Abstract
Cancer immunotherapy has shown great promise as a new standard cancer therapeutic modality. However, the response rates are limited for current approach that depends on enhancing spontaneous antitumor immune responses. Therefore, increasing tumor immunogenicity by expressing appropriate cytokines should further improve the current immunotherapy. Interleukin-33 is a member of the IL-1 family of cytokines and is released by necrotic epithelial cells or activated innate immune cells and is thus considered a “danger” signal. The role of IL-33 in promoting type 2 immune responses and tissue inflammation has been well established. However, whether IL-33 drives antitumor immune responses is controversial. Our previous work established that IL-33 promoted the function of CD8+ T cells. Here, we showed that the expression of IL-33 in two types of cancer cells potently inhibited tumor growth and metastasis. Mechanistically, IL-33 increased numbers and IFNγ production by CD8+ T and NK cells in tumor tissues, thereby inducing a tumor microenvironment favoring tumor eradication. Importantly, IL-33 greatly increased tumor-antigen-specific CD8+ T cells. Furthermore, both NK and CD8+ T cells were required for the antitumor effect of IL-33. Moreover, depletion of regulatory T cells (Treg) worked synergistically with IL-33 expression for tumor elimination. Our studies established “alarmin” IL-33 as a promising new cytokine for tumor immunotherapy through promoting cancer-eradicating type 1 immune responses.
Introduction
Tumor-antigen-specific immune responses are either present spontaneously in human cancer patients as a critical component of tumor immune surveillance or can be elicited by cancer vaccination or adoptive T-cell transfer (1–3). Type 1 immune responses, mediated by Th1, CD8+ T, NK, NKT, and γδ T cells, are thought to be a critical component of cell-mediated immunity against cancer (4). In humans, the presence of Th1 and CD8+ T within the tumor can be a favorable prognostic indicator (4). Blockade of immune checkpoint molecules as well as TIL-based immunotherapy have achieved great success with melanoma (5–7). It is well known, however, that many tumor infiltrating Th1 and CD8+ T cells are in a state of non-responsiveness due to local mechanisms of immune suppression in the tumor microenvironment (8, 9). Many mechanisms are responsible for the apparent failure of antitumor immunity including the active immunosuppression by the tumor microenvironment and the lack of sufficient immune stimulatory signals. Therefore, reversing immune suppression in the tumor microenvironment is a key step for a successful immunotherapy of cancer.
IL-33 is a member of the IL-1 family of cytokines (10). Its receptor complex consists of ST2 (also known as IL1RL1) and IL-1RAcP (11, 12). IL-33 is constitutively produced by structural and lining cells, such as epithelial cells and endothelial cells, where the first line of host defense against pathogens normally arises (13). Besides in epithelial cells, IL-33 can also be induced in myeloid cells and tissue stromal cells during infection. These properties of IL-33 make it a likely candidate “alarmin” for tissue damage and infection (14). IL-33 has been well established as a potent cytokine that promotes Th2-mediated immune responses(10). Recent evidence also supports its role in type 1 immune responses defined by the predominant production of IFNγ. We have shown that IL-33 synergized with both TCR and IL-12 to enhance IFNγ production by CD8+ T and Th1 cells (15). In addition, IL-33 promotes IFNγ production by NK cells and NKT cells (16–18). IL-33 signaling has also been shown to be required for eradication of viral infection through CD8+ T cells (19). Therefore, IL-33 is a candidate cytokine for reversing the immunosuppressive tumor microenvironment.
Since IL-33 is a danger signal released at the damaged tissue, we set out to determine whether tumoral expression of active IL-33 can render effective antitumor immune responses. In this study, we expressed IL-33 in two types of tumor cell lines and compared the growth upon transplantation to syngeneic mice. We found that overexpression of IL-33 in these tumor cells strongly inhibited tumor growth. IL-33 greatly increased numbers of tumor infiltrating NK cells and CD8+ T cells as well as their IFNγ production. In addition, we showed that the inhibition of tumor growth by IL-33 was dependent on CD8+ T cells and NK cells as well as IFNγ and perforin. Moreover, depletion of Treg further improved the antitumor effect of IL-33. Taken together, our study establishes IL-33 as a promising cytokine for improving tumor immunotherapy.
Materials and methods
Animals and tumor model
C57BL/6 (B6; H2Kb), BALB/c (H2Kd), and Rag2−/− IL2rg−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). BALB/c St2−/−mice (20) and B6.CD4-cre T-bet−/− Eomes−/− (DKO) mice were bred for experimental use at the University of Pittsburgh. All mice were housed in the specific pathogen-free facility of the University of Pittsburgh School of Medicine. Experiments were conducted under an institutional animal care and use committee-approved protocol and in accordance with National Institutes of Health guidelines.
B16 cells were injected intradermally to B6 mice and size of the tumor was monitored every two days. 4T1 cells were injected into the mammary fat pad of BALB/c mice and size of the tumor was monitored every two days. Five mice in each group were injected with 200 μg anti-CD8 (Clone: 53-6.72) (BioXcell, Lebanon, NH), or 15 μl anti-asialo GM1 (Wako chemicals) antibodies or control normal Rat IgG (Bioxcell) four times before and after tumor inoculation (day -2, 1, 7, 14). Metastatic 4T1 tumor nodules were enumerated after the India ink staining procedure, as previously reported (21). India ink solution was injected through the trachea to inflate the lung, and the lung was stained for 5 min. The lungs were then removed and placed in Fekete’s solution (70% alcohol, 10% formalin, and 5% acetic acid) for destaining. Tumor nodules did not absorb India ink, which resulted in the normal lung tissue staining black and the tumor nodules remaining white. Tumor nodules were counted blindly by two independent investigators.
Plasmid Construction
pcDEF3-Dap10 vector was obtained from Dr. Lawrence Kane (University of Pittsburgh). The full length IL-33 protein is a nuclear protein and only released by stressed cells. The mature peptide (S109 to I266) sequence of murine IL-33 was then amplified from cDNA generated from mouse lung RNA. The IL-33 expression construct was generated by fusing the nucleotide sequence encoding human CD8α signal sequence to the 5′ end of IL-33 (S109 to I266) sequence, then ligated into pcDEF3-Dap10 vector via BamH1 and EcoR1. IL-12 was generated by fusing the (G4S)3 linker sequence to the 3′ end of murine IL-12p40 sequence and 5′ end of murine IL-12p35 sequence. And then this was ligated into pcDNA3.1+ vector or pcDEF3 via Kpn1 and Xba1 or BamH1 and Not I respectively. All of the constructs were confirmed to be correct by DNA sequencing analysis (available upon request).
Cell culture
B16 cells and 4T1 cells were cultured in DMEM plus 10% FCS. IL-33 and IL-12 expression vectors were transfected into B16 cells and 4T1 cells, respectively, using Lipofectamine 2000 (Invitrogen Life Technologies) per the manufacturer’s instructions. Empty vector (pcDNA3.1+ or pcDEF3) was transfected into B16 cells and 4T1 cells as control. 24 hours post-transfection, cells were diluted into culture plates and selected with G418 (Sigma) at a concentration of 600 mg/L. The stable cell lines were selected by further subcloning. Expression levels of IL-33 and IL-12 in the subclones were determined by ELISA. Three independently generated B16-IL-33 and three independently generated B16-IL-12 clones, which produced around 5 to 50 ng/ml/24h cytokines, were used in our experiments.
ELISA measurement of mouse IL-33
Purified anti-mouse IL-33 antibody (Goat Polyclonal IgG, Poly5165, Biolegend) was used as the capturing antibody. Biotin anti-mouse IL-33 antibody (Poly5165, Biolegend) was used as the detection antibody. Recombinant mouse IL-33 (ELISA Std., Biolegend) was used as standard.
Analysis of Tumor-infiltrating Lymphocytes
Tumor masses were removed, minced, and digested with collagenase and hyaluronidase digestion solution (2.5 mg/ml collagenase I, 1 mg/ml collagenase IV, 0.25 mg/ml hyaluronidase IV-S, 300 μg/ml DNase I, and 0.01% HEPES in RPMI 1640 medium) at 37°C for two hours. The pieces were then gently pressed between the frosted edges of two sterile glass slides, and the cell suspension was filtered through a 40-μm cell strainer (BD Biosciences). Tumor-infiltrating lymphocytes (TILs) were further purified by using a gradient as per manufacturer protocol, washed, and re-suspended in HBSS for analysis. The various cell populations were analyzed by flow cytometry. Flow cytometric analysis was performed using a FACS flow cytometer (BD Biosciences, San Jose, CA). For intracellular cytokine staining, harvested cells were stimulated with PMA (10 ng/ml) and ionomycin (1 μg/ml) for 4 h and incubated for the last one hour with brefeldin A (10 μg/ml). Cells were subjected to intracellular cytokine analysis with anti–IFNγ antibody (clone XMG1.2; eBioscience).
Evaluation of specific CD8+ T cell responses ex vivo
CD8+ T cells were isolated from spleen of tumor bearing Balb/c mice by positive selection using immunomagnetic beads according to the manufacturer’s protocol (CD8α (Ly-2) MicroBeads, Miltenyi Biotec, Auburn, CA) and passed through a magnetic cell sorting column (Miltenyi Biotec, Auburn, CA). Antigen presenting cells (APC) were prepared from normal splenocytes by depleting CD4+ and CD8+ T cells and then irradiated at 3000 Gy. Purified CD8+ T cells (2 × 106/well) were co-cultured with 6000 Gy-irradiated 4T1 tumor cells (5 × 105/well) and irradiated APC (5 × 104/well) in presence of 20 IU/ml recombinant huIL-2 (obtained from the BRB Preclinical Repository) in 96-well round-bottom plates in a humidified incubator at 37°C and 5% CO2. After 96 hours, cell-free supernatants were harvested and assayed for IFNγ by a murine IFNγ ELISA kit (BD Biosciences Pharmingen, San Diego, CA).
Results
IL-33 exerts a strong antitumor effect when expressed in the tumor cells
Our results, as well as others’, have shown that IL-33 enhanced the function of effector CD8+ T cells and NK cells (15). We then postulated that IL-33 might have potent antitumor effects in vivo through CD8+ T cells and NK cells. In order to test this hypothesis, we sought to determine whether expression of IL-33 in tumor cells could inhibit tumor growth in vivo. We used the B16 melanoma cells because they represent an aggressive murine tumor model and are highly resistant to various immunotherapies. Thus, cytokines that have a significant effect on B16 tumor growth should have greater efficacy on other more immunogenic tumor cell lines. We generated B16 cell clones that express mouse IL-33. As control for the therapeutic efficacy, we also generated B16 clones that express mouse IL-12. Because full-length IL-33 is normally expressed in the nucleus, with the exception of neutrophils and macrophage that secret IL-33 by unknown mechanisms (22), we designed a secretable IL-33 by adding a leader sequence to IL-33. This allowed for IL-33 secretion from tumor cells (data not shown). The expression plasmid was transfected into B16F0 cells to generate stable cell lines, which were subjected to the limiting dilution culture to obtain B16-IL-33 clones. IL-33 was not detected in B16 cells, but 2 to 50 ng/ml IL-33 was made by B16-IL-33 clones (supplementary figure 1). IL-33 did not inhibit B16 proliferation and survival in vitro during culture (supplementary figure 2). Expression of IL-33 did not increase levels of MHC class I on B16 cells either. We injected control B16, B16-IL12 or B16-IL-33 into C57/BL6 mice intradermally (i.d.) and monitored tumor growth every two days. Consistent with the established Th1-promoting function of IL-12, B16-IL-12 tumor grew much slower than the control B16 tumor (Figure 1A). Importantly, tumor growth was greatly inhibited upon IL-33 expression (Figure 1A). Thus, tumoral expression of IL-33 showed a potent anti-tumor effect similar to that of IL-12.
Figure 1. Expression of IL-33 in tumor cell lines inhibited tumor growth and metastasis in vivo.
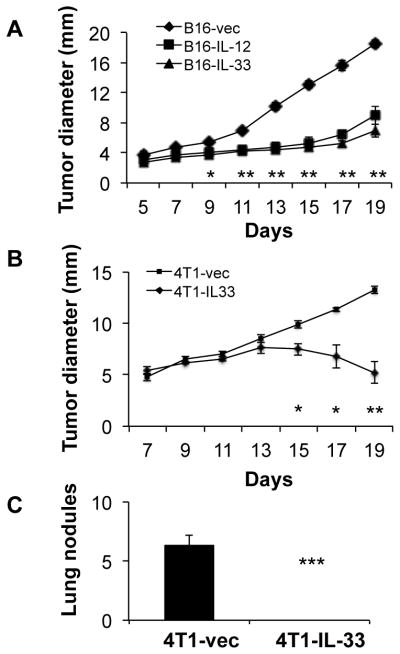
(A) 2×105 B16-vector (B16), B16-IL-12, or B16-IL-33 cells were injected intradermally into B6 mice and the size of the tumor was monitored every two days. Data (mean ±SEM) are representative of three independent experiments. Five mice were in each group. ** P<0.01,*** P<0.001, determined by Mann-Whitney Test. Comparison was performed between the B16-vec and B16-IL-33 groups. (B) 1×105 4T1-vector or 4T1-IL-33 cells were injected into the mammary fat pad of BALB/c mice and the size of the tumor was monitored every two days. Data (mean ±SEM) are representative of three independent experiments. Five mice were in each group. ** P<0.01,*** P<0.001, determined by Mann-Whitney Test. (C) Metastatic tumor nodules in the lung were quantified 30 days post 4T1 and 4T1-IL-33 tumor inoculation. Data (mean ±SEM) are representative of three independent experiments. Five mice were in each group. ** P<0.01,*** P<0.001, determined by Mann-Whitney Test.
In addition to B16, we also tested IL-33 on the growth of transplanted 4T1 breast cancer cell line in BALB/c mice. IL-33 did not inhibit 4T1 proliferation and survival in vitro during culture (supplementary figure 2). Nevertheless, similar to what we observed in the B16 model, 4T1 cells overexpressing IL-33 grew at a much slower rate than 4T1-vector controls (Figure 1B). Thus, our data indicated that IL-33 exerted potent antitumor effects when expressed in tumor cells in BALB/c mice. 4T1 breast cancer cells metastasize mainly to the lung. We then examined lung metastasis after we sacrificed the mice around 31 days after tumor cell inoculation. Many tumor nodules were observed in lungs from 4T1-bearing mice. In sharp contrast, no tumor nodule was found in the lungs of 4T1-IL-33-bearing mice (Figure 1 C). These data support the idea that IL-33 has pronounced antitumor function when expressed and secreted from tumor cells.
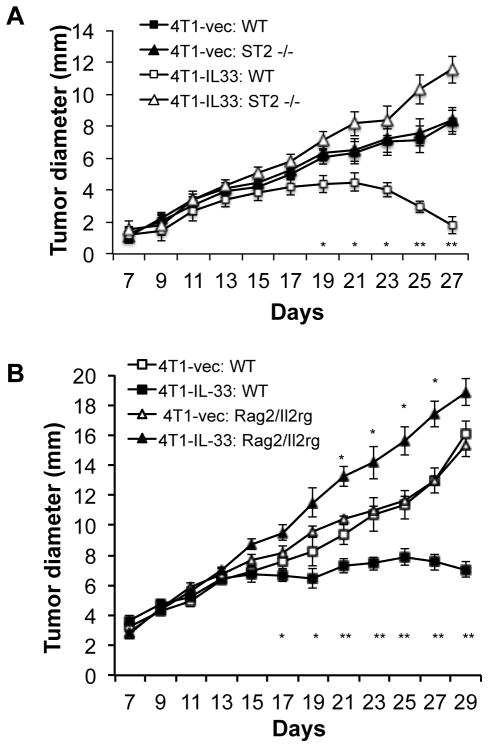
The antitumor effect of IL-33 is dependent on ST2 expression on host cells and the presence of lymphocytes
Our RT-PCR analysis showed no ST2 expression on B16 cells or 4T1 cells (data not shown), thus supporting the hypothesis that IL-33 exerts its antitumor effect through direct action on host cells. To further confirm this, we inoculate WT and ST2−/− mice with 4T1 and 4T1-IL-33 cells and examined tumor growth. In wild type mice, the growth of 4T1-IL-33 tumors was inhibited compared to 4T1 tumors. In contrast, 4T1 and 4T1-IL-33 grew at similar rates in ST2−/− mice. Additionally, there was no difference in the growth rate between 4T1 cells inoculated in WT and ST2−/− mice (Figure 2A) Thus, host cell ST2 signaling was crucial for the antitumor effect of IL-33. In order to further determine the cell types that are important for the tumor-inhibitory function of IL-33, we inoculated WT and Rag2−/−IL2rg−/− mice, which lack T, B and NK cells, with 4T1 and 4T1-IL-33 cells. 4T1-IL-33 tumors grew at a faster rate than that of 4T1 tumors in Rag2−/−IL2rg−/− mice (Figure 2B). These data suggest that lymphoid cells were elicited by IL-33 to inhibit tumor growth. IL-33 accelerated tumor growth in the absence of lymphocytes.
Figure 2. The antitumor effect of IL-33 is dependent on ST2 signaling and lymphocytes.
(A) 1×105 4T1-vector or 4T1-IL-33 cells were injected into the mammary fat pad of BALB/c and ST2−/− mice and the size of the tumor was monitored every two days. Data (mean ±SEM) are representative of three independent experiments. Five mice were in each group. * P<0.05, **P<0.01, determined by Mann-Whitney Test. Significance difference was found between 4T1-IL-33: WT and 4T1: WT groups. (B) 1×105 4T1-vector or 4T1-IL-33 cells were injected into the mammary fat pad of BALB/c and Rag2−/−IL2rg−/− mice and the size of the tumor was monitored every two days. Data (mean ±SEM) are representative of three independent experiments. Five mice were in each group. * P<0.05, **P<0.01, determined by Mann-Whitney Test. Significant difference was found between 4T1-IL-33: WT and 4T1: WT groups as well as between 4T1-IL-33: Rag2 −/− IL2rg −/− and 4T1: Rag2 −/− IL2rg −/− groups.
IL-33 promotes type 1 immune responses in the tumor site
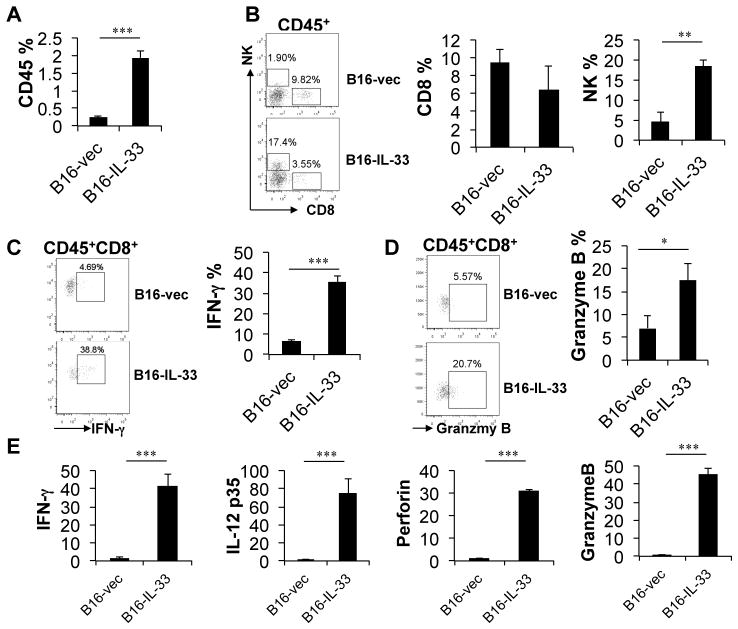
Type 1 lymphocytes, particularly CD8+ T cells and NK cells, are involved in tumor immunosurveillance. In order to further understand the underlying mechanisms of the tumor inhibitory effect of IL-33, we characterized tumor-infiltrating CD8+ and NK cells in B16-vec and B16-IL-33 tumors by flow cytometry. We first examined the CD8+ and NK TILs from tumors around 10 days after inoculation. At this time point, the sizes of B16-IL-33 tumors and control B16 tumors were similar. Strikingly, we found that CD45+ TIL were present at much greater number in B16-IL-33 tumors when compared to B16 tumors (Figure 3A). This is consistent with a pro-inflammatory role of IL-33. In addition, within the CD45+ TIL, the percentage of NK cells was much greater in B16-IL-33 tumors when compared to control B16 tumors (Figure 3B). Although the percentage of CD8+ T cells was not significantly different between these tumors (Figure 3B), much higher percentages of CD8+ TIL in B16-IL-33 tumors produced IFNγ and granzyme B when compared to those in B16 tumors (Figure 3C and D). This effect was local, as no systematic changes of CD8+ T or NK cells were found in the spleen, lymph nodes or tumor draining lymph nodes. Quantitative RNA analysis further revealed that the mRNA levels of type 1 effector genes such as IFNγ, IL-12, perforin, and granzyme B were highly up-regulated in B16-IL-33 tumors when compared to B16 tumors (Figure 3E). In addition, we found similar increases in CD8 and NK cells as well as IFNγ in B16-IL-33 tumors around 20 days after tumor cell inoculation (supplementary Fig 3). These data suggest that IL-33, when overexpressed in tumor cells, promotes type 1 immune responses in tumor tissues.
Figure 3. Expression of IL-33 in B16 cells enhanced type 1 immune responses in the tumor microenvironment.
2×105 B16-vector or B16-IL-33 cells were injected i.d. into B6 mice. On day 10, tumors were resected and processed to generate single cell suspension. (A) Percentages of CD45+ cells in tumor cell suspension. (B) Flow cytometric plots showing NK and CD8+ T cells in tumor and percentage of CD8+ or NK1.1+ cells within the CD45+ population. (C) Representative flow cytometric plots showing IFNγ+CD8+ T cells in tumor and percentage of IFNγ+ cells in CD8+ TILs. (D) Representative flow cytometric plots showing Granzyme B+CD8+ T cells in tumor and percentage of Granzyme B+ cells in CD8+ TILs. (E) Quantitative RNA analysis of IFNγ, IL-12, perforin and granzyme B in B16-IL-33 tumors compared to B16 control tumors. Results are mean ± SEM of three independent experiments. ** P<0.01 and *** P<0.001, two-tailed unpaired Student’s t-test.
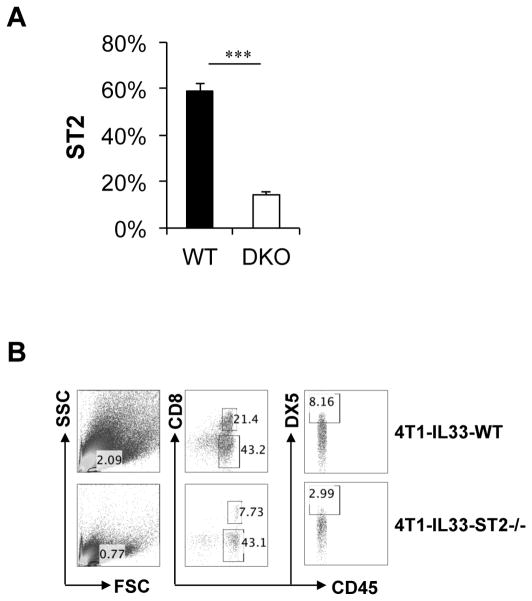
T-bet and Eomes are master transcriptional factors essential for the antitumor activities of CD8+ T cells (23–25). We have shown that T-bet and Eomes were critical for ST2 expression in activated CD8+ T cells in vitro, suggesting ST2 expression is tightly linked to effector CD8+ T cell differentiation. In order to determine whether T-bet and Eomes regulate ST2 expression in vivo, particularly during antitumor immune responses, we inoculated WT and T-bet−/− Eomes−/− mice with 4T1-IL-33 cells and analyzed the expression of ST2 on tumor infiltrating CD8+ T cells. ST2 was highly upregulated in CD8+ TIL isolated from WT mice. In contrast, ST2 levels were greatly reduced in TIL from T-bet−/− Eomes−/− (DKO) mice (Figure 4A). Thus, ST2 expression on CD8+ TIL required T-bet and Eomes.
Figure 4. ST2 expression was regulated by T-bet and Eomes in vivo and required for CD8+ T cells and NK cell infiltration of IL-33-expressing tumors.
(A) 2×105 B16-IL-33 cells were injected i.d. into B6 and T-bet−/− Eomes−/− (DKO) mice. 20 days after inoculation, tumors were harvested and processed to generate a single cell suspension. The surface expression of ST2 was analyzed by flow cytometry. Results are mean ±SEM of three independent experiments. ***P<0.001, two-tailed unpaired Student’s t-test. (B) 1×105 4T1-vector or 4T1-IL-33 cells were injected into the mammary fat pad of BALB/c and ST2−/− mice. 20 days after inoculation, tumors were harvested and processed to generate a single cell suspension. Representative flow cytometric plots showing CD8+ T and NK cells in tumor (TIL).
We then further investigated whether ST2 expression was required for tumor infiltration by CD8+ T cells. We inoculated WT and ST2−/− mice with 4T1-IL-33 cells and analyzed the tumor infiltrating CD8+ T cells by flow cytometry. We found both the percentage of lymphocytes as well as percentage of CD8+ T cells was greater in WT mice than that of ST2−/− mice (Figure 4B). In addition, the percentage of NK cells was also increased in tumors from WT mice when compared to those from ST2−/− mice (Figure 4B). These data are consistent with the idea that IL-33 signaling is required for the accumulation of CD8+ T and NK cells in tumor.
IL-33 elicits tumor antigen-specific adaptive immune responses
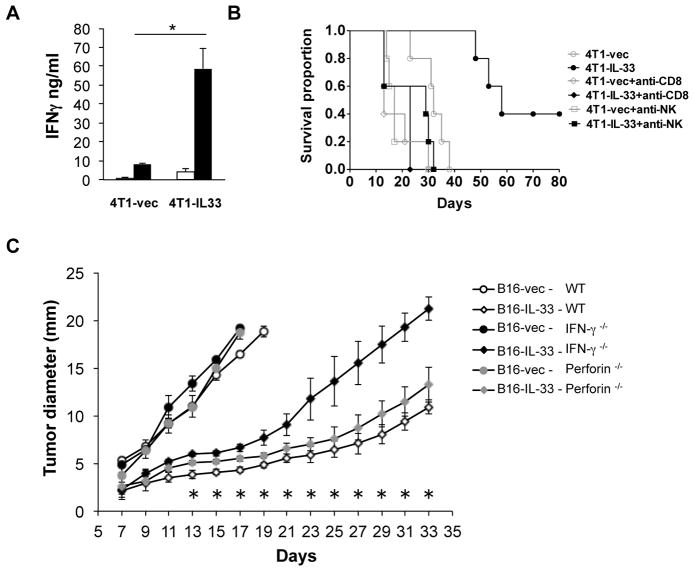
We showed that tumor cell expression of IL-33 elicited greatly increased tumor infiltrating CD8+ T and NK cells. These findings prompted us to explore whether systemic tumor-antigen-specific T cells can be induced to a higher level upon tumoral expression of IL-33. To address this question, we isolated CD8+ T cells from the spleen of 4T1 or 4T1-IL-33-tumor bearing mice. We then co-cultured these CD8+ T cells with irradiated tumor cells in the presence of APC isolated from non-tumor-bearing mice. We then measured the levels of IFNγ in these cultures by ELISA. We found less than 10 ng/ml IFNγ was produced by CD8+ T cells from 4T1-bearing mice. In contrast, approximately 60 ng/ml IFNγ was produced by CD8+ T cells isolated from 4T1-IL-33-bearing mice (Figure 5A). These data demonstrated that tumoral expression of IL-33 led to a significant increase of tumor-specific CD8+ T cells, supporting the role of IL-33 in eliciting a tumor-specific adaptive immune response.
Figure 5. NK and CD8+ T cells as well as IFNγ and perforin were required for the antitumor effect of IL-33.
(A) 1×105 4T1-vector or 4T1-IL-33 cells were injected into the mammary fat pad of BALB/c mice. 30 days after inoculation, CD8+ T cells were purified from the spleens of these mice and re-stimulated with irradiated 4T1 cells for 72h. The level of IFNγ was then measured by ELISA. Results are mean ±SEM of three independent experiments. *P<0.05, two-tailed unpaired Student’s t-test. (B) Mice were inoculated i.d. with 1×105 4T1 tumor cells (n = 5). These mice were injected intraperitoneally with anti-CD8, or anti-asialo GM1 antibodies, or control IgG four times before and after tumor inoculation (day -2, 1, 7, 14). Kaplan-Meier survival curves are shown and the Log-Rank test was performed. P=0.0018. (C) 2×105 B16-vector or B16-IL-33 cells were injected i.d. into WT B6, IFNγ−/− B6 or perforin−/− B6 mice and the size of the tumor was monitored every two days. Tumor diameters (mean ±SEM) were presented here. Five mice were in each group. * P<0.05, determined by Mann-Whitney Test. Comparison was performed between the B16-IL-33: WT and B16-IL-33: IFNγ−/− groups. The difference between the B16-IL-33: WT and B16-IL-33: perforin−/− groups was significance from days 13 to 19.
IL-33 exerts its antitumor effects through CD8+ T cells and NK cells as well as IFNγ and perforin effector molecules
Since CD8+ T cells and NK cells were increased in 4T1-IL-33 tumors and tumor-specific CD8+ T cells were increased in 4T1-IL-33-bearing mice, we then determined whether CD8+ T cells and/or NK cells are required for the antitumor effect of IL-33. We used anti-CD8 and NK antibodies to deplete CD8+ T cells and NK cells respectively and then examined tumor progression in these mice compared to mice injected with control antibodies. 4T1-IL-33-inoculated mice survived much longer compared to 4T1-inoculated mice (Figure 5B). Injection of either anti-NK or anti-CD8 mAbs shortened the survival of 4T1-bearing mice compared to mice injected with control mAbs. Importantly, injection of either anti-NK or anti-CD8 mAbs into 4T1-IL-33-bearing mice greatly reduced the survival of these mice (Figure 5B). Thus, both NK and CD8+ T cells are required for the antitumor function of IL-33.
Since IFNγ, perforin and granzyme B were all increased in B16-IL-33 tumors compared to B16 tumors during both acute and chronic phases, we sought to determine whether these type 1 effector molecules were required for the antitumor effect of IL-33. We injected control B16, or B16-IL-33 into WT, IFNγ −/−, or perforin −/− mice intradermally (i.d.) and monitored tumor growth every two days. The growth inhibition by IL-33 was partially reversed in IFNγ −/− mice. Perforin −/− mice also showed a more modest reversal of suppression (Figure 5C). Therefore, IFNγ played an important role in mediating antitumor effect of IL-33.
IL-33 regulates tumor associated myeloid cells and promotes MHC II expression
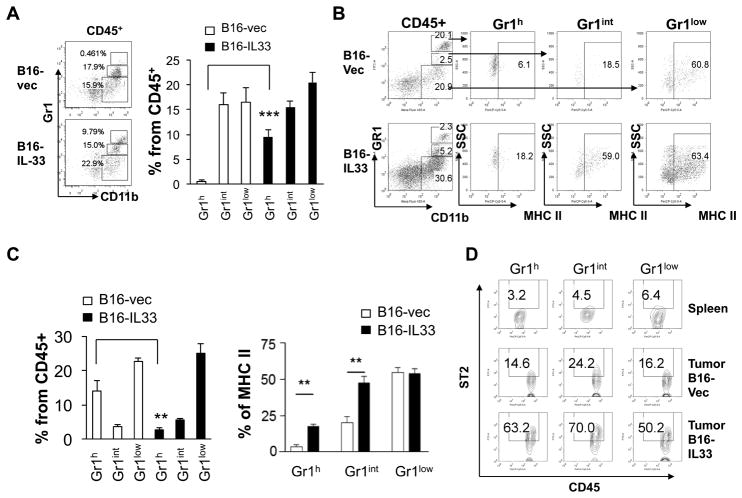
In order to gain further insight into how IL-33 impacts the cellular network within tumor, we examined the myeloid compartment with particular focus on monocytes, neutrophils and macrophages using CD11b and Gr1 markers. We first examined cells from tumors that formed about ten days after inoculation and sizes were comparable between B16 and B16-IL-33 tumors. We found that percentage of Gr1high CD11b+ cells, largely neutrophils, was much higher in B16-IL-33 tumors than B16 tumors (Figure 6A). The percentage of other CD11b+ myeloid subsets (Gr1intCD11b+ monocytic cells and Gr1-CD11b+ macrophages) was not different between these tumors. Taken into account of an increase in total CD45+ immune cells, all CD11b+ myeloid cells were increased in B16-IL-33 tumors compared to B16 control tumors. Our data support previous reports showing that tumor associated neutrophils in the type 1 environment inhibit tumor growth (26) (27). We also characterized the CD11b+ myeloid cells at a later time point (20 days after inoculation). Although we observed lower percentages of Gr1high CD11b+ myeloid cells within CD45+ immune cells in the B16-IL-33 tumor, the number of these cells was actually higher in B16-IL-33 tumors due to greater numbers of CD45+ TIL in B16-IL-33 tumors compared to B16 tumors. The percentage of other subsets of CD11b+ cells was also higher in B16-IL-33 tumors if taking into account of elevated levels of CD45+ immune cells in B16-IL-33 tumors compared to B16 tumors. In the mouse tumor microenvironment, many Gr1+CD11b+ (including both Gr1high and Gr1int) cells are considered myeloid-derived suppressor cells (MDSC) and these cells don’t express MHC II(28). Our analysis revealed that the level of MHC II was much higher in Gr1high and Gr1int cells in the B16-IL-33 tumor when compared to the B16 tumor, consistent with an increase of immunogenicity of the B16-IL-33 tumor microenvironment (Figure 6B and C). This is likely due to IFNγ produced by CD8+ T cells and NK cells. ST2 was highly expressed in all sub-populations of CD11b+ cells isolated from B16-IL-33, suggesting that IL-33 might directly modulate the function of these cells (Figure 6D). Thus, we showed here that IL-33 promoted type 1 immune responses in the tumor microenvironment, increased the flux of myeloid cells and their MHC class II expression.
Figure 6. IL-33 regulated tumor associated myeloid cells and upregulated MHC II on tumor associated myeloid cells.
2×105 B16-vector or B16-IL-33 cells were injected i.d. into B6 mice. On day 10 or 20, tumors were resected and processed to generate a single cell suspension. (A) Representative flow cytometric analysis of CD11b+ sub-populations and percentages of CD11b+ subpopulations within CD45+ cells on day 10. (B) Representative flow cytometric analysis of CD11b+ sub-populations on day 20. (C) Percentages of CD11b+ subpopulations in CD45+ cells and percentages of MHC class II expression on three CD11b+ sub-populations. Results are mean ±SEM of three independent experiments. (D) Surface ST2 expression on Gr1high CD11b+, Gr1int CD11b+, and Gr1low CD11b+ cells from spleen and tumor. Results are mean ±SEM of three independent experiments. **P<0.01 and ***P<0.001, two-tailed unpaired Student’s t-test.
Elimination of Treg further improves antitumor function of IL-33
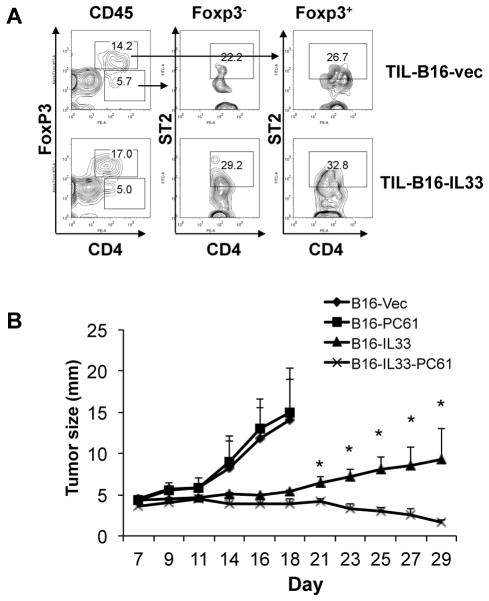
Despite the apparent presence of strong type 1 immune responses and inhibition of tumor growth, the B16-IL-33 tumor continues to increase in size. This prompted us to examine whether self-limiting immune regulatory mechanisms were also induced in the B16-IL-33 tumor. We focused on CD4 T cells for two reasons. First, Th2 cells have been shown to be upregulated by IL-33 (29). Second, Treg are a dominant group of CD4+ T cells within the tumor and we showed that IL-33 promoted Treg proliferation in the spleen (30–32). Despite reports that IL-33 induces Th2 responses (11), we did not find significant numbers of IL-4+, IL-5+ or IL-13+ CD4+ TIL (not depicted). In contrast, we found more than 60% of CD4+ TILs were Foxp3+, presumably Treg (Figure 7A and supplementary figure 4). Interestingly, approximately 30% of these Treg from B16-IL-33 tumor were ST2+. Similar percentages of Treg from B16 tumor also expressed ST2. However levels seemed lower. The fact that many Treg express ST2 suggested that IL-33 might have a direct effect on these cells (Figure 7A), thus, the anti-tumor effect of IL-33 could be inhibited by IL-33-influenced Treg. To test this hypothesis, we depleted Treg in mice using anti-CD25 (PC61) antibody. We found B16 grew at a similar rate in B6 mice regardless of anti-CD25 application, consistent with previous findings (33). In contrast, Treg depletion resulted in a much greater shrinkage of B16-IL-33 tumors (Figure 7B). These data indicated that IL-33 induced type 1 antitumor immune responses, but such responses were kept in check by Treg.
Figure 7. Synergistic effect of IL-33 expression and depletion of Treg for eliminating tumors.
(A) Representative flow cytometric analysis of ST2 on Foxp3+CD4+ and Foxp3-CD4+ in B16 tumor and B16-IL-33 tumor. (B) B6 mice were injected with control Rat IgG or PC61, 200 μg per mouse twice a week. One week after antibody injection, 2×105 B16-vector (B16) or B16-IL-33 cells were injected i.d. into these mice. Tumor sizes were measured every two days. Tumor diameters (mean ±SEM) were presented here. Five mice were in each group. * P<0.05, determined by Mann-Whitney Test. Comparison was performed between B16-IL-33 and PC61 B16-IL-33 groups.
Discussion
Tumor growth can trigger inflammatory responses by releasing danger signals and expression of tumor antigens. Tumor progression is inhibited by type 1 lymphocytes such as Th1, CD8+ T, NK, and γδ T cells, as well as collaborating innate cells such as dendritic cells (DC) and type 1 macrophages and neutrophils (M1 and N1) (4, 23) (26) (27). Nevertheless, tumors progress by enlisting and driving the dominance of immune suppressive cell types such as Treg and MDSC, as well as myeloid cells that produce cancer-promoting factors. Our study showed that high-level expression of IL-33 in tumors led to increases in antitumor immune responses and promoted the type 1 lymphocyte-dominated tumor immunogenic microenvironment. This was due to IL-33-induced increases in the production of type 1 effector molecules, such as IFNγ and granzymes, by CD8+ and NK cells in the tumor microenvironment.
Our study establishes an antitumor function of IL-33. This finding is in keeping with recent studies that also show a potent function of IL-33 in driving type 1 immune responses in tumor, viral infection and autoimmune models. Published work by our lab and others showed that activated CD8+ T cells and Th1 cells produce larger amounts of IFNγ in response to IL-33 (15, 34). In addition, antiviral immune responses require IL-33 signaling in CD8+ T cells (19). Furthermore, Th1, NK and NKT cells respond to IL-33 in a similar fashion to increase their IFNγ production (16, 17, 35). Overexpression of IL-33 systemically in transgenic mice promotes NK and CD8+ T cell function globally and inhibits tumor growth (36). IL-33 has also been used to further boost antitumor vaccination (37). Additionally, in the ConA-induced hepatitis model, IL-33 has been shown to be pathogenic (38) by inducing type 1 immune responses. These studies clearly established a critical role of IL-33 in promoting type 1 immune responses. Nevertheless, there are studies that show an opposite function of IL-33. One study shows that systemic injection of low levels of IL-33 leads to increases in tumor metastasis (39). In the ConA-induced hepatitis model, IL-33 has also been shown to be protective (40). In the transplantation setting, intraperitoneal (i.p.) injection of IL-33 protects cardiac allografts through expansion regulatory cells, particularly Treg (32). We believe that differences in the level of IL-33 and the primary target cells of IL-33 in these experiments are accountable for the different in vivo effects. Persistent and higher levels of IL-33 in tumor tissues, as well as during infection, promote type 1 immune responses. In addition, other cytokines, such as IL-2 and IL-12, both of which were shown to synergize with IL-33 to induce IFNγ, can help shape the nature of IL-33-driven immune responses (15, 34). In contrast, low-level and systemic delivery of IL-33 protein into resting or tumor-bearing mice resulted in immune tolerance (32, 39, 40). In the ConA-induced hepatitis model, IL-33 has been shown to be protective in one and pathogenic in another (38, 40). In the conA-induced hepatitis model, higher doses and frequencies of IL-33 injection elicited strong type 1 immune responses, whereas lower doses of IL-33 resulted in immune tolerance. Thus, the immune phenotype of IL-33 is likely shaped by dose, the local environment, and the target cells. Regulatory effects may be achieved through the systemic actions of IL-33 which expand and activate MDSC, tolerogenic DC, and Treg (32, 41). However, high-level and tumor-site delivery of IL-33 can mediate potent anti-tumor responses mediated by CD8+ T cells, NK and NKT and IFNγ. This knowledge will be key for the clinical application of this cytokine for cancer immunotherapy. This can potentially be achieved by inducible expression of IL-33 in tumor-specific T cells for enhancing adoptive T cell therapy or tumor-antigen-specific antibody-mediated IL-33 delivery (42).
CTLA-4 and PD-1 mAb-based immunotherapies have achieved great clinical success for melanoma (5–7). Nevertheless, the response rate for this type of therapy for other solid cancers will likely be limited due to its dependence on spontaneous T cell responses to tumor. Introducing the right inflammation to the tumor microenvironment should help further increase the response rate of immunotherapy in solid cancer by making the tumor more immunogenic. Cytokine-mediated immune therapy of cancer should provide new opportunities for enhancing the immune “check-point”-based approach, thus IL-33 should be a promising cytokine for enhancing cancer immunotherapy.
Supplementary Material
Acknowledgments
We thanks Drs Lisa Bufferfield, Olivera Finn and Lindsay Stollings for critical reading of the manuscript.
The project was mainly supported by the National Institutes of Health through Grant Numbers R21CA167229 (BL), UL1 RR024153 (BL), UL1TR000005 (BL), 1P50 CA097190 (BL), the RPCI-UPCI Ovarian Cancer SPORE (P50CA159981) (BL), and R00HL097155 (HRT). The work is partly supported by NSFC grant: 31428005 (to BL and JJ) 31320103918 (to X. Zhang and B. Lu) and NSFC grant support 81273208 (to Y. Zhu). GL and XG are supported by scholarships from China Scholarship council. WQ is supported by Shanghai Municipal Program of International Cooperation in Science and Technology grant 12410709800. QY and XC are supported by Twelfth-Fifth Mega-Scientific Project on “prevention and treatment of AIDS, viral hepatitis and other infectious diseases”, (2012ZX10003002), Natural Science Foundation of China Grant (81301389, 81273140), Shenzhen science and technology innovation project (JCYJ20130329171031738).
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Finn OJ. Cancer immunology. The New England journal of medicine. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T Cell Transfer Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;11:11. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY) 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 9.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 10.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. Journal of immunology (Baltimore, Md : 1950) 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 13.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PloS one. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8(+) T cells. Eur J Immunol. 2011;2:201141629. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 17.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeois EA, Levescot A, Diem S, Chauvineau A, Berges H, Milpied P, Lehuen A, Damotte D, Gombert JM, Schneider E, Girard JP, Gourdy P, Herbelin A. A natural protective function of invariant NKT cells in a mouse model of innate-cell-driven lung inflammation. Eur J Immunol. 2011;41:299–305. doi: 10.1002/eji.201040647. [DOI] [PubMed] [Google Scholar]
- 19.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Lohning M, Pinschewer DD. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science (New York, NY) 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 20.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. The Journal of experimental medicine. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis JD, Shearer MH, Kennedy RC, Bright RK. Surrogate tumor antigen vaccination induces tumor-specific immunity and the rejection of spontaneous metastases. Cancer research. 2005;65:2938–2946. doi: 10.1158/0008-5472.CAN-04-2874. [DOI] [PubMed] [Google Scholar]
- 22.Lefrancais E, Cayrol C. Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. European cytokine network. 2012;23:120–127. doi: 10.1684/ecn.2012.0320. [DOI] [PubMed] [Google Scholar]
- 23.Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, Lu BF, Jiang JT. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer immunology, immunotherapy : CII. 2013;62:553–561. doi: 10.1007/s00262-012-1358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Chen L, Liu L, Zhu Y, Wu C, Jiang J, Zhang X. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50:269–275. doi: 10.1007/s12026-011-8227-9. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. Journal of immunology (Baltimore, Md : 1950) 2010;185:3174–3183. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 26.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. The Journal of experimental medicine. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanchot C, Terme M, Pere H, Tran T, Benhamouda N, Strioga M, Banissi C, Galluzzi L, Kroemer G, Tartour E. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2013;6:147–157. doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PloS one. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, Demetris AJ, Liew FY, Wood KJ, Thomson AW. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. Journal of immunology (Baltimore, Md : 1950) 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008;333:167–179. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngoi SM, St Rose MC, Menoret AM, Smith DE, Tovey MG, Adler AJ, Vella AT. Presensitizing with a Toll-like receptor 3 ligand impairs CD8 T-cell effector differentiation and IL-33 responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10486–10491. doi: 10.1073/pnas.1202607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seltmann J, Werfel T, Wittmann M. Evidence for a regulatory loop between IFN-gamma and IL-33 in skin inflammation. Experimental dermatology. 2013;22:102–107. doi: 10.1111/exd.12076. [DOI] [PubMed] [Google Scholar]
- 36.Gao K, Li X, Zhang L, Bai L, Dong W, Gao K, Shi G, Xia X, Wu L, Zhang L. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer letters. 2013;335:463–471. doi: 10.1016/j.canlet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Villarreal DO, Wise MC, Walters JN, Reuschel EL, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP, Weiner DB. Alarmin IL-33 Acts as an Immunoadjuvant to Enhance Antigen-Specific Tumor Immunity. Cancer research. 2014;74:1789–1800. doi: 10.1158/0008-5472.CAN-13-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Duan L, Xiong A, Zhang H, Zheng F, Tan Z, Gong F, Fang M. Blockade of IL-33 ameliorates Con A-induced hepatic injury by reducing NKT cell activation and IFN-gamma production in mice. Journal of molecular medicine (Berlin, Germany) 2012;90:1505–1515. doi: 10.1007/s00109-012-0938-4. [DOI] [PubMed] [Google Scholar]
- 39.Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. International journal of cancer. Journal international du cancer. 2014;134:1669–1682. doi: 10.1002/ijc.28481. [DOI] [PubMed] [Google Scholar]
- 40.Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, Pejnovic N, Arsenijevic N, Lukic ML. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. Journal of hepatology. 2012;56:26–33. doi: 10.1016/j.jhep.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q, Turnquist HR. Implications for Interleukin-33 in solid organ transplantation. Cytokine. 2013;62:183–194. doi: 10.1016/j.cyto.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, Rosenberg SA, Morgan RA. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.