (LAMP1, clone H4A3; 1:100; Biolegend, San Diego, CA), anti-microtubule-associated protein 1 light chain 3 (LC3, clone 166AT1234; 1:100; Abgent, San Diego, CA), and AF488-goat anti-mouse immunoglobulin G (IgG; 1:400; Invitrogen, Carlsbad, CA). For colocalization studies, THP-1 cells (1.5 × 105) were seeded onto 18 mm glass coverslips and incubated at 37 °C (with 5% CO2) for 72 hrs in the presence of 10 ng/ml PMA. Purified PmpG-1-vaults vaults were labeled with DyLight 650 according to the manufacturer’s instructions (Pierce, Thermo Scientific, Rockford, IL). Coverslips containing primed THP-1 cells were incubated with 30 μg of DyLight 650-labeled PmpG-1-vaults vaults for 15 min, 30 min, and 1 h. Cells were fixed in 3.7% paraformaldehyde in 1× PHEM buffer (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, 2 mM MgCl2) for 15 minutes at room temperature. Cells were washed 3 times in 1× PHEM buffer before permeabilization for 10 minutes in 0.25% Triton X-100 in 1× PHEM buffer. Following permeabilization, the cells were washed 3 times in 1× PHEM buffer prior to incubation in blocking solution (10% normal goat serum in 1× PHEM buffer) for 1 h at room temperature. Cells were further incubated with the appropriate primary antibody diluted in blocking solution for 1 h at room temperature, rinsed 3 times in 1× PHEM buffer and further incubated for 1 h in secondary antibody prepared in blocking solution. Following staining with the secondary antibody, the cells were washed 3 times with 1× PHEM buffer and mounted in VectaShield Hard Mount with DAPI (Vector Labs, Inc., Burlingame, CA) and visualized using a Yokagawa CSU-22 spinning disc confocal scanner and a Hamamatsu C9100–13 EMCCD camera mounted on a Zeiss Axiovert 200m stand. The images were captured using Slidebook 5 software (Intelligent Imaging Innovations, Inc., Denver, CO). The optimal conditions including the number of vault particles used for each experiment were determined empirically. Images were acquired with a 100× oil immersion objective and were processed using ImageJ (http://rsb.info.nih.gov/ij/). In addition, 10 images were used to determine colocalization by applying the Pearson’s correlation coefficient located in the JACoP Plugin module.
3. RESULTS
3.1 PmpG-1-vault-vaccines stimulate secretion of IL-1β and activated caspase-1 from monocytes
Treatment of THP-1 monocytes with PMA (phorbol-12-myristate-13-acetate) stimulates their differentiation into macrophages. PMA-primed THP-1 cells also synthesize pro-IL-1β, making them good models for studying inflammasome activation. To evaluate whether PmpG-1-vault-vaccines could affect inflammasome activation, we measured IL-1β secretion from PMA-primed THP-1 cells incubated with recombinant vaults containing the chlamydial epitope, PmpG. A significantly higher level of IL-1β was detected in the supernatants after 6 hrs of incubation with the PmpG-1-vaults “vaults” than from cells without vaults (Figure 1A). The empty vaults (without any epitope) were also tested but do not induce an immune response (data not shown) [29], and therefore were not tested further here. To determine whether the IL-1β secretion is dependent on caspase-1 activation, we incubated the cells with a caspase-1 inhibitor, zWEHDfmk [49]. This inhibitor also blocks caspase-4 and caspase-5, which could potentially modulate inflammasome activity [50]. When cells are pre-treated with the caspase inhibitor before adding the vaults, a dramatic decrease in IL-1β secretion and processing was observed (Figure 1A). ELISA of secreted (activated) caspase-1 and Western blot analysis confirmed that the inhibitor also blocked caspase-1 activation (Figure 1C), as expected.
Figure 1. PmpG-1-vaults activate the NLRP3 inflammasome and induce IL-1β secretion, as measured by an ELISA assay.
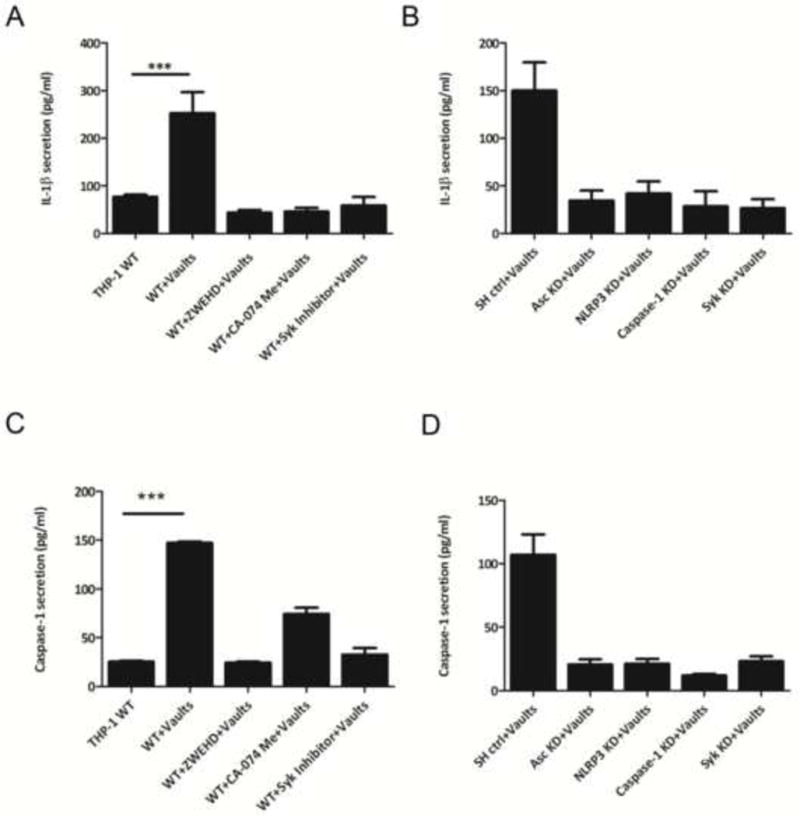
THP-1 (1×106) wild type (WT) cells (A) were incubated in 6-well plates with RPMI 1640 media. Inhibitors of caspase-1 (ZWEHD) or cathepsin B (CA-074) were added individually 1.5 h prior to incubation with PmpG-1-vaults, and the Syk inhibitor was added 0.5 h prior to incubation with PmpG-1-vaults. THP-1 knockdown (KD) cells (B) were incubated with media alone, and 500 μg of PmpG-1-vaults were added to each well, except the WT control. Culture supernatants were collected 6 hrs post-incubation and IL-1β was measured by ELISA. IL-1β levels secreted by cells with inhibitors vs cells without inhibitors and by WT vs KD cells was significantly different (p<0.001). (C) THP-1 (1×106) WT cells were incubated in 6-well plates. ZWEHD or CA-074 was added individually 1.5 h prior to incubation with PmpG-1-vaults, and the Syk inhibitor was added 0.5 h prior to incubation with PmpG-1-vaults. (D) THP-1 knockdown (KD) cells were incubated with media alone, and 500 μg of PmpG-1-vaults were added to each well, except the WT control. Culture supernatants were collected 6 hrs post-incubation and caspase-1 was measured by ELISA kit. The mean ±SD of a representative experiment from six times was analyzed by ANOVA. Caspase-1 levels secreted by cells with inhibitors vs cells without inhibitors and by WT vs KD cells was significantly different (p<0.001). In all cases, cell supernatants were measured in triplicate.
3.2 Incubation of cells with PmpG-1-vaults activates the NLRP3 Inflammasome
The NLRP3 inflammasome can be activated by a broad range of stimuli, including nanoparticles and crystals [51]. We therefore examined whether PmpG-1-vaults may induce IL-1β secretion and caspase-1 activation through the NLRP3 inflammasome. We focused on several representative NLRP3 components such as the adaptor ASC, the NLR family member NLRP3, the protease caspase-1, and the mediators Syk and cathepsin B. To test whether these components may play a role in vault-induced IL-1β secretion, we applied inhibitors of each component and also depleted some components by RNA interference.
When CA-074 Me, an inhibitor of cathepsin B, was incubated with cells 1.5 hrs before incubation with the PmpG-1-vaults, there was a large inhibition of IL-1β secretion (Figure 1A). The inhibitor alone had no effect on IL-1β secretion (data not shown). Similarly, pre-incubation with a Syk inhibitor for 30 minutes significantly decreased PmpG-1-vault-induced IL-1β secretion (Figure 1A). These results suggest that both Syk recruitment and lysosomal destabilization are involved in vault-induced inflammasome activation.
To confirm NLRP3 inflammasome activation by the PmpG-1-vault vaccine, we also depleted ASC and NLRP3 using shRNA method delivered using lentiviral particles. THP-1 cells were treated with a non-target shRNA control, and lentiviral particles to deplete ASC, Syk, caspase-1, and NLRP3 individually. The efficiency of ASC reduction was evaluated by qPCR (Supplementary Figure S1), which also confirmed specificity of the depletion. When cells were incubated with PmpG-1-vaults, IL-1β secretion decreased dramatically in each depleted cell line, compared to the control group (Figure 1B). These results further strengthen the conclusion that PmpG-1-vaults activate the NLRP3 inflammasome.
We next measured caspase-1 activation in the presence of inhibitors against upstream mediators of the NLRP3 inflammasome. The cathepsin B inhibitor, CA-074 Me, dampened PmpG-1-vault activation by approximately half, suggesting that lysosomal disruption may be involved in this process. The Syk inhibitor also strongly decreased caspase-1 activation (Figure 1A).
The effects of the inhibitors were confirmed by depleting the respective target genes by RNA interference (data not shown). Thus, there was significantly less vault-induced caspase-1 activation when THP-1 cells were depleted of ASC, NLRP3 or Syk. As expected, there was also less caspase-1 activation when the cells were depleted of caspase-1.
The results of processed IL-1β and activated caspase-1 secretion obtained by ELISA (Figure 1) were confirmed by measuring mature IL-1β and activated caspase-1 in the supernatant by Western blot (Figure 2). Incubation of THP-1 cells with vaults stimulated secretion of mature IL-1b in the supernatant, which could be inhibited by pre-incubation with the caspase-1 inhibitor zWEHDfmk (Figure 2A and B). Similarly, activated caspase-1 could be observed in the supernatant of vault-stimulated THP1 cells, and secretion of activated caspase-1 could be inhibited by zWEHDfmk (Figure 2C and D).
Figure 2. PmpG-1-vaults activate the NLRP3 inflammasome and caspase-1, as measured by Western blot.
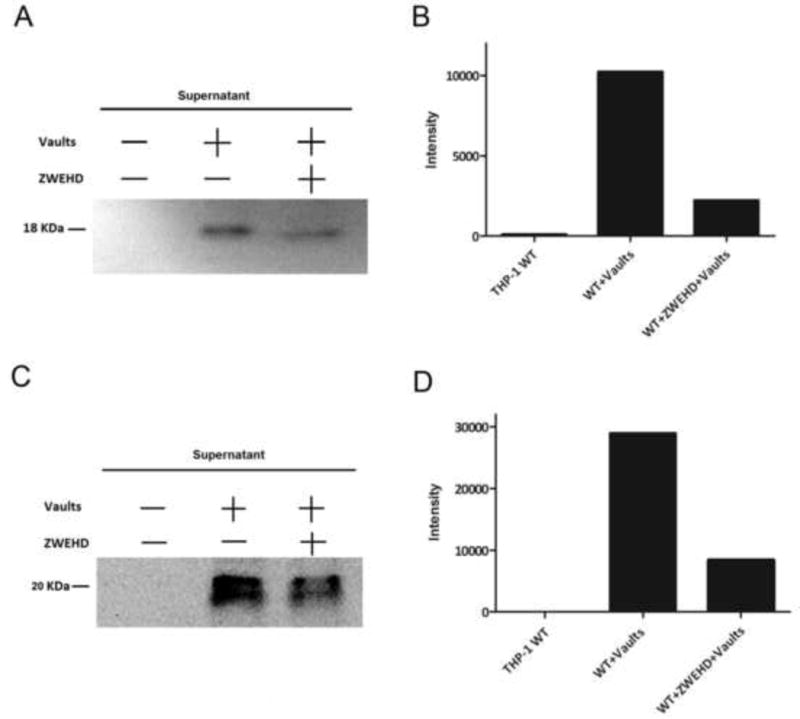
THP-1 (1×106) wild type (WT) cells (A) were incubated in 6-well plates with RPMI 1640 media. ZWEHD was added 1.5 h prior to incubation with PmpG-1-vaults. THP-1 knockdown (KD) cells (B) were incubated with media alone, and 500 μg of PmpG-1-vaults were added to each well, except the WT control. Culture supernatants were collected 6 hrs post-incubation and IL-1β or caspase-1 were detected by Western blot. (A) Western blot of the supernatant probed for IL-1β. (B) Histogram showing the intensity of the bands in the Western blots. (C) Western blot of the supernatant probed caspase-1. (D) Histogram showing the intensity of the bands in the Western blots.
To confirm the functional specificity of the shRNA depletion of caspase-1, ASC, NLRP3 or Syk, the THP-1 cell lines were primed with 10 μg/ml LPS, and TNF-γ secretion was measured. Secretion of this cytokine takes place through an inflammasome-independent pathway, and the results demonstrated that depletion of inflammasome-associated components had no effect (Figure 3). These results showed that depletion of caspase-1, ASC, NLRP3 and Syk by shRNA affected caspase-1 activation and IL-1 secretion, but not cytokine secretion through inflammasome-independent pathways. Taken together, these results imply that the PmpG-1-vault vaccines can activate caspase-1 and stimulate IL-1β secretion through a process involving the NLRP3 inflammasome.
Figure 3. TNF-α levels were not affected by depletion of inflammasome-related genes.
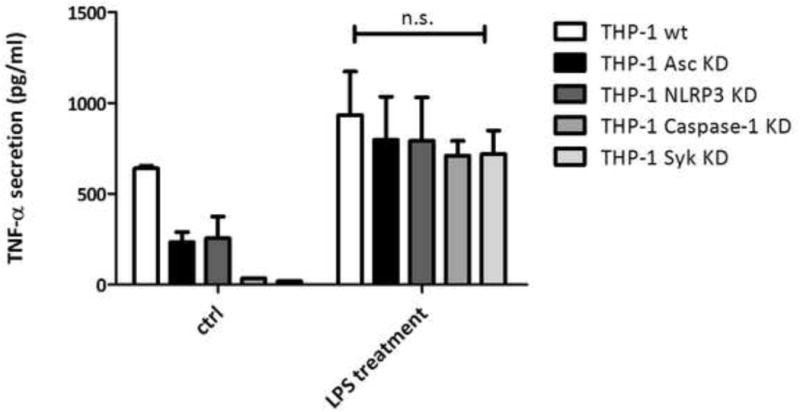
THP-1(1×106) knockdown (KD) cells were incubated in 6-well plates with RPMI 1640 media. LPS (100 ng/ml) was added as stimulator. Culture supernatants were collected 24 hrs post-incubation, and TNF-α was measured by ELISA. TNF-α levels from WT cells were compared to KD cells stimulated by LPS. The values for the WT and KD cells were not statistically significant: p < 0.5 for WT vs caspasd-1 KD, and p < 0.5 for WT vs Syk KD cells.
3.3 Internalized vaults co-localize with lysosomes
PmpG-1-vaults were labeled with FITC (green) and TRITC (red) (Figure 4A) in order to study their intracellular trafficking by flow cytometry (Figure 4B). The fluorescence of FITC is sensitive to pH, which allowed us to determine whether the particles entered acidic compartments after internalization. One group of cells was treated with bafilomycin (to prevent re-acidification of vesicles) before incubation with PmpG-1-vaults. The results indicated that the majority of PmpG-1-vaults were in acidic compartments after 6 hrs of incubation, but most were at neutral pH after 24 hrs (Figure 4C). These experiments suggest that following phagocytosis, the majority of PmpG-1-vaults are internalized initially into acidic compartments (endolysosomes or phagolysosomes), from which they escape into the cytosol.
Figure 4. PmpG-1-vaults are internalized into an acidic compartment.

(A) PmpG-1-vaults dual-labeled with FITC and TRITC fluorophores and incubated with PMA-activated THP-1 cells. (B) Gating scheme (black circle) showing the dot plot of PMA-activated THP-1 cells after PmpG-1-vaults incubation. FITC can only fluorescence when inside acidified chambers and fluorescence can be modified with bafilomycin which prevents re-acidification of vesicles while TRITC constitutively fluoresces. (C) Overlay histogram of FITC-labeled vault fluorescence +/− bafilomycin after 6 hrs and 24 hrs post vault exposure.
This possibility was also addressed by confocal fluorescence microscopy, using DyLight650-labeled PmpG-1-vaults and antibodies against EEA1 (early endosomal marker), Lamp1 (marker of lysosomes), and LC3 (marker of autophagosomes) (Figure 5). The results showed that after 15 min, 30 min and 60 min, approximately 40% of PmpG-1-vaults co-localized extensively with lysosomes (Figure 5), with a significant Pearson’s coefficient. This indicates that the majority of PmpG-1-vaults were internalized into lysosomes, which led to lysosomal disruption. These results are consistent with previous results showing that inhibitors of cathepsin B block NLRP3 inflammasome activation in cells incubated with vaults.
Figure 5. Uptake of PmpG-1-vaults and colocalization within the endocytic pathway.
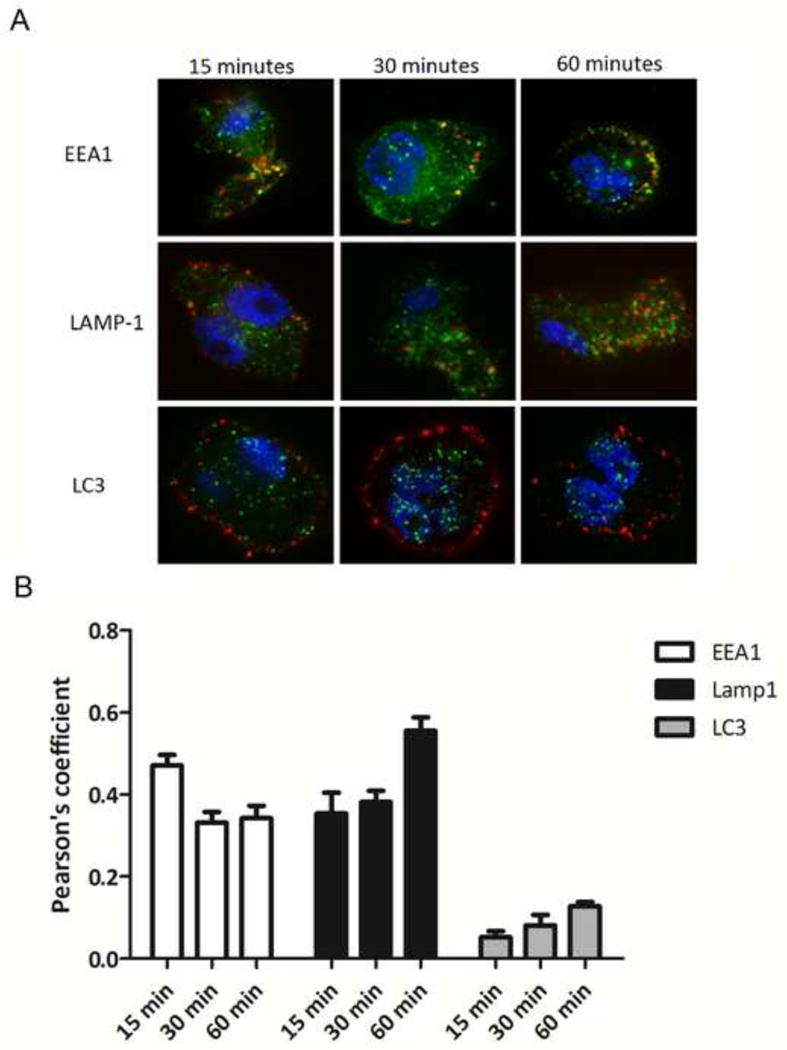
(A) THP-1 cells were grown on 18 mm glass cover slips and treated with 30 ug of DyLight 650 labeled PmpG-1-vaults for 15, 30, and 60 min and imaged by confocal microscopy. For immunofluorescence staining, THP-1 cells were reacted with either anti-EEA1 mouse mAb, anti-Lamp1 mouse mAb, or anti-LC3 mouse mAb followed by Alexa Fluor 488-conjugated goat anti-mouse to identify endocytic compartments. (B) Colocalization of PmpG-1-vaults within each compartment was determined by calculation of the Pearson’s correlation coefficient of the red and green channels using ImageJ.
3.4 Immunization with PmpG-1-vaults induces an immune response in vivo
We examined the immune response of mice vaccinated vaginally with the PmpG-1-vault vaccine. Spleen cells were harvested from naïve mice as well as from mice immunized with PmpG-1-vaults three times. Two weeks after the last immunization, all mice were sacrificed and the lymphocytes were isolated from spleens and stimulated in vitro overnight. Single-cell suspensions were analyzed by flow cytometry for expression of CD3, CD4, and IFN-γ, which are markers for TH1 helper cells, and gated on cells that are specific for MHC-peptide tetramers containing a peptide derived from PmpG-1 (Figure 6). We observed that the cells from immunized mice have a larger percentage of specific TH1 cells within the CD4+ cell compartment, compared to cells from naïve mice. Taken together, these results show that the immune system can recognize the foreign epitope incorporated into the PmpG-1-vault vaccine which could be used in a subsequent immune response to antigen-expressing Chlamydia
Figure 6. PmpG-1-vaults immunization induces a cellular immune response in vivo.
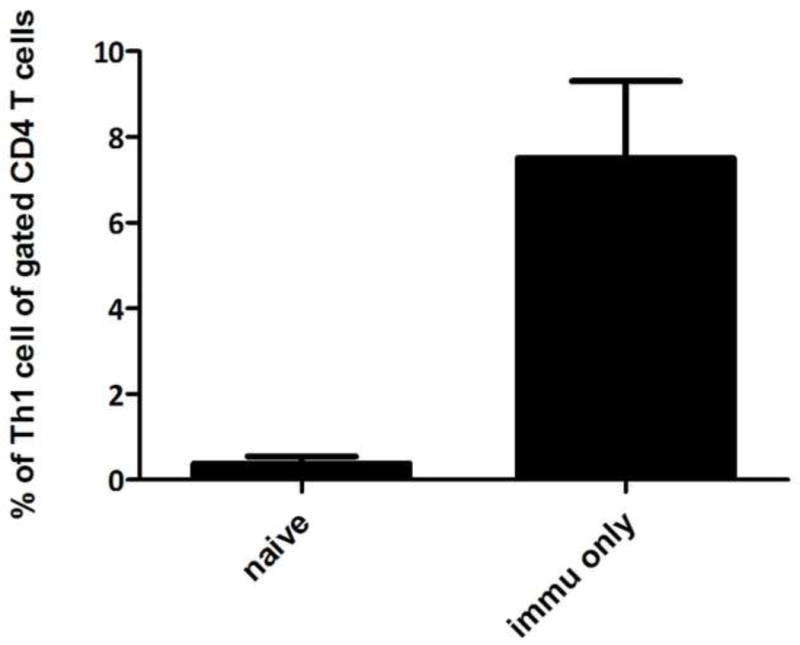
Spleen cells were harvested from naïve mice as well as mice immunized with PmpG-1-vaults containing a total of 15 μg PmpG-1 peptide, 7 days after challenge. Bars indicate percentage of CD3+CD4+IFNγ+ (Th1) cells out of CD4+ cells following in vitro stimulation with PmpG-peptide (mean % ± SEM). n=4, p<0.001 by Student’s t-test.
DISCUSSION
Vaccines that prevent significant infection of the female genital tract are essential to reduce the incidence of PID following C. trachomatis infection. We have shown that vaults containing a chlamydial protein (MOMP) markedly reduces infection early after infection suggesting that the self-adjuvanting vault vaccine is activating innate immunity while not producing excessive inflammation as measured by TNF-α production [29]. In this study, we characterized this innate immunity as involving inflammasome activation. The results demonstrate that incubation of PMA-primed THP-1 cells with PmpG-1-vaults can activate caspase-1 and stimulate IL-1β secretion through a process requiring the NLRP3 inflammasome. We found that the cathepsin B inhibitor CA-074 Me could partially inhibit this process. Interestingly, when internalized PmpG-1-vaults were visualized in cells, we found that the vaults co-localize at early times with lysosomes. The lysosomal permeabilization assay showed that the PmpG-1-vaults are in acidic compartments at early times, but then transfer to an environment with neutral pH. Once lysosomes are ruptured, they release proteases such as cathepsin B, which have been previously shown to activate the NLRP3 inflammasome.
Syk also modulates vault-mediated inflammasome activation. While the mechanism for this dependence is not yet known, the Syk kinase is known to be recruited into lipid rafts when phagosomes form [52]. It had also been proposed that MVP is involved in intracellular transport and concentrated in lipid rafts [53]. Considering that vaults are phagocytosed by cells during incubation, we speculate that PmpG-1-vaults might enter the cells though lipid rafts and then interact with Syk kinase and, simultaneously, lysosomes, in order to activate the NLRP3 inflammasome. Alternatively, the PmpG-1-vaults were engineered with a 33 amino acid-peptide called the “Z” domain. This peptide was derived from a staphylococcal binding domain that can bind the Fc portion of IgG at a site distinct from the binding site for the Fc receptor (FcR). It was also previously shown that vaults with a “Z” domain increase binding of mouse IgG [29]. We expected that these vaults would be internalized through the FcR, which also stimulates the Syk pathway [54]. Further studies should elucidate the mechanisms whereby PmpG-1-vaults can stimulate Syk- and cathepsin B-dependent NLRP3 inflammasome activation.
Taken together, these findings support a model whereby in vivo administered vault-vaccines are phagocytosed by antigen presenting cells as we have shown in vitro using BMDC [47]. Following internalization, we showed in this study, that incubation of monocytes with PmpG-1-vaults can activate caspase-1 and stimulate IL-1β secretion through a process requiring the NLRP3 inflammasome. Inhibitors of the lysosomal protease, cathepsin B, prevented inflammasome activation, implying that lysosomal disruption likely plays a role in caspase-1 activation. This interpretation is consistent with the observation that the PmpG-1-vaults are internalized through a pathway that is transiently acidic and leads to destabilization of lysosomes. PmpG-1-vault interaction within cells are unique from other reported activators of NLRP3 inflammasomes, in that Syk was also shown to be involved in PmpG-1-vault-induced inflammasome activation, which may be due to vault interactions with lipid rafts. Vault vaccines can also be engineered to induce specific adaptive immunity, as we have shown here that immunization of mice with PmpG-1-vaults induces generation of PmpG-1-responsive CD4+ cells immune cells. Vaults can also be engineered to deliver drugs and promote anti-tumor responses [26, 27, 29]. These studies define vault-vaccines as unique among other vaccines that induce NLRP3 inflammasomes, such as alum, as they are also able to induce specific marked T cell responses against antigens incorporated in the vault body.
Supplementary Material
HIGHLIGHTS.
Vault-vaccines activate NLRP3 inflammasomes following phagocytosis
Syk is necessary for NLRP3 inflammasome activation by vault-vaccines
Vault-vaccines are internalized into acidic compartments commonly associated with lysosomes
Vault-vaccines appear in the endocytic pathway following internalization
Vault-vaccines induce specific T cell responses in vivo following administration without an adjuvant
Acknowledgments
We thank Guangchao Liu (Dept of Pathology and Laboratory Medicine, UCLA) for testing the vault-vaccine preparations in vivo and Hedi Roseboro (Dept Biological Chemistry, UCLA) for making the vault-vaccine preparations.
*These studies were funded by NIH grant AI079004, BalanceFrom Products, and a University of California Presidential Chair.
Abbreviations
- C. trachomatis
Chlamydia trachomatis
- PmpG
Polymorphic membrane protein G
- CARD
Caspase recruitment domain
- MOMP
Major outer membrane protein
- PAMPs
Pathogen associated molecular patterns
- DAMPs
Danger associated molecular patterns
- NLR
Nod-like receptor
- MSU
Monosodium urate
- ASC
Apoptosis-associated speck-like protein containing a CARD
- Syk
Spleen tyrosine kinase
- PMA
Phorbol 12-myristate 13-acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflict of interest.
References
- 1.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of Sequelae after Chlamydia trachomatis Genital Infection in Women. Journal of Infectious Diseases. 2010;201:S134–S55. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 2.Westrom L. Effect of Acute Pelvic Inflammatory Disease on Fertility. Obstetrical & Gynecological Survey. 1975;30:552–3. [Google Scholar]
- 3.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic Inflammatory Disease and Fertility: A Cohort Study of 1,844 Women with Laparoscopically Verified Disease and 657 Control Women with Normal Laparoscopic Results. Sexually Transmitted Diseases. 1992;19:185–92. [PubMed] [Google Scholar]
- 4.Heinonen PK, Miettinen A. Laparoscopic study on the microbiology and severity of acute pelvic inflammatory disease. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1994;57:85–9. doi: 10.1016/0028-2243(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 5.Taylor BD, Haggerty CL. Management of Chlamydia trachomatis genital tract infection: screening and treatment challenges. Infect Drug Resist. 2011;4:19–29. doi: 10.2147/IDR.S12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiesenfeld HC, Sweet RL, Ness RB, Krohn MA, Amortegui AJ, Hillier SL. Comparison of Acute and Subclinical Pelvic Inflammatory Disease. Sexually Transmitted Diseases. 2005;32:400–5. doi: 10.1097/01.olq.0000154508.26532.6a. [DOI] [PubMed] [Google Scholar]
- 7.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infection and Immunity. 1995;63:4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito JI, Lyons JM. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infection and Immunity. 1999;67:5518–21. doi: 10.1128/iai.67.10.5518-5521.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infection and Immunity. 2001;69:2643–9. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infection and Immunity. 2000;68:6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karunakaran KP, Yu H, Foster LJ, Brunham RC. Development of a Chlamydia trachomatis T cell Vaccine. Human Vaccines. 2010;6:676–80. doi: 10.4161/hv.6.8.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infection and Immunity. 1997;65:1993–9. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsey KH, Soderberg LS, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infection and Immunity. 1988;56:1320–5. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kickhoefer VA, Poderycki MJ, Chan EKL, Rome LH. The La RNA-binding Protein Interacts with the Vault RNA and Is a Vault-associated Protein. Journal of Biological Chemistry. 2002;277:41282–6. doi: 10.1074/jbc.M206980200. [DOI] [PubMed] [Google Scholar]
- 15.Kedersha NL, Rome LH. Isolation and characterization of a novel ribonucleoprotein particle: large structures contain a single species of small RNA. The Journal of Cell Biology. 1986;103:699–709. doi: 10.1083/jcb.103.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolli S, Zito CI, Mossink MH, Wiemer EA, Bennett AM. The major vault protein is a novel substrate for the tyrosine phosphatase SHP-2 and scaffold protein in epidermal growth factor signaling. The Journal of biological chemistry. 2004;279:29374–85. doi: 10.1074/jbc.M313955200. [DOI] [PubMed] [Google Scholar]
- 17.Yu Z, Fotouhi-Ardakani N, Wu L, Maoui M, Wang S, Banville D, et al. PTEN associates with the vault particles in HeLa cells. The Journal of biological chemistry. 2002;277:40247–52. doi: 10.1074/jbc.M207608200. [DOI] [PubMed] [Google Scholar]
- 18.Yi C, Li S, Chen X, Wiemer EA, Wang J, Wei N, et al. Major vault protein, in concert with constitutively photomorphogenic 1, negatively regulates c-Jun-mediated activator protein 1 transcription in mammalian cells. Cancer research. 2005;65:5835–40. doi: 10.1158/0008-5472.CAN-05-0423. [DOI] [PubMed] [Google Scholar]
- 19.Steiner E, Holzmann K, Pirker C, Elbling L, Micksche M, Sutterluty H, et al. The major vault protein is responsive to and interferes with interferon-gamma-mediated STAT1 signals. Journal of cell science. 2006;119:459–69. doi: 10.1242/jcs.02773. [DOI] [PubMed] [Google Scholar]
- 20.Kim E, Lee S, Mian MF, Yun SU, Song M, Yi KS, et al. Crosstalk between Src and major vault protein in epidermal growth factor-dependent cell signalling. The FEBS journal. 2006;273:793–804. doi: 10.1111/j.1742-4658.2006.05112.x. [DOI] [PubMed] [Google Scholar]
- 21.Scheffer GL, Wijngaard PL, Flens MJ, Izquierdo MA, Slovak ML, Pinedo HM, et al. The drug resistance-related protein LRP is the human major vault protein. Nature medicine. 1995;1:578–82. doi: 10.1038/nm0695-578. [DOI] [PubMed] [Google Scholar]
- 22.Scheffer GL, Schroeijers AB, Izquierdo MA, Wiemer EA, Scheper RJ. Lung resistance-related protein/major vault protein and vaults in multidrug-resistant cancer. Current opinion in oncology. 2000;12:550–6. doi: 10.1097/00001622-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Gopinath SC, Matsugami A, Katahira M, Kumar PK. Human vault-associated non-coding RNAs bind to mitoxantrone, a chemotherapeutic compound. Nucleic acids research. 2005;33:4874–81. doi: 10.1093/nar/gki809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowalski MP, Dubouix-Bourandy A, Bajmoczi M, Golan DE, Zaidi T, Coutinho-Sledge YS, et al. Host resistance to lung infection mediated by major vault protein in epithelial cells. Science. 2007;317:130–2. doi: 10.1126/science.1142311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephen AG. Assembly of Vault-like Particles in Insect Cells Expressing Only the Major Vault Protein. Journal of Biological Chemistry. 2001;276:23217–20. doi: 10.1074/jbc.C100226200. [DOI] [PubMed] [Google Scholar]
- 26.Kar UK, Jiang J, Champion CI, Salehi S, Srivastava M, Sharma S, et al. Vault Nanocapsules as Adjuvants Favor Cell-Mediated over Antibody-Mediated Immune Responses following Immunization of Mice. PLoS ONE. 2012;7:e38553. doi: 10.1371/journal.pone.0038553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buehler DC, Marsden MD, Shen SH, Toso DB, Wu X, Loo JA, et al. Bioengineered vaults: self-assembling protein shell-lipophilic core nanoparticles for drug delivery. ACS Nano. 2014;8:7723–32. doi: 10.1021/nn5002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger W, Steiner E, Grusch M, Elbling L, Micksche M. Vaults and the major vault protein: novel roles in signal pathway regulation and immunity. Cell Mol Life Sci. 2009;66:43–61. doi: 10.1007/s00018-008-8364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Champion CI, Kickhoefer VA, Liu G, Moniz RJ, Freed AS, Bergmann LL, et al. A Vault Nanoparticle Vaccine Induces Protective Mucosal Immunity. PLoS ONE. 2009;4:e5409. doi: 10.1371/journal.pone.0005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogura Y, Sutterwala FS, Flavell RA. The Inflammasome: First Line of the Immune Response to Cell Stress. Cell. 2006;126:659–62. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death and Differentiation. 2006;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 32.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 33.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–65. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A Critical Role for Hemolysins and Bacterial Lipoproteins in Staphylococcus aureus-Induced Activation of the Nlrp3 Inflammasome. The Journal of Immunology. 2009;183:3942–8. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saïd-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomed J. 2012;35:437–49. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nature Immunology. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 38.Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, et al. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–9. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical Role for NALP3/CIAS1/Cryopyrin in Innate and Adaptive Immunity through Its Regulation of Caspase-1. Immunity. 2006;24:317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 Forms an IL-1β-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 41.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 42.Ciraci C, Janczy JR, Sutterwala FS, Cassel SL. Control of innate and adaptive immunity by the inflammasome. Microbes and infection/Institut Pasteur. 2012;14:1263–70. doi: 10.1016/j.micinf.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moayeri M, Sastalla I, Leppla SH. Anthrax and the inflammasome. Microbes and infection/Institut Pasteur. 2012;14:392–400. doi: 10.1016/j.micinf.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimada K, Crother TR, Arditi M. Innate immune responses to Chlamydia pneumoniae infection: role of TLRs, NLRs, and the inflammasome. Microbes and infection/Institut Pasteur. 2012;14:1301–7. doi: 10.1016/j.micinf.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kickhoefer VA, Siva AC, Kedersha NL, Inman EM, Ruland C, Streuli M, et al. The 193-Kd Vault Protein, Vparp, Is a Novel Poly(Adp-Ribose) Polymerase. The Journal of Cell Biology. 1999;146:917–28. doi: 10.1083/jcb.146.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poderycki MJ, Kickhoefer VA, Kaddis CS, Raval-Fernandes S, Johansson E, Zink JI, et al. The Vault Exterior Shell Is a Dynamic Structure that Allows Incorporation of Vault-Associated Proteins into Its Interior†. Biochemistry. 2006;45:12184–93. doi: 10.1021/bi0610552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kickhoefer VA, Han M, Raval-Fernandes S, Poderycki MJ, Moniz RJ, Vaccari D, et al. Targeting Vault Nanoparticles to Specific Cell Surface Receptors. ACS Nano. 2009;3:27–36. doi: 10.1021/nn800638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maxion HK, Liu W, Chang M-H, Kelly KA. The Infecting Dose of Chlamydia muridarum Modulates the Innate Immune Response and Ascending Infection. Infection and Immunity. 2004;72:6330–40. doi: 10.1128/IAI.72.11.6330-6340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, et al. A Combinatorial Approach Defines Specificities of Members of the Caspase Family and Granzyme B: FUNCTIONAL RELATIONSHIPS ESTABLISHED FOR KEY MEDIATORS OF APOPTOSIS. Journal of Biological Chemistry. 1997;272:17907–11. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 50.Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 51.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, et al. Receptor-Independent, Direct Membrane Binding Leads to Cell-Surface Lipid Sorting and Syk Kinase Activation in Dendritic Cells. Immunity. 2008;29:807–18. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu S, Huo J, Gunawan M, Su I-H, Lam K-P. Activated Dectin-1 Localizes to Lipid Raft Microdomains for Signaling and Activation of Phagocytosis and Cytokine Production in Dendritic Cells. Journal of Biological Chemistry. 2009;284:22005–11. doi: 10.1074/jbc.M109.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kowalski MP, Dubouix-Bourandy A, Bajmoczi M, Golan DE, Zaidi T, Coutinho-Sledge YS, et al. Host Resistance to Lung Infection Mediated by Major Vault Protein in Epithelial Cells. Science. 2007;317:130–2. doi: 10.1126/science.1142311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mócsai A, Ruland J, Tybulewicz VLJ. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nature Reviews Immunology. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


