Abstract
Background
The role of Plasmodium falciparum malaria in EBV transmission among infants early in life remain elusive. We hypothesized that infection with malaria during pregnancy could cause EBV reactivation leading to high EBV load in circulation, which could subsequently enhance early age of EBV infection.
Method
Pregnant women in Kisumu, where P. falciparum malaria is holoendemic, were actively followed monthly through antenatal visits (up to 4 per mother) and delivery. Using real-time quantitative (Q) – PCR, we quantified and compared EBV and P. falciparum DNA levels in the blood of pregnant women with and without P. falciparum malaria.
Results
Pregnant women that had malaria detected during pregnancy were more likely to have detectable EBV DNA than pregnant women who had no evidence of malaria infection during pregnancy (64% vs. 36%, p=0.01). EBV load as analyzed by quantifying area under the longitudinal observation curve (AUC) was significantly higher in pregnant women with P. falciparum malaria than in women without evidence of malaria infection (p =0.01) regardless of gestational age of pregnancy. Increase in malaria load correlated with increase in EBV load (p <0.0001). EBV load was higher in third trimester (p =0.04) than first and second trimester of pregnancy independent of known infections.
Conclusion
Significantly higher frequency and elevated EBV loads were found in pregnant women with malaria than in women without evidence of P. falciparum infection during pregnancy. The loss of control of EBV latency following P. falciparum infection during pregnancy and subsequent increase in EBV load in circulation could contribute to enhanced shedding of EBV in maternal saliva and breast milk postpartum, but further studies are needed.
Keywords: EBV load, mother, pregnancy, Plasmodium falciparum malaria
Introduction
Epstein–Barr virus (EBV) is a human gammaherpesvirus that infects and persists in >90% of the world adult population clinically manifesting either as an asymptomatic infection or as acute infectious mononucleosis (1, 2). However, EBV infection is also associated with malignant transformation of infected cells and is etiologically linked to endemic Burkitt's lymphoma (eBL), the most common childhood cancer in equatorial Africa(3, 4). Previous epidemiologic studies (5-7) as well as recent studies done in Kenya (7) reported a striking overlap between elevated incidence of malaria transmission and eBL, and confirmed that holoendemic malaria was associated with an increased risk for eBL.
Geographical differences in age of primary EBV infections exist with infants in sub-Saharan Africa getting infected early in life than their counterparts in the developed world where infections occur in adolescence and adulthood (8, 9). We have previously reported that there is a significantly earlier age of primary EBV infection in infants from Kisumu – a malaria holoendemic region of Kenya – compared to infants from Nandi, an area with little malaria transmission (9), suggesting a role for malaria in increasing EBV risk in early infancy. Strikingly, 35% of children in Kisumu were infected before 6 months of age (9), supporting the long-held hypothesis that EBV infection early in life and holoendemic malaria are risk factors for eBL (34).
Cellular immunity, particularly CD8+ cytotoxic T lymphocytes (CTL) play a pivotal role in the immunosurveillance of EBV infections (10-12). As pregnancy is thought to be a state of repressed cellular immune responses (13, 14), it is possible that the immunological control of EBV infections may be naturally altered. Using serology as a marker of EBV reactivation, early studies showed EBV reactivation during pregnancy (15-17). However, Meyohas et al (18) measured EBV DNA in pregnant women and found no significant differences in the prevalence of EBV DNA in pregnant women than non-pregnant women. Moreover, EBV has rarely been implicated in in utero infection and few studies reported the risk of mother-to-child transmission of EBV (18, 19). Pregnant women are at a greater risk of infection with P. falciparum with substantial adverse effects on both maternal and fetal health (20, 21). P. falciparum malaria is known to interfere with both EBV biology and EBV-specific immunity (11, 22, 23) and can induce EBV reactivation (24, 25). To the best of our knowledge, no study has been done on the impact of malaria on EBV reactivation in pregnant women residing in malaria holoendemic areas where many women are exposed to malaria throughout the course of their pregnancies (20).
Though both EBV infection early in life and holoendemic malaria are known risk factors for eBL, why children are infected early in life with EBV, and what role malaria plays in EBV transmission remain elusive. We hypothesized that infection with malaria during pregnancy could cause EBV reactivation leading to high EBV load in circulation, which in turn enhance early age of EBV infection. In this prospective study in Western Kenya where malaria is endemic and the eBL risk is high, pregnant women were actively followed through delivery, to test the hypothesis that P. falciparum malaria during pregnancy could cause EBV reactivation. Our data describe the frequency and quantity of EBV in pregnant women with and without P. falciparum infection and suggest that women infected with malaria during pregnancy were more likely to have indicators of EBV reactivation compared to women without evidence of P. falciparum infection.
Methods
Study participants
Pregnant women, of all gravidities, attending the antenatal clinic (ANC) were recruited from Chulaimbo sub-district hospital, in Kisumu District of western Kenya. The hospital serves predominantly rural populations. Transmission of malaria in this area is holoendendemic (e.g. perennial and intense malaria transmission) with two seasonal peaks, June to August and November to December (22, 26). Plasmodium falciparum malaria species is the predominant species in the study region (27). All pregnant women were tested for HIV as part of Maternal-to-Child-Transmission (MCTC) of HIV programs in accordance with the Kenya Ministry of Health national guidelines.
The inclusion criteria consisted of pregnant women of all gravidities, less than 30 weeks gestation, having a normal full blood count, HIV negative, residence within a 10km distance of the hospital and willing to return to the clinic for follow up clinical procedures and laboratory testing. Gestational age was evaluated by measurement of fundal height and history of the last menstrual period. All pregnant women were enrolled in a six-month period from June to November 2011. As part of the study participant's health care, the pregnant women were closely monitored for any illness and all cases were reported to the study clinicians for treatment as per Kenya Ministry of Health guidelines. A total of 200 pregnant women were screened for inclusion into the study. Of these, 25 were HIV positive and were excluded. The remaining 175 pregnant women were longitudinally evaluated in an active monthly follow-up visits through antenatal visits (up to 4 per mother) and delivery. Of these, there were 93 pregnant women that delivered at Chulaimbo sub-district hospital and had samples collected at delivery.
Pregnant women enrolled showed no signs of symptomatic malaria during the follow up visits
All pregnant women were provided with insecticide treated bed nets, intermittent preventive treatment and deworming during pregnancy in accordance with the Kenya Ministry of Health guidelines.
Approval of this study was obtained from both the Kenya Medical Research Institute and SUNY Upstate Medical University Ethical Review Boards. Written informed consent was obtained from the study participants before any sample collection.
Sample collection and processing
At enrolment, participants provided 2–4 ml of venous blood sample collected in EDTA tubes and measurements of hemoglobin (Hb) levels were determined using a portable β-hemoglobin photometer (Hemocue AB Angelholm, Sweden), while complete blood counts were performed with a Beckman coulter AcT diff2 (Beckman-coulter corporations, Miami, FL, USA), calibrated regularly according to the manufacturer's instructions and use of appropriate controls. Urine and stool samples were obtained and examined for schistosoma hematobium and for the presence of intestinal parasites (pathogenic protozoans and helminthes) respectively. During follow-up visits, finger-prick blood was collected in EDTA tubes for malaria surveillance and EBV load. Less than 24 hours after delivery, mothers provided 2–4 ml of venous blood sample collected in EDTA tubes for malaria diagnosis, and EBV load.
All samples were transported to the SUNY Upstate research laboratory located at the Center for Global Health Research, KEMRI in Kisumu for processing within 1 hour of blood collection. The plasma fraction was separated from peripheral blood cells by centrifugation and aliquots were then stored at -80°C until use.
Detection of P. falciparum Infection
Because real-time quantitative (Q)-PCR has high sensitivity and allows for identification, quantification and speciation of malaria parasite(28, 29), we performed Q-PCR on DNA extracted from peripheral blood as described previously (28).
Measurement of EBV load
DNA was extracted from 200 µL of whole blood by use of a Qiagen DNAeasy kit (Qiagen, Valencia CA), in accordance with the manufacturer's protocol. DNA was eluted off the column in an equivalent volume of elution buffer and stored at -20°C. EBV DNA levels were determined using primers and probes designed to detect a 70 base pair region of the EBV BALF5 gene and β-actin gene as a control for DNA input using Q– PCR as described previously (9, 22). EBV load was normalized to the number of beta-actin DNA multiplexed and then calculated on the basis of copies of EBV genome per microgram of DNA.
Measurement of EBV-VCA antibodies
Plasma samples were analyzed for the presence and titers of antibodies to EBV-specific IgG Viral capsid antigen (VCA) using a synthetic peptide based enzyme-linked immunosorbent assay (ELISA) for immunodominant epitopes derived from VCA-p18 as described previously (23, 30). The cut-off value was defined as a mean of OD from EBV negative control, plus three standard deviations.
Statistical analysis
All statistical analyses were performed using Stata, IC software (13.1, StataCorp LP, College Station, TX) and setting 2-tailed alpha to reject the null hypothesis at 0.05. Differences in proportions were evaluated using Fisher'exact tests. Malaria and EBV viral load data were measured longitudinally during pregnancy, with each observation contribution to an overall area under the curve (AUC), (by trapezoidal methods) for statistical analysis(31). These AUC data were correlated using the Somer's D non-parametric measure of association for our primary hypothesis testing. Somer's D was also used for comparing EBV AUC between women with and without any positive malaria test results. Subsequent comparisons of repeated EBV viral load observations during the first and second versus third trimesters of pregnancy were log-transformed and compared via unpaired t-tests.
Results
Demographic, and other characteristics of study participants at enrolment
To investigate the impact of malaria on EBV reactivation during pregnancy, pregnant women from the Chulaimbo Sub-District Hospital in the Kisumu District of western Kenya were enrolled in this study. The hospital serves a predominantly rural population and malaria transmission in this area is holoendemic (26). The data obtained from 175 HIV-negative women were analyzed. The demographic, obstetric, clinical and laboratory characteristics of the study participants are presented in Table 1. The women had a mean age of 22.3 years (SD [5.6]) and 40% of the women were primigravidae. The majority (58%) of the women had at least upper primary level of education, with 82% being of the Luo ethnic group. Sixty five percent of the women were married.
Table 1. Demographic, and other characteristics of the participants (n=175) at enrolment.
| Characteristics | Summary |
|---|---|
| Mean age in years (± S.D.) at enrolment | 22.3 (5.6) |
|
| |
| Education | |
|
| |
| Lower Primary | 20%(35) |
| Upper Primary | 58%(101) |
| Secondary | 16%(28) |
| Village Polytechnic | 1% (1) |
| Other | 5%(10) |
|
| |
| Ethnic group | |
|
| |
| Luo | 82% (144) |
| Luhya | 14% (26) |
| Kalenjin | 1% (1) |
| Other | 2% (4) |
|
| |
| Marital Status | |
|
| |
| Single | 31% (55) |
| Married | 65% (114) |
| Separated | 1% (1) |
| Widowed | 2% (3) |
| Widowed, inherited | 1% (1) |
|
| |
| Parity | |
|
| |
| Multiparous | 60%(105) |
| Primiparous | 40%(70) |
|
| |
| Gestation Age at enrolment | |
|
| |
| 1st trimester (<14 weeks) | 15%(27) |
| 2nd trimester (14-27 weeks) | 61%(107) |
| 3rd trimester (≥28 weeks) | 24%(41) |
|
| |
| Mean hemoglobin (g/dl) (± S.D) at enrolment | 10.9(1.7) |
|
| |
| EBV seroprevalence by ELISA VCA positive at enrolment | 100%(175) |
|
| |
| EBV DNA prevalence by q-PCR at enrolment | 44%(77) |
|
| |
| P. falciparum malaria prevalence at enrolment by qPCR | 33% (57) |
| Prevalence of helminthes infections at enrolment* | 10%(18) |
|
| |
| Prevalence of pathogenic protozoan Infections at enrolment** | 27%(47) |
|
| |
| P. falciparum and helminthes co-infections | 7%(13) |
Prevalence of specific helminthes infections: Hookworm (n=9 [5%]); Ascaris lumbricoides (n=4 [2%]); Trichuris trichiura (n=3 [2%]); Schistosoma mansoni (n=2 [1%]).
Pathogenic protozoan Infections: Trichomonas vaginalis (n=5 [3%]), Entamoeba histolytica (n=29 [17%]), and Giardia lamblia (n=13 [7%]).
To determine the prevalence of EBV in the study participants, we tested for EBV-VCA antibodies – a marker for past EBV infection – by ELISA and EBV DNA by Q-PCR. All (100%) participants were EBV seropositive, confirming results of previous studies that showed a high rate of EBV seroprevalence among adults in Africa (22). In addition, EBV DNA was detectable in blood in 44% of study participants at enrolment.
Because Q-PCR has high sensitivity and has allowed for identification, quantification and speciation of malaria parasite (28, 29), we next tested for P. falciparum infection using Q-PCR assay. We found that 33% of the pregnant women at enrolment were Q-PCR positive for P. falciparum. This is consistent with previous reported malaria PCR prevalence of 33.1% among pregnant women from Western Kenya (32). The prevalence of intestinal helminthes and pathogenic protozoans were 10% and 27% respectively.
Malaria status of study participants during pregnancy
We actively followed the pregnant women monthly through antenatal visits (up to 4 per mother) and delivery and assessed whether they had P. falciparum malaria infection at any time during their antenatal visits or at delivery. We observed that 94 (54%) of the pregnant women had malaria, i.e. at least one positive Q-PCR P. falciparum infection test during follow-up. One hundred and fifty-three of 617 (25%) total follow-up visits had P. falciparum malaria infection as detected by Q-PCR.
Because of the variation in maternal age, parity, bed net use and gestational age influencing the prevalence of malaria among pregnant women (33, 34), we tested whether these variables had an impact on the prevalence of P. falciparum infection in our cohort. We found no statistically significant associations between pregnant women who had P. falciparum malaria detected versus those who had no evidence of P. falciparum infection at anytime during pregnancy relative to their age, hemoglobin level, parity, bed net use and gestational age at enrolment (Table 2).
Table 2. Malaria Status of Pregnant Women During Pregnancy Versus Demographic, obstetric, clinical and laboratory history at follow-up.
| Variable | Had malaria detected during pregnancy, 94(54%) | Had no evidence of malaria infection during pregnancy, 81(46%) | P value* |
|---|---|---|---|
| Mean Gestation Age, weeks (±SD) at enrolment | 21.3 ± 6.4 | 21.4±6.3 | 0.93 |
| Parity | |||
| Primiparous | 41(58.6%) | 29(41.4%) | 0.35 |
| Multiparous | 53(50.4%) | 52(49.5%) | |
| Hemoglobin (Hb) level at enrolment** | |||
| Moderate Anemia (Hb <11g/dl) | 49(56.3%) | 38(43.7%) | 0.54 |
| No Anemia (Hb ≥11g/dl) | 45(51.1%) | 43(48.9%) | |
| Age at enrolment | |||
| <20 years | 46(57.8%) | 36(43.9%) | 0.66 |
| 20 – 30 years | 38(50.0%) | 38(50.0%) | |
| >30 years | 10(58.8%) | 7(41.2%) | |
| Bed net use at enrolment | |||
| Yes | 71(53%) | 64(47%) | 0.71 |
| No | 23(57%) | 17(43%) | |
| EBV DNA Prevalence at enrolment | |||
| EBV DNA Positive | 50(64%) | 28(36%) | 0.01 |
| EBV DNA Negative | 44(45%) | 53(55%) | |
All estimated using Fisher's exact test except gestation age, which was estimated by unpaired t-test. All tests of statistical significance are two sided.
Cut-off as defined by the World Health Organization (WHO) for pregnant women(35)
Prevalence of EBV DNA is higher in pregnant women with evidence of P. falciparum infection compared to those without P. falciparum infection
To assess whether the frequency of EBV DNA detection differs with P. falciparum infection status at enrolment, we performed EBV DNA detection by Q-PCR. We observed that pregnant women who had detectable P. falciparum infection were more likely to have a detectable EBV DNA at enrolment than pregnant women who had no evidence of P. falciparum infection during pregnancy (64% vs. 36%, p=0.01), Table 2.
EBV load is higher in the third trimester among pregnant women without known infections
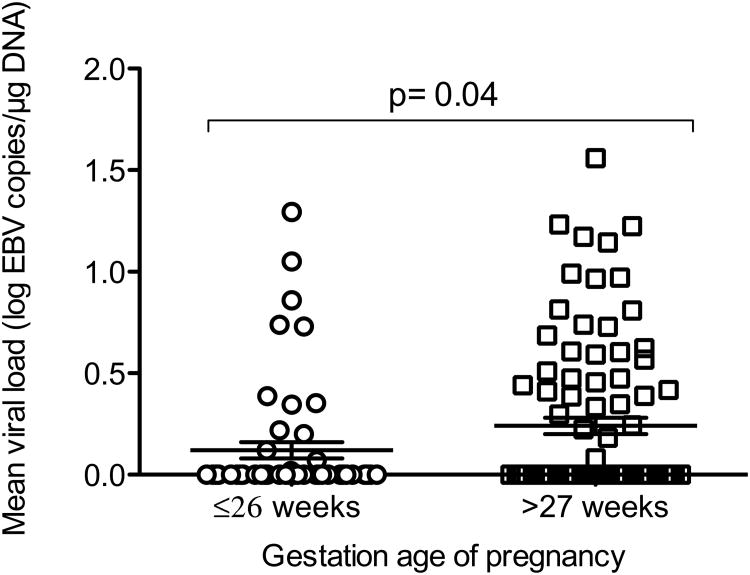
To investigate the dynamics of EBV load in pregnancy independent of known infections, we measured EBV load in whole blood by Q-PCR. We included in the analysis only pregnant women with no known infections: P. falciparum, helminthes and pathogenic protozoans (n=44). Because the resultant data had few counts at first semester, we combined the data for first and second trimester (≤26 weeks of gestation). We next categorized the data according to gestational age, ≤26 weeks versus >27 weeks, and compared the log transformed EBV load data among groups using unpaired t-tests. We found higher EBV load at >27 weeks of gestation (p= 0.04) relative to the ≤26 weeks of gestation (figure 1).
Figure 1.
Comparison of the levels of EBV load in ≤26 weeks of gestation versus >27 weeks of gestation (n=44), independent of known infections. We excluded EBV load data of pregnant women with known infections during pregnancy. Then we categorized the data according to gestational age (first and second, versus third trimesters) and compared the log transformed EBV load data among groups using unpaired t-tests. P value of unpaired t-test test is indicated in the figure.
EBV viral load is not affected by presence of anemia
A significant portion of the pregnant women in the study were identified as having mild anemia (Table 2). Because anemia could be caused by a number of factors that potentially modulate EBV viral load, pregnant women were categorized into non-anemic (Hb ≥11) and anemic Hb <11 g/dl (anemic) as per WHO classification of anemia (35). None of the study participants had Hb <7.5 g/dl (for severe anemia), hence the anemia documented in our participants were all mild to moderate anemia. Next, we compared the EBV load of pregnant women with (n=87) and without anemia (n=88) at enrolment. We found no significant difference in EBV load between pregnant women with and without anemia (p= 0.88, by Mann–Whitney U test). Furthermore, to control for the effect of infections during pregnancy on anemia, we excluded data of pregnant women with known infections: malaria, helminthes and pathogenic protozoan and then compared EBV load of pregnant women with anemia (n=47) and those without anemia (n=50). Again we found no significant difference in EBV load between women with and without anemia, independent of known infections (p= 0.73, by Mann–Whitney U test).
EBV load is higher in pregnant women with P. falciparum infection compared to those without P. falciparum infection
To determine whether there were differences in levels of EBV load between pregnant women with or without P. falciparum infection during pregnancy, we measured EBV viral load and P. falciparum in whole blood by Q-PCR. We then compared levels of EBV load of pregnant women that had P. falciparum detected during pregnancy, i.e., at least one positive Q-PCR test during pregnancy, to those that had no evidence of P. falciparum infection during pregnancy using EBV load area under the longitudinal observation curve (AUC). AUC analysis is a method for evaluating the overall response of a continuously scaled outcome that can increase and decrease over time. Repeated observations of detectable EBV load were recorded throughout the course of study, and these values for any given individual may increase or decrease given their exposure and immune response. In order to “capture” the effect of their exposure and immune response in an overall sense, we literally calculate the areas under the curve that would be drawn for each individual given all of their recorded viral loads, and used this one number (their area under the curve) to represent the entirety of their viral challenge (31). We found that EBV load as analyzed by quantifying AUC was significantly higher in women that had malaria detected than in women that had no evidence of malaria infection during pregnancy (p= 0.01).
Increase in P. falciparum load is associated with increase in EBV load
We next assessed whether P. falciparum load correlated with EBV load AUC. Since these data are highly skewed and zero-inflated we used a non-parametric measure of association (Somers d) (31). We found a highly significant positive association: increase in P. falciparum load correlated with increase in level of EBV load (p <0.0001).
EBV viral load is higher in pregnant women with P. falciparum infection Independent of gestation age of pregnancy
Because pregnancy associated malaria morbidity is gestational age dependent where the prevalence of malaria infection and parasite density are highest during the first half of pregnancy with gradual decline during the second half (33, 34); we assessed whether there was gestational age-related difference in EBV load between pregnant women with and without evidence of P. falciparum infection during pregnancy. To eliminate the confounding effect of known infections e.g. helminthes and pathogenic protozoans, we excluded from analysis data of pregnant women with any known infections besides P. falciparum malaria. We then categorized the data according to gestational age, ≤26 weeks versus >27 weeks relative to P. falciparum infection status. Median EBV load was higher at both ≤26 weeks (p= 0.02) and >27 weeks (p= 0.05) of gestation in pregnant women with malaria in comparison to women without malaria.
Discussion
Despite the well-established link and our recent support of the long-held hypothesis that EBV infection early in life and holoendemic malaria being risk factors for eBL (9, 36), little is known about why children are infected early in life with EBV, and what role malaria plays in EBV transmission. We hypothesized that infection with malaria during pregnancy could cause EBV reactivation leading to high EBV load in circulation, which could subsequently enhance early infant age of EBV infection. To test the first part of our hypothesis, we conducted a cohort study, which actively followed up pregnant women during pregnancy, and categorized these women according to their malaria history during pregnancy. We observed that significantly higher frequency and elevated EBV loads were found in pregnant women with malaria compared to women without malaria during pregnancy independent of gestational age of pregnancy.
Plasmodium falciparum malaria has been linked to EBV reactivation in children following acute malaria infection(24). In addition, in vitro studies show that P. falciparum can induce reactivation of EBV latently infected B cells (37). Thus, the correlation of elevated EBV loads in woman infected with P. falciparum during pregnancy could be due to a direct effect of P. falciparum malaria on EBV infected B cells. Alternatively, immune control of EBV is critical in maintaining stable viral loads (38) and because pregnancy itself could modulate immune function especially T-cell functions, which appear to be down-regulated during pregnancy (13, 14), it is plausible that the synergistic effect of P. falciparum malaria and pregnancy could significantly alter and shift the dynamic balance between EBV and the host immunity in favor of the virus. Moreover, increased malaria load was associated with increased EBV load, suggesting that the level of EBV load is proportional to the parasite density. This dovetails with our previous findings of increased viral load in peripheral blood of children living in a malaria holoendemic area, an area characterized by high parasite densities, compared to children living in malaria sporadic area (22).
Consistent with our findings, previous studies (24, 39, 40) albeit in children, reported significantly elevated peripheral blood EBV DNA loads – indicative of viral reactivation – following malaria infection, suggesting that malaria has inherent effects on EBV-host balance. The possible mechanisms through which P. falciparum malaria induce EBV reactivation is through a multiplicity of pathways: an interaction between a specific malaria antigen, the cysteine rich interdomain 1 alpha (CIDR1α) of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) protein, and B cells (41); leading to activation of memory B cells, which in turn results in increased production of EBV in virus-positive cell line (37). Secondly, expansion of latently infected B cells during acute malaria has also been postulated (12, 39). Thirdly, malaria parasite is also known to possess unmethylated CpG DNA, a ligand for Toll like receptor (TLR) 9 (42), which can potentially enhance EBV induced proliferation and activation of B cells (43).
Although induction of EBV reactivation during pregnancy following P. falciparum infection seen in our cohort may not result in apparent EBV-related diseases in pregnant women, the significant increase of EBV load in the maternal circulation may have ramifications for their infants early in life. One possible consequence of this is the increased shedding of infectious EBV in maternal saliva and possibly breast milk postpartum, the probable routes of EBV transmission. Moreover, pregnancy associated P. falciparum malaria not only influences pregnancy outcomes but also the development of neonatal immune responses (44-47) suggesting that the ability of infants – born to mothers that had malaria during pregnancy – to effectively control EBV and other infections early in life may be impaired. We previously found that 35% of children in this area were infected before 6 months of age and that there was evidence of repeated infections with EBV during the first year of life, implying a failure to control the virus in infants living in malaria holoendemic area (9).
We observed higher EBV load during the third trimester of pregnancy independent of known infections, suggesting that the dynamics of EBV load during pregnancy is affected by gestational age of pregnancy. A rather intriguing report of increase of CD4+ CD25+ T regulatory cells (Tregs) at early pregnancy, dramatically peaking during the second and third trimesters, with subsequent decline postpartum (48), may perhaps explain the observed phenomenon. Tregs are known potent suppressors of T-cell functions (49), and T cells particularly CD8+ cytotoxic T lymphocytes (CTL) play a pivotal role in maintenance of persistent EBV infections (10-12). Thus, elevated EBV load observed during the last phase of pregnancy could have been secondary to increased levels of Tregs, which in turn compromised host control of EBV persistence.
Previous studies reported pregnancy associated malaria morbidity being dependent on covariates such as, parity, bed net use and gestational age of pregnancy (33, 34), however, we found no significant association between these variables and malaria in our cohort. This lack of finding is not surprising as we were investigating the impact of malaria on EBV reactivation and we, therefore, accordingly selected for only pregnant women with normal blood count and no apparent known infections besides malaria. The rather stringent inclusion criteria we employed may have consequently circumvented the prospects of finding any significant association with the foregoing variables.
Earlier studies to examine the effects of pregnancy on EBV persistence only focused on healthy pregnant women from the developed world, often correlating EBV reactivation with adverse pregnancy outcomes (50, 51), seldom examining in the context of infectious diseases such as malaria. Though using different approach in determining EBV reactivation, some studies (15-17) but not others (18, 51) reported increased EBV reactivation during pregnancy among pregnant women.
The potential strength of this study is that we actively followed up pregnant women monthly during pregnancy through to delivery, and categorized them according to their malaria history. We were therefore able to document accurate malaria infection histories for each pregnant woman. Secondly, because pregnancy itself could modulate immune functions, we compared one group of pregnant women with malaria with another without malaria, with a view to control for the possibility of pregnancy itself playing a confounding role and having an influence on the results. Lastly, we used Q-PCR for malaria detection, which in addition to detecting submicroscopic parasitaemia, also allows for accurate quantification as well as speciation of malaria parasites.
In summary, significantly higher frequency and elevated EBV loads were found in pregnant women with malaria than in women without malaria during pregnancy. It remains to be determined whether the loss of control of EBV latency following P. falciparum malaria during pregnancy and subsequent increase of EBV load in circulation could contribute to enhanced shedding of EBV in saliva and breast milk postpartum; probable conduits of EBV transmission in infants early in life. In addition, because malaria during pregnancy may alter the development of neonatal immune responses, it remains to be determined whether the ability of infants to control EBV early in life is impaired in infants born to mothers that had malaria during pregnancy.
Acknowledgments
Funding was provided through National Cancer Institute CA102667 (RR) and National Institute of Allergy and Infectious diseases AI 098511 (AD) at the National Institutes of Health. I. I. Daud is a doctoral trainee supported by a D43 training grant (NCI 153707). AD was supported by Burroughs Wellcome CAMS 1006818. We would like to acknowledge the Chulaimbo Sub-District Hospital for allowing us to use their facilities to perform this study. We also thank our clinical officers, medical officers, data entry and field staff involved in the project. We are particularly grateful to the mothers for their participation in this study. This manuscript has been approved for publication by the Director of the Kenya Medical Research Institute.
Footnotes
The authors do not have any commercial or other association that might pose a conflict of interest.
References
- 1.Silins SL, Sherritt MA, Silleri JM, et al. Asymptomatic primary Epstein-Barr virus infection occurs in the absence of blood T-cell repertoire perturbations despite high levels of systemic viral load. Blood. 2001;98(13):3739–44. doi: 10.1182/blood.v98.13.3739. [DOI] [PubMed] [Google Scholar]
- 2.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 3.Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annual review of pathology. 2006;1:375–404. doi: 10.1146/annurev.pathol.1.110304.100209. [DOI] [PubMed] [Google Scholar]
- 4.Magrath I. The pathogenesis of Burkitt's lymphoma. Adv Cancer Res. 1990;55:133–270. doi: 10.1016/s0065-230x(08)60470-4. [DOI] [PubMed] [Google Scholar]
- 5.Dalldorf G. Lymphomas of African children. JAMA. 1963;183:619–20. doi: 10.1001/jama.1963.03700070147030. [DOI] [PubMed] [Google Scholar]
- 6.Morrow RH., Jr . Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt's lymphoma. 60. IARC Sci Publ; 1985. pp. 177–86. [PubMed] [Google Scholar]
- 7.Rainey JJ, Mwanda WO, Wairiumu P, et al. Spatial distribution of Burkitt's lymphoma in Kenya and association with malaria risk. Trop Med Int Health. 2007;12(8):936–43. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 8.Biggar RJ, Henle G, Bocker J, et al. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int J Cancer. 1978;22(3):244–50. doi: 10.1002/ijc.2910220305. [DOI] [PubMed] [Google Scholar]
- 9.Piriou E, Asito AS, Sumba PO, et al. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis. 2012;205(6):906–13. doi: 10.1093/infdis/jir872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callan MF. The evolution of antigen-specific CD8+ T cell responses after natural primary infection of humans with Epstein-Barr virus. Viral Immunol. 2003;16(1):3–16. doi: 10.1089/088282403763635401. [DOI] [PubMed] [Google Scholar]
- 11.Moormann AM, Chelimo K, Sumba PO, et al. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis. 2007;195(6):799–808. doi: 10.1086/511984. [DOI] [PubMed] [Google Scholar]
- 12.Njie R, Bell AI, Jia H, et al. The effects of acute malaria on Epstein-Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J Infect Dis. 2009;199(1):31–8. doi: 10.1086/594373. [DOI] [PubMed] [Google Scholar]
- 13.Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21(24):3352–7. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- 14.Motran CC, Diaz FL, Gruppi A, et al. Human pregnancy-specific glycoprotein 1a (PSG1a) induces alternative activation in human and mouse monocytes and suppresses the accessory cell-dependent T cell proliferation. J Leukoc Biol. 2002;72(3):512–21. [PubMed] [Google Scholar]
- 15.Haeri S, Baker AM, Boggess KA. Prevalence of Epstein-Barr virus reactivation in pregnancy. Am J Perinatol. 2010;27(9):715–9. doi: 10.1055/s-0030-1253098. [DOI] [PubMed] [Google Scholar]
- 16.Purtilo DT, Sakamoto K. Reactivation of Epstein-Barr virus in pregnant women: social factors, and immune competence as determinants of lymphoproliferative diseases-a hypothesis. Med Hypotheses. 1982;8(4):401–8. doi: 10.1016/0306-9877(82)90033-0. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto K, Greally J, Gilfillan RF, et al. Epstein-Barr virus in normal pregnant women. Am J Reprod Immunol. 1982;2(4):217–21. doi: 10.1111/j.1600-0897.1982.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 18.Meyohas MC, Marechal V, Desire N, et al. Study of mother-to-child Epstein-Barr virus transmission by means of nested PCRs. J Virol. 1996;70(10):6816–9. doi: 10.1128/jvi.70.10.6816-6819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baschat AA, Towbin J, Bowles NE, et al. Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J Matern Fetal Neonatal Med. 2003;13(6):381–4. doi: 10.1080/jmf.13.6.381.384. [DOI] [PubMed] [Google Scholar]
- 20.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Hviid L, Duffy PE, et al. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7(2):105–17. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 22.Moormann AM, Chelimo K, Sumba OP, et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191(8):1233–8. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- 23.Piriou E, Kimmel R, Chelimo K, et al. Serological evidence for long-term Epstein-Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J Med Virol. 2009;81(6):1088–93. doi: 10.1002/jmv.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donati D, Espmark E, Kironde F, et al. Clearance of circulating Epstein-Barr virus DNA in children with acute malaria after antimalaria treatment. J Infect Dis. 2006;193(7):971–7. doi: 10.1086/500839. [DOI] [PubMed] [Google Scholar]
- 25.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt's lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3(2):182–7. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 26.Ndenga B, Githeko A, Omukunda E, et al. Population dynamics of malaria vectors in western Kenya highlands. Journal of medical entomology. 2006;43(2):200–6. doi: 10.1603/0022-2585(2006)043[0200:pdomvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Beier JC, Perkins PV, Onyango FK, et al. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in western Kenya in preparation for malaria vaccine trials. Journal of medical entomology. 1990;27(4):570–7. doi: 10.1093/jmedent/27.4.570. [DOI] [PubMed] [Google Scholar]
- 28.Hermsen CC, Telgt DS, Linders EH, et al. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol. 2001;118(2):247–51. doi: 10.1016/s0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 29.Kamau E, Tolbert LS, Kortepeter L, et al. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol. 2011;49(8):2946–53. doi: 10.1128/JCM.00276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fachiroh J, Paramita DK, Hariwiyanto B, et al. Single-assay combination of Epstein-Barr Virus (EBV) EBNA1- and viral capsid antigen-p18-derived synthetic peptides for measuring anti-EBV immunoglobulin G (IgG) and IgA antibody levels in sera from nasopharyngeal carcinoma patients: options for field screening. J Clin Microbiol. 2006;44(4):1459–67. doi: 10.1128/JCM.44.4.1459-1467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newson R. Parameters behind “nonparametric” statistics: Kendall's tau, Somers' D and median differences. Stata Journal. 2002;2(Number 1, 1st Quarter 2002):45–64. [Google Scholar]
- 32.Perrault SD, Hajek J, Zhong K, et al. Human immunodeficiency virus co-infection increases placental parasite density and transplacental malaria transmission in Western Kenya. Am J Trop Med Hyg. 2009;80(1):119–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Okoko BJ, Enwere G, Ota MO. The epidemiology and consequences of maternal malaria: a review of immunological basis. Acta Trop. 2003;87(2):193–205. doi: 10.1016/s0001-706x(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 34.Ibhanesebhor SE, Okolo AA. Placental malaria and pregnancy outcome. Int J Gynaecol Obstet. 1992;37(4):247–52. doi: 10.1016/0020-7292(92)90324-c. [DOI] [PubMed] [Google Scholar]
- 35.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. [accessed [10 December 2013]]. (WHO/NMH/NHD/MNM/11.1), ( http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 36.de-The G. Is Burkitt's lymphoma related to perinatal infection by Epstein-Barr virus? Lancet. 1977;1(8007):335–8. doi: 10.1016/s0140-6736(77)91137-0. [DOI] [PubMed] [Google Scholar]
- 37.Chene A, Donati D, Guerreiro-Cacais AO, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 2007;3(6):e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cesarman E. Gammaherpesviruses and lymphoproliferative disorders. Annual review of pathology. 2014;9:349–72. doi: 10.1146/annurev-pathol-012513-104656. [DOI] [PubMed] [Google Scholar]
- 39.Lam KM, Syed N, Whittle H, et al. Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet. 1991;337(8746):876–8. doi: 10.1016/0140-6736(91)90203-2. [DOI] [PubMed] [Google Scholar]
- 40.Yone CL, Kube D, Kremsner PG, et al. Persistent Epstein-Barr viral reactivation in young African children with a history of severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2006;100(7):669–76. doi: 10.1016/j.trstmh.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Donati D, Zhang LP, Ch√™ne A, et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect and Immunol. 2004;72(9):5412–8. doi: 10.1128/iai.72.9.5412-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X, Gowda NM, Kumar S, et al. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol. 2010;184(8):4338–48. doi: 10.4049/jimmunol.0903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iskra S, Kalla M, Delecluse HJ, et al. Toll-like receptor agonists synergistically increase proliferation and activation of B cells by epstein-barr virus. J Virol. 2010;84(7):3612–23. doi: 10.1128/JVI.01400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adegnika AA, Kohler C, Agnandji ST, et al. Pregnancy-associated malaria affects toll-like receptor ligand-induced cytokine responses in cord blood. J Infect Dis. 2008;198(6):928–36. doi: 10.1086/591057. [DOI] [PubMed] [Google Scholar]
- 45.Brustoski K, Moller U, Kramer M, et al. IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J Immunol. 2005;174(3):1738–45. doi: 10.4049/jimmunol.174.3.1738. [DOI] [PubMed] [Google Scholar]
- 46.Ismaili J, van der Sande M, Holland MJ, et al. Plasmodium falciparum infection of the placenta affects newborn immune responses. Clin Exp Immunol. 2003;133(3):414–21. doi: 10.1046/j.1365-2249.2003.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rachas A, Le Port A, Cottrell G, et al. Placental malaria is associated with increased risk of nonmalaria infection during the first 18 months of life in a Beninese population. Clin Infect Dis. 2012;55(5):672–8. doi: 10.1093/cid/cis490. [DOI] [PubMed] [Google Scholar]
- 48.Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 50.Avgil M, Diav-Citrin O, Shechtman S, et al. Epstein-Barr virus infection in pregnancy--a prospective controlled study. Reprod Toxicol. 2008;25(4):468–71. doi: 10.1016/j.reprotox.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Eskild A, Bruu AL, Stray-Pedersen B, et al. Epstein-Barr virus infection during pregnancy and the risk of adverse pregnancy outcome. BJOG. 2005;112(12):1620–4. doi: 10.1111/j.1471-0528.2005.00764.x. [DOI] [PubMed] [Google Scholar]