Abstract
Purposes
To establish the efficacy and toxicities of concurrent bevacizumab and docetaxel with radiation for locally advanced HNSCC.
Materials/Methods
Patients with previously untreated HNSCC received standard daily radiotherapy with concurrent weekly docetaxel (20 mg/m2) and biweekly bevacizumab (5 mg/kg). Biweekly bevacizumab was then continued for up to one year following radiotherapy. The primary objective was progression-free survival (PFS). Secondary objectives included overall survival, patterns of failure, and toxicities of treatment.
Results
Thirty patients were recruited. With median follow-up of 38 months, the 3 year PFS, OS, locoregional recurrence free survival and distant metastasis free survival was 61.7%, 68.2%, 84.5%, and 80.5%, respectively. The most common local toxicities were mucositis and dermatitis. Two patients developed hemorrhage. There was no grade 5 toxicity.
Conclusions
The combination of bevacizumab, docetaxel and radiotherapy is tolerable and effective in HNSCC. This regimen is worthy of further study in appropriate subset of patients receiving chemoradiation therapy.
Keywords: head and neck cancer, radiation, bevacizumab, docetaxel, chemoradiation
Introduction
Head and neck cancer is the eighth most common cancer in the United States (1). For locally advanced HNSCC, concurrent chemoradiotherapy (chemo-RT) is the standard treatment established with multiple randomized trials (2–4). The survival benefit of chemo-RT in locally advanced HNSCC has been confirmed by meta-analyses, which revealed a 19% reduction in mortality and an absolute survival benefit of 8% at 5 years when chemotherapy was administered concurrently with radiation (5, 6).
Despite incremental improvements in local regional control and survival in HNSCC patients treated with chemo-RT, a substantial fraction of patients suffer persistent or recurrent diseases. Combining novel agents with radiation therapy therefore remains of great interest to further improving the treatment outcomes of HNSCC.
Cisplatin is the most commonly used chemotherapeutic agent given concurrently with radiation. However, introduction of additional effective radiosensitizing agents is urgently needed. A significant percentage of patients appropriate for definitive concurrent chemo-RT are not candidates for cisplatin based treatment. Furthermore, recent data have emerged that cisplatin might not be the most optimal cytotoxic radiosensitizing agent when addition of novel targeted agents to concurrent chemo-RT is evaluated.
Radiation Therapy Oncology Group (RTOG) 0234 is a phase II randomized clinical trial evaluating postoperative radiation plus concurrent docetaxel and cetuximab versus postoperative radiation plus cisplatin and cetuximab for high-risk HNSCC after surgery. The results showed an impressive improvement in overall survival and disease-free survival of the docetaxel arm compared to the cisplatin arm (79% versus 69% and 66% versus 57% respectively (7). These results are pointing to the possibility that a non-cisplatin based regimen chemo-RT should be explored for further development of novel targeted agents and led to a recently opened phase III randomized RTOG trial, RTOG 1216, which compares postoperative radiation with concurrent cisplatin versus docetaxel versus docetaxel and cetuximab for high-risk HNSCC patients (8).
Vascular endothelial growth factor (VEGF) is one of the most important regulators of angiogenesis. Up-regulation of VEGF has been found in many HNSCC and was shown to be associated with radioresistance and poor prognosis (9, 10). Bevacizumab is a recombinant humanized anti-VEGF monoclonal antibody and has been shown in preclinical models to be a radiation sensitizer and can enhance anti-tumor efficacy of radiation and chemotherapeutic agents (11–13).
Our group has extensive experience in studying the efficacy and toxicities of targeted therapies added to a docetaxel-based chemo-RT both in phase I and phase II setting (14, 15). Initial selection of docetaxel was based on its documented potent radiosensitizing effects and favorable toxicity profile when compared to cisplatin. We developed the first protocol combining RT with the doublet of docetaxel/bevacizumab in the effort to identify a non-cisplatin concurrent chemo-RT regimen. Herein we report the results of this phase II clinical trial.
Materials and Methods
This is a nonrandomized open label phase II study opened in University Hospitals Case Medical Center (UHCMC) and University of Pittsburgh Medical Center (UPMC). The study was approved by the Institutional Review Boards (IRB) of Case Comprehensive Cancer Center and UPMC. All patients provided IRB-approved written informed consent prior to study enrollment.
Patient Eligibility and Baseline Assessment
Eligible patients had histologically confirmed previously untreated stage III to IVA/B HNSCC without distant metastatic disease. Patients were evaluated by a multidisciplinary team that included otolaryngologists, medical oncologists, and radiation oncologists. Each case was presented at a multidisciplinary tumor board for consensus treatment recommendations and to assess appropriateness for clinical trial. Pretreatment evaluation included history and physical examination, triple endoscopy, and radiographic imaging of the head and neck, and chest prior to enrollment to the study. All patients underwent dental evaluation prior to treatment. If dental extractions were required, at least 7 days of healing was required prior to initiation of radiation. All patients were required to have an ECOG performance status of 0–1 and adequate bone marrow, renal and hepatic functions. Percutaneous gastrotomy tubes were placed in the majority of patients (26/30) prior to the start of radiation therapy unless refused by the patient per institutional practice.
Squamous cell carcinoma of salivary gland and paranasal carcinomas were excluded from the study. Excluded also patients with ≥ grade 2 pre-existing peripheral neuropathy, history of allergic reactions to the chemotherapeutic agents, and uncontrolled intercurrent diseases as well as HIV positive patients. With respect to bevacizumab specific concerns, patients with history of stroke or myocardial infarction within 6 months, blood pressure >150/100, urine protein:creatinine ratio ≥ 1.0, New York Heart Association (NYHA) grade II or greater congestive heart failure, unstable angina, or evidence of bleeding diathesis or coagulopathy, major surgery or significant traumatic injuries within 28 days prior to Day 0 of treatment were also excluded from study. Those who had close proximity or involvement of major blood vessel by the tumor or nodes were also excluded.
Protocol Treatment
The treatment schema is summarized in Figure 1.
Figure 1.
The treatment schema
Chemotherapy
Treatment was administered on an outpatient basis. Bevacizumab was administered 5mg/kg intravenously over a 90 minute infusion once every two weeks during the course of radiation and up to one year following the completion of radiation therapy. If neck dissection was performed, bevacizumab was stopped for at least 8 weeks prior to surgery and then reinitiated at a minimum of 4 weeks after neck dissection. Docetaxel was administered intravenously once per week during the course of radiation. A dose of 20mg/m2 was given over 60 minute infusion either alone or following bevacizumab administration. Premedication included dexamethasone 4 mg orally daily for 3 doses 24 hours prior to docetaxel, benadryl 50 mg and ranitidine 50 mg intravenously 30 minutes prior to docetaxel administration. Antiemetic agents were administered 30 minutes prior to docetaxel.
Radiation Therapy
Concurrent with chemotherapy, radiation therapy was delivered using standard once-daily fractionation, five days a week (excluding weekends and holidays). Both conventional radiation technique and intensity-modulated radiation therapy (IMRT) were allowed. For conventional technique, the primary region and adjacent lymphatic areas were treated to 39.6 Gy after which appropriate off-cord field reductions were made. The posterior neck was then treated to a total dose of 50.4 Gy. The off-cord field was given an additional 10.8 Gy. Subsequently the primary tumor and involved nodes received an additional 19.8 Gy utilizing a conformal technique. The total planned dose to the primary tumor and the involved nodes was 70.2 Gy. The supraclavicular area received 50.4 Gy in 28 fractions of 1.8 Gy. For patients treated with IMRT, the primary tumor and involved nodes were treated to 70 Gy, the high-risk lymphatic regions to 63 Gy, and the intermediate risk areas to 56 Gy. All these volumes were treated simultaneously using dose painting in 35 fractions.
Study Evaluation and Assessment of Response
Adverse events were evaluated weekly using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3. Computer tomography (CT) of the head and neck and chest (and optional fluorodeoxyglucose positron emission tomography scans) were obtained 8 to 12 weeks following completion of concurrent chemoradiation therapy, and every two months thereafter during adjuvant treatment with bevacizumab up to one year, and at the end of study. Salvage surgery was performed for patients with persistent or recurrent disease at least 8 weeks from last infusion of bevacizumab. Patients were seen monthly if they remained on adjuvant bevacizumab for up to one year, and every 3 months thereafter.
Study Objectives and Statistical Considerations
The primary objective of the study was to determine the 3 year progression-free survival (PFS) of patients with locally advanced HNSCC treated with the combination of decetaxel, bevacizumab and concurrent radiotherapy. Secondary endpoints included objective response rate, locoregional control rate, duration of response, patterns of failure, and overall survival. Based on phase II/III clinical trials of chemoradiation in the treatment of locally advanced HNSCC at the time the study was designed, it was estimated that the probability of disease-progression free survival at 3 years after treatment was about 50%. To detect an improvement in the 3-year PFS rate from 50% to 75%, the study required 27 patients (type I error of 0.05, a power of 80% and a follow-up period of 24 months after enrollment of the last patient).
Time to progression free survival was calculated from the start of treatment to the date of disease progression or the date of death, whichever comes first, and censored at the date of last follow-up for those alive and without disease progression. Overall survival (OS) was calculated from the start of treatment to the date of death and censored at the date of last follow-up for survivors. Distant metastases free survival (DMFS) was calculated from the start of treatment to the date of distant metastases and censored at the date of last follow-up for those without metastases. Local-regional failure free survival (LRFFS) was calculated from the start of treatment to the date of local-regional failure and censored at the date of last follow-up for those without local-regional failure. Probability of survival was estimated by Kaplan-Meier method and the difference of survival between groups was examined by log-rank test. All tests are two-sided and p-values ≤ 0.05 were considered statistically significant.
Results
Patient Characteristics
From December 2005 through September 2008, 30 patients were enrolled on study (27 from UHCMC and 3 from UPMC). Patient characteristics are summarized in table 1. The median age was 55.5 years (range 43 – 73) and the majority of patients were male (87%). The primary site included 20 oropharynx, 7 larynx, 2 hypopharynx, and 1 oral cavity (retromolar trigone). The majority of patients had stage IVA/B disease (80%). Primary T-stage was split between T1/T2 tumors and T3/T4 tumors at 47% and 53%, respectively. The majority of patients had advanced nodal disease (N2/N3, 73%).
Table 1.
Patient Characteristics
| Characteristic | Number of Patients |
|---|---|
| Gender | |
| Male | 26 |
| Female | 4 |
| Age | |
| median 55.5 Range 43–73 | |
| Primary Tumor Site | |
| Oropharynx | 20 |
| Larynx | 7 |
| Hypopharynx | 2 |
| Oral cavity | 1 |
| T Stage | |
| T1 | 4 |
| T2 | 10 |
| T3 | 10 |
| T4 | 6 |
| N Stage | |
| N0 | 4 |
| N1 | 4 |
| N2 | 20 |
| N3 | 2 |
| AJCC Stage | |
| III | 6 |
| IVA and IVB | 24 |
| HPV Status | |
| Positive | 12 |
| Negative | 6 |
| Unknown | 12 |
HPV status was determined retrospectively on 18 patients that had tissue available for further testing. Of these patients, 12 were HPV positive, all with oropharynx primary with the exception of one patient with supraglottic laryngeal cancer.
Treatment Delivery and Toxicity
Of the 30 patients enrolled on study, 28 completed a full course of radiation therapy. One patient developed aspiration pneumonia during radiation therapy requiring prolonged hospitalization after he had received 59.4 Gy. Due to the prolonged treatment break and the patient’s general condition, the treatment was discontinued. A second patient experienced a grade 4 oropharyngeal hemorrhage at 27 Gy requiring hospital admission and radiation therapy was discontinued at that time.
Median dose intensities of planned chemotherapy were 20 mg/m2/qwk, × 8 doses for docetaxel, and 5 mg/kg/qow, × 4 doses for bevacizumab during concurrent chemoradiation. Only 10 patients did not receive any adjuvant bevacizumab treatments. The remaining twenty patients received a median of 8 treatments (range 2 – 22).
Selected toxicities of chemoradiotherapy are shown in table 2. The most common non-hematologic grade 3 or higher toxicity was dysphagia likely related to radiation induced mucositis. There was no grade 3 or higher leukopenia however a substantial number of patients had ≥ grade 3 lymphopenia (80%). There were two episodes of grade 4 bleeding seen in the study. One patient experienced an oropharyngeal hemorrhage from the lingual artery during chemoradiation which occurred in the radiation field. A second patient experienced hemorrhagic cholecystis requiring a cholecystectomy. One patient developed a deep vein thrombosis (DVT) requiring anticoagulation. There were no grade 5 toxicities.
Table 2.
Related Toxicity
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Mucositis | 3% | 36% | 23% | 0% | 0% | 62% |
| Dermatitis | 6% | 40% | 30% | 0% | 0% | 76% |
| Dysphagia | 13% | 16% | 36% | 0% | 0% | 66% |
| Infection | 3% | 23% | 13% | 0% | 0% | 40% |
| Leukopenia | 23% | 0% | 0% | 0% | 0% | 23% |
| Lymphopenia | 0% | 3% | 80% | 3% | 0% | 86% |
| Dehydration | 3% | 10% | 6% | 0% | 0% | 19% |
| Weight Loss | 26% | 46% | 23% | 0% | 0% | 96% |
| Bleeding | 3% | 0% | 0% | 6% | 0% | 9% |
| Hypertension | 3% | 0% | 0% | 0% | 0% | 3% |
| Thrombosis | 0% | 0% | 3% | 0% | 0% | 3% |
Regarding late toxicity, one patient experienced radiation necrosis of the larynx requiring total laryngectomy. Pharyngoesophageal stenosis requiring esophageal dilatation (grade 3 dysphagia) was observed in 23% of patients.
Survival and Patterns of Failure
The median follow-up was 38 months (range: 2–64) for the whole group. At last follow-up, 21 patients were alive without evidence of disease progression. The 3 year PFS was 61.7% (95% CI: 41.5–75.7%). Of those completing chemoradiation therapy, the 3 year PFS was 66.2% (95% CI: 45–80.8%). The median progression free survival was not reached at the last follow-up (> 64 months, Figure 2). The 3 year OS was 68.2% (95% CI: 47.5–82.1%). Of those completing chemoradiation therapy, the 3 year OS was 73.2% (95% CI: 51.7–86.3%). Again, the median overall survival was not reached at last follow-up (> 64 months, Figure 3).
Figure 2.
Kaplan-Meier estimation of progression free survival with 95% confidence interval.
Figure 3.
Kaplan-Meier estimation of overall survival with 95% confidence interval.
For the analysis of patterns of failure, information was available on 27 patients. There were four locoregional failures. One patient had local recurrence only, two with both local and regional recurrences, and one with local and distant recurrences. The 3 year LRFFS was 84.5% (95% CI: 63.7–93.9%, Figure 4). All local recurrences occurred during the first two years of follow-up. The patient who had local recurrence only underwent a salvage laryngectomy and remained alive with no evidence of disease in last follow-up. The patients who had both local and regional recurrences underwent salvage surgery and postoperative re-irradiation, but both succumbed to the disease later.
Figure 4.
Kaplan-Meier estimation of local-regional failure free survival with 95% confidence interval.
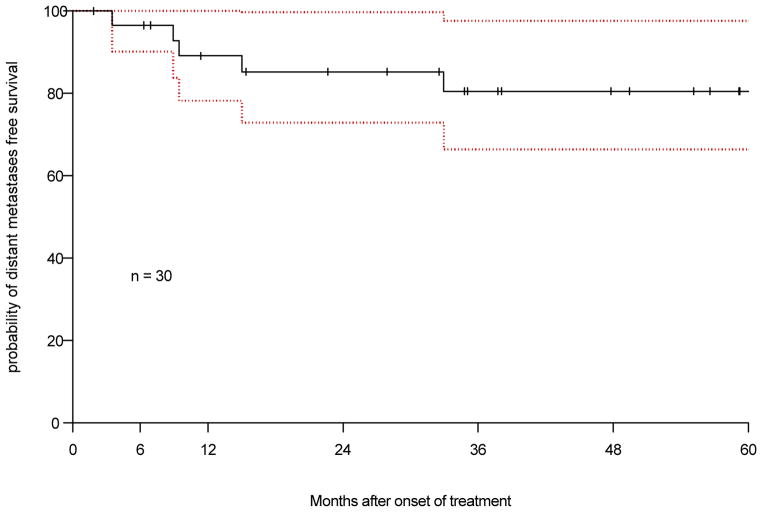
There were 6 patients developed distant metastasis, all had lung metastases. Five of these patients had distant metastases as the first and only failure. The 3 year DMFS was 80.5% (95% CI: 59–91.5%, Figure 5).
Figure 5.
Kaplan-Meier estimation of distant metastasis free survival with 95% confidence interval.
Discussion
We reported here a non-randomized phase II clinical trial using a novel combination of concurrent bevacizumab, docetaxel and radiation in the management of locally advanced HNSCC. There are four other studies that have been published recently combining bevacizumab and other chemotherapeutic agent(s) with radiation in HNSCC (16–19). Salama et al (16) in a phase II randomized trial compared 5-fluoruracil, hydroxyurea and twice-daily radiotherapy with or without bevacizumab (FHX vs. BFHX). Fury et al (17) combined bevacizumab with standard concurrent cisplatin and radiation. Haisworth et al (18) combined bevacizumab, erlotinib, paclitaxel and radiation. In their non-randomized phase II trial, patients were treated with two cycles of neoadjuvant chemotherapy with bevacizumab, carboplatin, paclitaxel and 5-fluoruracil before concurrent chemo-RT. Yoo et al (19) combined bevacizumab, erlotinib, cisplatin and radiation. Radiation treatment was given twice daily at 1.25 Gy per fraction with a 6 hours interfraction interval and a planned one week break during week 4.
Our study is the first evaluating the combination of bevacizumab and docetaxel administered concurrently with radiation therapy. In the setting of emerging data derived from RTOG 0234 showing postoperative radiation plus docetaxel plus cetuximab resulted in clinically meaningful improvement in overall survival and disease-free survival when compared to radiation plus cisplatin plus cetuximab for high-risk HNSCC (7), the need to evaluate the efficacy of bevacizumab added to a radiation therapy and docetaxel backbone is easily understood and justified.
This trial also provides valuable data on the ongoing discussion of the optimal docetaxel weekly dose given concurrently with radiation. In this trial, docetaxel administered at a weekly dose of 20 mg/m2 during concurrent radiation treatment was effective and well tolerated. Our group has also completed phase I and II studies combining radiation, docetaxel, and the anti-EGFR tyrosine kinase inhibitor erlotinib and has demonstrated that docetaxel at 20 mg/m2/week is well tolerated in combination with erlotinib and radiation (13, 14). It is of interest to note that a lower docetaxel dose (15 mg/m2/week) was selected in the phase III RTOG 1216 clinical trial.
This trial also raises the question of the appropriate dose of bevacizumab. Among the five trials, this is the only one that bevacizumab at 5mg/kg administered every 2 weeks was used, which is half of the dose used in the other 4 trials. Although there are no definitive data for the appropriate dose in combination with radiation therapy, both doses are approved to be used in combination with various cytotoxic regimens (19). We decided to use the same dose intensity (5 mg/kg administered every two weeks) used in the first in humans clinical trial evaluating the bevacizumab effects in combination with radiation and cytotoxic chemotherapy (21)
The role of adjuvant treatment and its potential duration following definitive concurrent chemoradiation is an area of active clinical research. Use of bevacizumab in the adjuvant setting to slow down metastasis and improve response to treatment is appealing and needs to be addressed in the clinical setting (22). However, planned administration of adjuvant bevacizumab was only included in the trial by Fury et al (6 months of maintenance treatment) and in our trial (12 months of maintenance treatment). However, data on the feasibility and tolerability of adjuvant bevacizumab are only available from this trial, as maintenance treatment was discontinued in an amendment after a grade 4 pulmonary hemorrhage event in patient 1 during maintenance treatment (17).
The major local side effects noted in our trial were mucositis and dermatitis. These are consistent with other reports as well as those reported in clinical trials using standard concurrent chemotherapy and radiation in HNSCC (2–4, 23, 24). This suggests that bevacizumab did not increase acute local toxicities of chemoradiation. The grade 3 dysphagia in our trial might be over-rated. We routinely placed PEG tube before treatment and the patients might tend to use tube feeding earlier in the treatment course, which is graded as grade 3 toxicity in CTCAE. There was no grade 5 toxicity in our patients. Fury et al (17) reported two deaths in 42 patients recruited in their trial and Haisworth et al (18) reported 1 death in 60 patients in their trial. Again, this is consistent with about 4% treatment-related death reported in combined chemoradiation in locally advanced HNSCC (2–4, 25).
After careful selection of 30 patients treated in this trial, two developed hemorrhage, one within the radiation field and the other outside the radiation field in the abdomen, which does not point to an unexpected toxicity profile of the combination regimen. Fury et al (17) also reported one patient with grade 4 pulmonary hemorrhage that occurred during the maintenance bevacizumab treatment after the completion of chemoradiation. Later, they amended the protocol and eliminated the maintenance bevacizumab treatment. Hemorrhage was not reported in three other published studies combining bevacizumab and radiation. In a study (26) using bevacizumab in combination of carboplatin and paclitaxel in previously untreated locally advanced and metastatic non-small cell lung cancer (without radiation), 6 patients experienced a major life-threatening bleeding (hemoptysis or hematemesis). They reported that life-threatening bleeding was associated with squamous cell histology, tumor necrosis and cavitations, and disease location close to major blood vessels. Therefore, extreme precaution is needed with strict selection criteria and early detection of any bleeding signs and symptoms when using bevacizumab.
Table 3 summarizes the treatment outcomes of published clinical trials in combination of bevacizumab and chemoradiation in locally advanced HNSCC. In our study, a good local-regional control was obtained. For those who completed radiation treatment, only 4 developed local-regional recurrences, one of them also with distant metastasis. However, there was a high distant metastasis rate with 3 year DMFS of only 80.5%, possible due to a large portion of our patients with high nodal stages (22 of 30 patients with N2/3 disease). As a result, the progress-free survival, which was the primary endpoint of the study, did not meet the expected efficacy (i.e. 3 year PFS of 75%).
Table 3.
Comparison of clinical trial combining bevacizumab and radiation in locally advanced head and neck cancer
| Study | Patient | Other Chemo Agents | BevacizumabDose | 3 Year LRFFS | 3 Year PFS | 3 Year OS |
|---|---|---|---|---|---|---|
| Salama (16) | 17 | 5 FU, Hydroxyurea | 10 mg/kg, every 2 weeks | 67% (2 year) | 59% (2 year) | 58% (2 year) |
| Fury (17) | 42 | Cisplatin | 15 mg/kg, every 3 weeks | 75.9% (2 year) | 88% (2 year) | |
| Hainsworth (18) | 60 | Paclitaxel Erlotinib | 15 mg/kg, every 3 weeks | 71% | 82% | |
| Yoo (19) | 29 | Cisplatin Erlotinib | 10 mg/kg, every 2 weeks | 85% | 82% | 86% |
| Current study | 30 | docetaxel | 5 mg/kg, every 2 weeks | 84.5% | 61.7% | 68.2% |
Abbreviation: LRFFS, local-regional failure free survival; PFS, progression-free survival; OS, overall survival.
Salama et al (16) terminated their randomized phase II study early due to an unexpected high locoregional recurrence in the bevacizumab arm. After 26 patients entered to the study, four of five T4 patients on the BFHX arm had locoregional disease progression versus zero of two patients on the FHX arm. They reported 2 year locoregional control was 67% in the BFHX arm and 100% in the FHX arm, and 2 year PFS was 59% in the BFHX arm versus 89% in the FHX arm. For patients treated in the BFHX arm, the doses of 5-fluoruracil and hydroxyurea had to been reduced compared to their standard doses in FHX regimen, which was required due to elevation of hepatic enzymes. These dose modifications may cause reduction of the radiosensitizing effect of the BFHX regimen.
Fury et al (17) and Yoo et al (19) incorporated bevacizumab in standard concurrent cisplatin and radiotherapy regimen. They reported encouraging treatment outcomes as compared to recent reports from RTOG clinical trials with cisplatin and radiation in HNSCC (23, 24). RTOG 0129 (23) is a phase III randomized trial comparing concurrent cisplatin with an accelerated concomitant boost (AFX-C) versus standard fractionation radiation (SFX) in locally advanced HNSCC. From July 2002 to May 2005, 743 cases were entered. There were no differences observed between two arms in 5 year overall survival (59% vs. 56%; p=0.18), 5 year disease-free survival (45% vs. 44%; p=0.42), 5 year local-regional failure (31% vs. 28%; p=0.76), or 5 year metastasis (18% vs. 22%; p=0.06). RTOG 0522 (24) is a randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for locally advanced HNSCC. In analysis of 895 entered the trial with a median follow-up of 2.4 years for surviving patients, 2 year progression-free survival was 63% vs. 64% (with cetuximab vs. without) and 2 year overall survival was 83% vs. 80% (with cetuximab vs. without), both without significant differences. Both Fury et al (17) and Yoo et al (19) reported better PFS and OS than the RTOG experience with cisplatin and radiation in locally advacned HNSCC. However, these were single institutional studies with a limited patient sample. Multi-institutional randomized studies with larger patient sample are needed to confirm these findings.
Although our study did not meet the primary pre-specified 3-year PFS of 75%, it is well recognized that patient selection and disease heterogeneity can significantly influence outcomes. Our results do not appear greatly different from RT/cisplatin as reported in RTOG 0129 and RTOG 0522. This regimen appears to be worthy of further study for the appropriate selected subgroup of patients, especially for those who are not candidates for cisplatin administration.
Acknowledgments
Supported in part by: Genentech, NIH grants P30 CA43703 and M01 RR-000080
Footnotes
Presented in part at: ASTRO 53rd Annual Meeting Miami Beach, Fla 2011.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharyngeal carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Adelstein D, Saxton J, Lavertu P, et al. Mature Results of A phase III randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Cancer. 2000;88(4):876–883. [Google Scholar]
- 4.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced larynx cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: Three meta-analyses of updated individual data. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 6.Pignon JP, le Maître A, Maillard E, et al. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Kies MS, Harris J, Rotman MZ, Myers JN, Foote RL, Machtay M, Khuntia D, Straube WL, Anq KK, Harari PM. Phase II Randomized Trial of Postoperative Chemoradiation Plus Cetuximab for High-risk Squamous Cell Carcinoma of the Head and Neck (RTOG 0234) Int J Rad Onc. 2009;75(3):S14–15. [Google Scholar]
- 8.RTOG 1216
- 9.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 10.Kyzas PA, Cunha IW, Ionnidis JPA. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434–1440. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 11.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 12.Sweeney CJ, Miller KA, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- 13.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of action. Clin Cancer Res. 2003;9:1957–1971. [PubMed] [Google Scholar]
- 14.Savvides P, Argiris A, Greskovich J, Bokar J, Hoppel C, Rezaee R, Fu P, Wasman J, Lavertu P, Remick S. Phase I study of the EGFR tyrosine kinase inhibitor erlotinib in combination with docetaxel and radiation in locally advanced squamous cell cancer of the head and neck (SCCHN). AACR Annual meeting; Los Angeles CA. April 2007. [Google Scholar]
- 15.Savvides P, Yao M, Rezaee R, Bokar J, Fu P, Wasman J, Sarlis N, Dowlati A, Machtay M, Lavertu P. Phase II study of Erlotinib in combination with Docetaxel and Radiation in locally advanced squamous cell cancer of the head and neck (SCCHN). AHNS 2010 Research Workshop on the biology, Prevention and Treatment of Head and Neck Cancer; Arlington, VA. October 28–30, 2010; (Oral presentation) [Google Scholar]
- 16.Salama JK, Haraf DJ, Stenson KM, et al. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compated with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancer. Ann Oncol. 2011;22:2304–2309. doi: 10.1093/annonc/mdq736. [DOI] [PubMed] [Google Scholar]
- 17.Fury MG, Lee NY, Sherman E, et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer. 2012;118:5008–5014. doi: 10.1002/cncr.27498. [DOI] [PubMed] [Google Scholar]
- 18.Hainsworth JD, Spigel DR, Greco FA, et al. Combined modality treatment with chemotherapy, radiation therapy, bevacizumab, and erlotinib in patients with locally advanced squamous carcinoma if the head and neck. Cancer J. 2011;17:267–272. doi: 10.1097/PPO.0b013e3182329791. [DOI] [PubMed] [Google Scholar]
- 19.Yoo DS, Kirkpatrick JP, Craciunescu O, et al. Prospective trial of synchronous bevacizumab, erlotinib, and concurrent chemoradiation in locally advanced head and neck cancer. Clin Cancer Res. 2012;18:1404–1414. doi: 10.1158/1078-0432.CCR-11-1982. [DOI] [PubMed] [Google Scholar]
- 20. [accessed on 10/25/2013];Avastin (Bevacizumab) Prescribing information. http://www.gene.com/download/pdf/avastin_prescribing.pdf.
- 21.Willett CG, Boucher Y, Di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004 Feb;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Reviews. 2011;10:417–27. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 23.Ang KK, Pajak T, Wheeler R, et al. A phase III trial to test accelerated versus standard fractionation in combined with concurrent cisplatin for head and neck carcinoma (RTOG 0129): report of efficacy and toxicity. Int J Radiat Oncol Biol Phys. 2010;77:1–2. [Google Scholar]
- 24.Ang KK, Zhang QE, Rosenthal DI, et al. A randomized phase III trial (RTOG 0522) of concurrenr accelerated radiation plus cisplatin with or without cetuximab for stage III–IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29(suppl):360s. doi: 10.1200/JCO.2013.53.5633. abstr 5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garden AS, Harris J, Vokes EE, et al. Preliminary results of radiation Therapy Oncology Group 97-03: a randomized phase II trial of concurrent radiation and chemotherapy for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22:2856–2864. doi: 10.1200/JCO.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]