Abstract
Mosquito eggs laid within two hours are necessary for transgenic (injection) studies, because mosquito eggs become hard after that period. Thus, in order to have eggs available within this two-hour window, it is important to understand the ovipositional behavior of Anopheles gambiae s.s.. In the present study, the ovipositional behavior of An. gambiae s.s. (Kisumu) was investigated in several different conditions: age of mosquitoes, time post blood meal to access oviposition substrate, and light conditions. Two groups of mosquitoes, 3–5 days old and 9–11 days old were blood-fed. For those mosquito groups, an oviposition dish was set either at 48 hours or 72 hours after the blood meal either in a light condition or in an artificial dark condition. The number of laid eggs was compared among the different conditions. The 3–5 day-old mosquitoes apparently produced a higher number of eggs than 9–11 day-old mosquitoes, while there was no significant difference between the two groups. The number of laid eggs per one surviving blood-fed mosquito in the dark condition was significantly higher than that in the light condition (p = 0.03). Providing an oviposition dish at 72 hours after blood meal resulted in a significantly higher number of laid eggs per one surviving blood-fed mosquito than at 48 hours after blood meal (p = 0.03). In conclusion, the optimal condition to have readily available egg supply for transgenic analysis was as follows: 3–5 day-old mosquitoes with an oviposition dish placed at 72 hours after the blood meal in a dark environment.
Keywords: Anopheles gambiae, ovipositional behavior, transgenic study
Introduction
Malaria is caused by Plasmodium, a protozoan parasite and transmitted by the bite of infected mosquitoes of genus Anopheles. Annually 250 million people are infected and close to one million people, mostly children in sub-Saharan Africa were killed by malaria [1]. Insecticide-based control such as massive use of insecticides and insecticide-impregnated bed nets has been one of the most successful methods to control malaria [2]. However, emergence and spread of insecticide-resistant Anopheles observed throughout the world including Africa pose a threat to the gains made in malaria control [3, 4]. Novel approaches to the control of vectors are therefore urgently needed to combat malaria.
Advances in insect molecular biology, in particular the development of germ-line transformation, have opened a new avenue for the control of malaria [5]. One of the major goals of gene-modification studies is to generate malaria parasite-resistant (refractory) An. gambiae s.s., the most important vector of human malaria in Africa, and to replace the wild-type An. gambiae s.s. population that is susceptible to malaria parasites with the genetically modified mosquito population.
The first key step for germ-line transformation is to obtain a requisite number of mosquito eggs for injection. Since mosquito eggshell becomes hard within two hours after being laid [6], it becomes difficult to inject genes into mosquito eggs using a glass needle after that period. Therefore clarification of the conditions in which mosquito eggs laid within two hours can be efficiently obtained is a crucial prerequisite for gene-modification studies. Compared to Anopheles stephensi, which is the predominant Anopheles vector in the Indian subcontinent, An. gambiae s.s. has been used less frequently in embryo transgenic studies and relatively little information is available regarding its ovipositional behavior [7]. In the present study, we analyzed ovipositional behavior of An. gambiae s.s. with reference to the following conditions: age of mosquitoes, time post blood meal to access ovipostion substrate, and light conditions at oviposition.
Methods
Mosquitoes
An. gambiae s.s. (Kisumu strain) mosquito colony was maintained by the following procedures. The mosquitoes were reared in a climate controlled insectary at a temperature of 28 ± 1°C, 12L:12D photoperiod and relative humidity of 70 ± 10%. The adults were kept in holding cages (30 × 30 × 30 cm) with access to 10% sucrose (WAKO) solution. 4–6 day-old females were blood-fed on rabbits and provided with oviposition dishes, covered with moist filter paper. The eggs laid on the filter paper were transferred to plastic trays. Hatched larvae were fed on fish meal (Tetramin®). Pupae were collected in small cups and transferred to holding cages.
Oviposition
Two groups of An. gambiae s.s. mosquitoes were used: 3–5 day-old mosquitoes and 9–11 day-old mosquitoes. For each group, approximately 100 female mosquitoes were kept in one cage (4 cages per group). The mosquitoes in each group were maintained in a photoperiod of 12L:12D and blood-fed at halfway of the light period, after being starved for at least 3 hours. An oviposition dish was then set in each cage either at 48 hours or at 72 hours after the blood meal either in a light condition or in an artificial dark condition. The number of eggs laid on filter papers was counted every 30 minutes up to 2 hours for each cage. All experiments were done at a temperature of 28 ± 1°C in triplicate.
Statistical analysis
The number of laid eggs was adjusted by the number of surviving blood-fed female mosquitoes. The obtained values (numbers of eggs per one surviving blood-fed mosquito) were compared separately for each condition: age of mosquitoes, time post blood meal to access oviposition substrate, and light conditions. Significant differences were analyzed by Student’s t-test (significance at p < 0.05).
Results and Discussion
In order to understand the ovipositional behavior of An. gambiae s.s., we compared the effect of mosquito age on egg-laying behavior. The 3–5 day-old mosquitoes apparently produced a higher number of eggs than the 9–11 day-old mosquitoes (Table 1), while there was no significant difference (p = 0.86) between the number of eggs produced by one female mosquito in the two groups. Mortality of Anopheles mosquitoes was reported to be age-dependent [8], and indeed the average mortality of 9–11 day-old mosquitoes in our study was significantly higher than that of 3–5 day-old mosquitoes (Table 1). The difference in the numbers of laid eggs between the two groups might therefore be due to the difference in the actual numbers of surviving mosquitoes.
Table 1.
Numbers of eggs produced by female An. gambiae mosquitoes in different conditions.
| Light |
Dark |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 hours |
72 hours |
48 hours |
72 hours |
|||||||||
| 3–5 day | 9–11 day | 3–5 day | 9–11 day | 3–5 day | 9–11 day | 3–5 day | 9–11 day | |||||
| Number of eggs laid at |
0–30 min | 0 ± 0 | 1.0 ± 1.0 | 13.3 ± 13.3 | 0.3 ± 0.3 | 63.7 ± 63.7 | 0.3 ± 0.3 | 301.0 ± 112.2 | 140.0 ± 67.6 | |||
| 30–60 min | 0 ± 0 | 0 ± 0 | 3.7 ± 3.7 | 1.7 ± 1.7 | 27.0 ± 27.0 | 25.0 ± 25.0 | 206.0 ± 91.0 | 50.0 ± 42.7 | ||||
| 60–90 min | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.0 ± 1.0 | 0 ± 0 | 1.0 ± 1.0 | 14.7 ± 10.4 | 0.3 ± 0.3 | ||||
| 90–120 min | 0 ± 0 | 0.3 ± 0.3 | 16.7 ± 8.4 | 47.3 ± 29.3 | 0 ± 0 | 17.0 ± 17.0 | 0 ± 0 | 46.0 ± 46 | ||||
| Number of eggs (total) | 0 ± 0 | 1.3 ± 1.3 | 33.7 ± 25.4 | 50.3 ± 32.3 | 90.7 ± 90.7 | 43.3 ± 43.3 | 521.7 ± 213.2 | 236.3 ± 156.6 | ||||
| Number of female (total) mosquitoes |
107.0 ± 3.0 | 88.0 ± 10.4 | 108 ± 6.5 | 85.3 ± 13.9 | 109.3 ± 4.1 | 88.7 ± 12.3 | 112.0 ± 3.8 | 84.3 ± 11.5 | ||||
| Number of blood-fed mosquitoes | 78.0 ± 3.5 | 61.3 ± 7.6 | 83 ± 4.2 | 55.3 ± 9.6 | 88.3 ± 3.7 | 61.0 ± 8.5 | 73.0 ± 13.0 | 59.0 ± 5.5 | ||||
| Dead (blood-fed) | 2.3 ± 1.9 | 7.3 ± 1.5 | 5 ± 2.9 | 10.3 ± 4.3 | 1.3 ± 0.9 | 11.0 ± 4.2 | 3.3 ± 1.5 | 11.7 ± 3.8 | ||||
| Dead (blood-unfed) | 1.0 ± 0 | 3.7 ± 1.5 | 2.7 ± 0.9 | 3.7 ± 0.3 | 0.7 ± 0.3 | 5.3 ± 2.6 | 1.0 ± 0.6 | 6.7 ± 2.2 | ||||
| Feeding Rates | 72.9% | 69.7% | 76.9% | 64.8% | 80.8% | 68.8% | 65.2% | 70.0% | ||||
| Mortality Rates | 3.1% | 12.5% | 7.1% | 16.4% | 1.8% | 18.4% | 3.9% | 21.7% | ||||
| Number of laid eggs (total) / number of blood-fed alive mosquitoes |
0 ± 0 | 0 ± 0 | 0.4 ± 0.3 | 1.2 ± 1.2 | 1.1 ± 1.0 | 1.1 ± 1.3 | 7.6 ± 3.9 | 5.8 ± 2.7 | ||||
Each value (mean ± SE) represents the mean of three independent experiments. Feeding rate was calculated as follows: number of blood-fed mosquitoes divided by number of female (total) mosquitoes. Mortality rate was calculated as follows: number of both blood-fed and -unfed dead mosquitoes divided by number of female (total) mosquitoes.
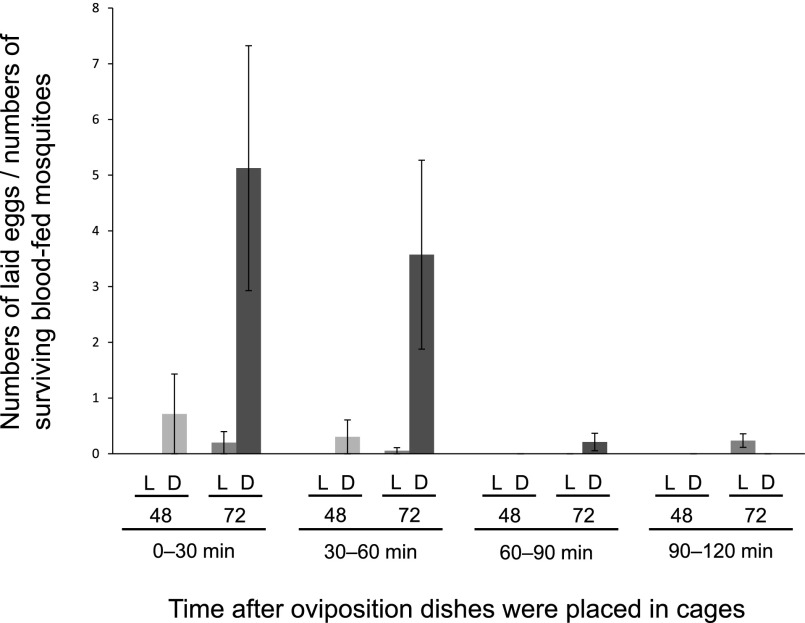
Secondly, light condition was compared. The number of laid eggs per one surviving blood-fed mosquito was significantly higher in the dark conditions than that in the light conditions (p = 0.03) (Table 1, Fig. 1), which was consistent with the previous report [9]. Since wild-type Anopheles mosquitoes were reported to lay eggs preferably at night [10], artificial dark environment seemed to promote the egg-laying behavior of An. gambiae s.s. mosquitoes.
Fig. 1.
Comparison of numbers of eggs per one surviving blood-fed mosquito at 0–30 min, 30–60 min, 60–90 min and 90–120 min in different conditions.
3–5 day-old mosquitoes. L denotes light condition. D denotes artificial dark condition. “48” and “72” denote that the oviposition dish was placed at 48 hours and 72 hours after blood feeding, respectively.
Finally, oviposition rates were compared between mosquito groups for which oviposition dishes were set at 48 hours and at 72 hours after the blood meal. Providing an oviposition dish at 72 hours after blood meal resulted in a significantly higher number of laid eggs per one surviving blood-fed mosquito compared to that at 48 hours after blood meal (p = 0.03) (Fig. 1). No significant difference was observed in hatching rate or viability of laid eggs between the two mosquito groups (data not shown). Since female mosquitoes require blood nutrients to produce eggs, the longer period after the blood meal may have allowed mosquitoes to digest the blood and consequently to utilize more nutrients for egg production. Alternatively, the longer period of holding matured eggs may have induced the more active egg laying of the mosquitoes when the oviposition dish was made available in the cage. Also possibly, it may have taken more than 48 hours for mosquitoes to fully recover from the effect of starvation before the blood meal. However, it seems unlikely that a holding period longer than 72 hours would result in higher numbers of eggs in total because mosquito mortality rates would increase [8].
From these results, the best condition for optimal oviposition rates of An. gambiae s.s. mosquitoes was determined as follows: 3–5 day-old mosquitoes for which an oviposition dish was placed at 72 hours after the blood meal in a dark environment. It would be also noteworthy that in this condition, most of the eggs were laid within 60 min after the placement of oviposition dishes. To our knowledge, this is the first report on the time-course ovipositional behavior of An. gambiae mosquitoes. While An. gambiae s.s. strain-specific difference may exist, this egg laying system could provide valuable information for transgenic studies of An. gambiae s.s. mosquitoes. Moreover, the above information may be useful for practical malaria vector control.
Acknowledgement
The study was supported in part by a grant-in aid from the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- 1.World Health Organization Global Malaria Program World Malaria Report 2013. Geneva: WHO Press; 2013. [Google Scholar]
- 2.Kleinschmidt I, Schwabe C, Benavente L, et al. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg 2009; 80(6): 882–888. [PMC free article] [PubMed] [Google Scholar]
- 3.Tchouassi DP, Quakyi IA, Addison EA, et al. Characterization of malaria transmission by vector populations for improved interventions during the dry season in the Kpone-on-Sea area of coastal Ghana. Parasit Vectors 2012; 5(212): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temu EA, Maxwell C, Munyekenye G, et al. Pyrethroid resistance in Anopheles gambiae, in Bomi county, Liberia, compromises malaria vector control. PLoS One 2012; 7(9): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto DS, Sumaitani M, Nagumo H, et al. Induction of antisporozoite antibodies by biting of transgenic Anopheles stephensi delivering malaria antigen via blood feeding. Insect Mol Biol 2012; 21(2): 223–233. [DOI] [PubMed] [Google Scholar]
- 6.Li JS, Li J. Major chorion proteins and their cross linking during chorion hardening in Aedes aegypti mosquitoes. Insect Biochem Mol Biol 2006; 36(12): 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan T, Bower TM, Brown AE, et al. piggyBac-mediated germline transformation of the malaria mosquito Anopheles stephensi using the red fluorescent protein dsRED as a selectable marker. J Biol Chem 2002; 277(11): 8759–8762. [DOI] [PubMed] [Google Scholar]
- 8.Dawes JE, Churcher ST, Zhuang S, et al. Anopheles mortality is both age- and Plasmodium-density dependent: implications for malaria transmission. Malar J 2009; 8(228): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz ML, Huang J, Walker ED, et al. Ovipositional periodicity of caged Anopheles gambiae individuals. J Circadian Rhythms 2008; 6(2): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumba LA, Okoth K, Deng AL, et al. Daily oviposition patterns the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. J Circadian Rhythms 2004; 2(1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]