Abstract
The antiphospholipid syndrome is characterized by venous or arterial thrombosis and/or recurrent fetal loss in the presence of circulating antiphospholipid antibodies. These antibodies cause activation of endothelial and other cell types leading to the release of microparticles with procoagulant and pro-inflammatory properties. The aims of this study were to characterize the levels of endothelial cell, monocyte, platelet derived, and tissue factor-bearing microparticles in patients with antiphospholipid antibodies, to determine the association of circulating microparticles with anticardiolipin and anti-β2-glycoprotein antibodies, and to define the cellular origin of microparticles that express tissue factor. Microparticle content within citrated blood from 47 patients with antiphospholipid antibodies and 144 healthy controls was analyzed within 2 hours of venipuncture. Levels of Annexin-V, CD105 and CD144 (endothelial derived), CD41 (platelet derived) and tissue factor positive microparticles were significantly higher in patients than controls. Though levels of CD14 (monocyte-derived) microparticles in patient plasma were not significantly increased, increased levels of CD14 and tissue factor positive microparticles were observed in patients. Levels of microparticles that stained for CD105 and CD144 showed a positive correlation with IgG (R = 0.60, p=0.006) and IgM anti-beta2-glycoprotein I antibodies (R=0.58, p=0.006). The elevation of endothelial and platelet derived microparticles in patients with APS and their correlation with anti-β2-glycoprotein I antibodies suggests a chronic state of vascular cell activation in these individuals and an important role for β2-glycoprotein I in development of the pro-thrombotic state associated with antiphospholipid antibodies.
Keywords: microparticles, antiphospholipid, thrombosis, endothelial cell, platelet, thrombosis
INTRODUCTION
The antiphospholipid syndrome (APS) is a multi-system disorder characterized by arterial and/or venous thrombosis or recurrent fetal loss in the presence of antiphospholipid antibodies (APLA) [1–5]. The majority of pathogenic APLA are actually directed against β2-glycoprotein I (β2GPI), an abundant plasma phospholipid binding protein [6,7]. However, the pathogenesis of APS remains incompletely understood. APLA/anti-β2GPI antibodies appear not only to be an important serologic marker of disease, but to play a central role in the pathogenesis of thrombosis [8,9]. Some APLA inhibit important anticoagulant pathways, such as the activation or activity of protein C or S [10–12] or the anticoagulant activity of annexin A5 [13,14]. APLA antibodies also activate vascular cells, including endothelial cells [15–17], monocytes [18,19], and platelets, particularly in the presence of other agonists [20–22]. Cellular activation results in a pro-adhesive and procoagulant phenotype characterized by expression of adhesion molecules [15,23,24], tissue factor (TF) [25] and vWF [26]. Antibodies reactive with endothelial cells occur in many patients with APS, induce endothelial cell activation in a β2GPI-dependent manner, and correlate with a history of thrombosis [26,27].
The release of microparticles from vascular cells activated by APLA has been implicated in the pathogenesis of thrombosis in patients with APS. Stimulation of microparticle release is a characteristic of activated cells and can be induced by stimuli such as terminal complement components, inflammatory cytokines and apoptosis [28–30]. Microparticles are submicron vesicles composed of anionic phospholipid that express or contain specific cellular proteins and nucleic acids that may mediate procoagulant activity [31]. Elevated levels of circulating microparticles have been observed in several disorders including cardiovascular disease [30], venous thrombosis [32–34], systemic lupus [35;36], and cancer [37–39], among others. Microparticles released in vitro in response to APLA-mediated endothelial activation, express prothrombotic properties [40].
The prothrombotic properties of microparticles may result from the expression of tissue factor [41] and anionic phospholipid on the microparticle surface [42]. Microparticles may also mediate procoagulant effects through their expression of inflammatory mediators such as IL-1β, which induce activation of other cells through autocrine or paracrine mechanisms [43,43].
The ability to identify individuals at greatest risk for thrombosis remains a challenge in the management of patients with APLA. Better understanding of the pathogenesis of APS in humans may provide insight into more specific approaches to prevent the clinical manifestations of these antibodies. Microparticles may also provide a potential surrogate marker of vascular dysfunction that may be useful in stratifying the risk of thrombosis associated with APS.
In this study, we have characterized circulating microparticles in a large cohort of patients with APLA, and determined their cellular origin, expression of tissue factor, correlation with clinical tests for APLA, and association with clinical events.
MATERIALS AND METHODS
Materials
Antibodies to CD105-PE and CD144-PE (to detect endothelial cell-derived microparticles), CD41-PECy4 (to detect platelet-derived microparticles) and CD14-PE (to detect monocyte-derived microparticles) were obtained from Abcam (Cambridge, MA). A FITC-conjugated monoclonal antibody against tissue factor (CD142; product #4507CJ) was obtained from American Diagnostica (Stamford, CT). Annexin V-Alexafluor 647 and Alignflow flow cytometry beads (2.5 µM) were from Life Technologies (Grand Island, NY). Latex, amine-modified polystyrene, fluorescent yellow-green beads (1 µm; product # L1030) and all chemicals used for preparation of buffers were from Sigma-Aldrich (St Louis, MO). Venipuncture tubes containing sodium citrate were purchased from BD (Franklin Lakes, NJ).
Patients and Controls
Forty-seven patients with APLA and 144 healthy controls were studied. Patients were recruited from the hematology clinics of the Cleveland Clinic and Case Western Reserve University. This study included patients with antiphospholipid antibodies, not specifically patients who met clinical criteria for APS. However, since the patients were largely recruited from hematology clinics, most of them met criteria for clinical APS. Complete clinical information was available for 46 of 47 patients. Thirty-eight met clinical criteria for APS (either thrombosis or pregnancy loss). Of the remaining 8 patients, 6 were referred for evaluation on the basis of a persistent APLA without clinical manifestations. In two cases this was detected during evaluation of SLE and in another case it was detected during evaluation of persistent migraine with aura. Patients with a history of thrombosis were enrolled at least three months after their most recent thrombotic event to minimize confounding of microparticle levels by acute thrombus. Control subjects consisted of normal, healthy individuals who did not have APLA, a history of thrombosis or other congenital or acquired thrombophilia and who did not smoke. The Institutional Review Boards at Cleveland Clinic and Case Western Reserve University approved this study.
Microparticle isolation and Flow Cytometry
Blood samples from patients and controls were collected by venipuncture into citrated tubes and processed within 60 minutes; the first 3 ml were discarded and not used for microparticle measurements. Blood was processed as previously described by Lee et al [44] and Dignat-George et al [45]. Briefly, blood was centrifuged at 1500 × G for 15 minutes. The supernatant “platelet poor plasma” was collected and centrifuged again at 13,000 × G for 2 minutes to remove residual platelets and cell fragments, yielding “platelet-free plasma”. Platelet-free plasma was added to individual tubes containing isotype and label-specific control IgG, specific fluorochrome-labeled antibodies to CD105, CD144, CD41, CD14-PE and tissue factor, or annexin V. After incubation for 60 minutes, 200 µl of a solution of 2.5 µM µm Alignflow flow cytometry beads (concentration = 3 × 106 beads/ml) were added to each tube and used for calibration to ensure analysis of an identical volume of each sample.
Samples were analyzed by flow cytometry using an LSRII flow cytometer (BD Biosciences). On a log forward scatter (FSC) vs. log side scatter (SSC) plot, 1 µm latex beads (Sigma-Aldrich) were used to define the MP gate. MP within this gate labeled with either annexin V or control or antigen-specific fluorochrome-labeled antibodies were counted; data collection for each sample was terminated following counting of 50,000 Alignflow beads in a separate gate distinct from that of microparticles. Background staining due to control antibodies was generally < 5% of that observed with antigen-specific antibodies and was subtracted from that caused by the latter.
In a randomly selected cohort of 19 control individuals and 12 patients, we also determined the contribution of microparticles from specific cell types to tissue factor expression by double staining microparticles using cell-specific and anti-tissue factor antibodies. These studies were performed in an identical-manner as those utilizing single antibodies, except for the inclusion of anti-mouse Ig, κ/negative control compensation beads to adjust for spectral overlap between the flourochrome emission spectra of the two labeled antibodies.
Measurement of APLA
A PTT based screening test (positive >32.4 seconds) and dilute Russell viper venom (DRVVT) confirmatory test (positive >46.9 seconds with DRVVT confirm ratio >1.20) were used to detect LAC. Determination of anticardiolipin antibodies (aCL) was performed by standardized ELISA for both IgG and IgM isotypes (INOVA Diagnostics Inc., USA) with bovine calf serum in the sample diluent as the source of β2GPI. Results are expressed as GPL units for the IgG (positive ≥ 23 GPL) and MPL units for the IgM (positive ≥ 11 MPL) aCL antibodies, with 1 GPL or MPL unit being equivalent to 1 µg/mL of an affinity-purified standard IgG or IgM aCL antibody sample. Determination of IgM and IgG anti-β2GPI antibodies was performed by ELISA with irradiated and chemically activated plastic microwell plates containing purified human β2GPI (INOVA Diagnostics Inc., USA). Results are expressed in standardized units, SGU for the IgG (positive ≥ 20 SGU) and SMU for the IgM (positive ≥ 20 SMU) anti-β2GPI antibodies. All tests met the quality control standards as determined by the manufacturer.
Statistical Analysis
Statistical analysis was performed using SPSS Software version 20.0 (IBM Corp. 2011). Descriptive statistics were calculated for MP measurements (mean, standard deviation). The distribution of MP and antiphospholipid antibody measurements was skewed, therefore a logarithmic transformation was performed prior to parametric analyses. Wilcoxon rank sum test was used to compare MP levels between patients and controls and between sub-groups of patients (with or without DVT/pregnancy morbidity). Additional adjustment of covariates was performed through the analysis of covariance method. Correlations between two variables were investigated using the Pearson’s correlation test. A P value of 0.05 was considered significant for all analyses
RESULTS
Demographics
The study cohort included 47 patients (52% women) with antiphospholipid antibodies and 147 controls (55% women). Complete demographic and clinical information describing the patient and control groups is depicted in Table 1. Patients were older than controls (median age 43 years versus 29 years, p=0.004). Thirteen patients had secondary APS, 29 had a history of venous thrombosis, 12 had experienced cerebrovascular events, and 6 of 24 female patients had a history of pregnancy loss. On testing for lupus anticoagulant and APLA, 25 patients (53.2%) were triple positive, i.e positive for lupus anticoagulant, anti-β2GPI antibodies and aCL. Eighteen (38.3%) patients were positive by 2 of three assays – 9 for LAC and aCL, 5 for LAC and anti-B2GPI, and 4 for aCL and anti-β2GPI antibodies. Finally, 4 patients (8.5%) were strongly positive for LAC but did not have significant titers of aCL and anti-β2GPI antibodies at the time of sampling associated with MP measurement (although these may have been positive in the past).
Table 1.
Clinical and demographic characteristics of patients and controls
| APS patients (n=47)* |
Controls (n=144) |
|
|---|---|---|
| Age | 43 (31,63) | 29 (24,42) |
| Female sex | 52% | 55% |
| Ethnicity | N=41 | N=137 |
| Caucasian | 34 | 104 |
| African American | 6 | 14 |
| Asian | 0 | 16 |
| Other | 1 | 3 |
| SLE | 13/46 (28.3) | N/A |
| DVT | 29/46 (63.0) | N/A |
| Pregnancy loss | 6/24(25.0) | N/A |
| DVT + pregnancy loss | 2/24 (8.3) | N/A |
| Cerebrovascular accident | 12/46 (26.0) | N/A |
| Arterial thrombosis (other than CVA) | 4/46 (8.7) | N/A |
| MI | 3/46(6.5) | N/A |
| Thrombocytopenia | 9/46 (19.6) | N/A |
| Treatment at enrollment | ||
| Aspirin | 12 | N/A |
| Clopidogrel | 4 | N/A |
| Warfarin | 23 | N/A |
| Lovenox | 9 | N/A |
| Fondaparinux | 2 | N/A |
Complete clinical data was available on 46 of 47 patients.
Levels of circulating microparticles in patients and controls
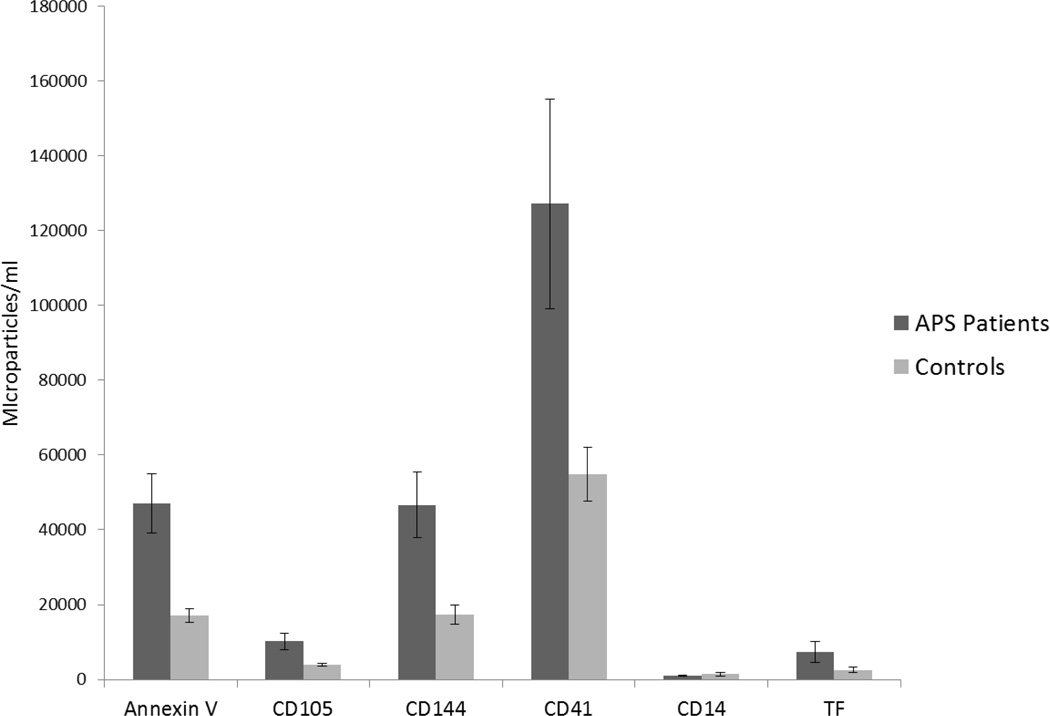
The levels of circulating microparticles in patients and controls is depicted in Figure 1 and summarized in Table 2. The mean levels of Annexin V positive microparticles were significantly higher in patients with APLA than controls [21386 (10448, 71368) vs. 8255 (3532, 19342) MP/ml, p<0.001]. Microparticles positive for CD105 and CD144 were also higher in patients with APLA compared to controls [CD105: 5020 (1202, 11362) vs. 2256 (687, 5444), p=0.008; CD144: 22402 (7137, 59710) vs. 8241 (2747, 18762) MP/ml], as were CD41 positive microparticles [38326 (17970, 104119) vs. 21975 (9516, 51230) MP/ml, p=0.006). Patients with APLA also demonstrated elevated levels of tissue factor positive microparticles [981 (196, 4325) vs. 327 (0, 1575) MP/ml, p=0.027). In contrast there was no significant difference in total levels of CD14 positive microparticles between patient and control groups [590 (294, 1378) vs. 589 (196, 1205), p=0.66].
Figure 1. Levels of circulating microparticles in patients and controls.
Levels of microparticles derived from endothelial cells (CD105 and CD144), platelets (CD41), monocytes (CD14) or those staining with an anti-tissue factor antibody were measured in fresh platelet-free plasma by flow cytometry as described in Materials and Methods.
Table 2.
Microparticle levels in patients with antiphospholipid antibodies and healthy controls [expressed as median(Q25,Q75)].
| Microparticle subpopulation |
Patients (n=47) | Controls (n=144) | P-value (Wilcoxon) |
|---|---|---|---|
| Annexin V | 21386 (10448, 71368) | 8255 (3532, 19342) | < 0.001 |
| CD 105 | 5020 (1202, 11362) | 2256 (687, 5444) | 0.008 |
| CD 144 | 22402 (7137, 59710) | 8241 (2747, 18762) | < 0.001 |
| CD 41 | 38326 (17970, 104119) | 21975 (9516, 51230) | 0.006 |
| CD14 | 590 (294, 1378) | 589 (196, 1205) | 0.660 |
| TF | 981 (196, 4325) | 327 (0, 1575) | 0.027 |
Since age was unbalanced between cases and controls, we additionally adjusted for the possible effect of age using Analysis of Covariance (ANCOVA) and found that age was not associated with microparticle levels detected using any of the markers employed (Table 3).
Table 3.
Relationship between age and microparticle levels by ANCOVA analysis
| Outcome variable* | Age Coeff. (SD) |
p-value |
|---|---|---|
| AV (log) | 0.00286 (0.00923) | 0.76 |
| CD 105 (log) | −0.00489 (0.00966) | 0.61 |
| CD 14 (log) | −0.00091 (0.0154) | 0.953 |
| CD 144 (log) | −0.00279 (0.00817) | 0.73 |
| CD 41 (log) | 0.0067 (0.0087) | 0.44 |
| TF (log) | −0.0227 (0.0218) | 0.30 |
ANCOVA = Analysis of Covariance
Microparticle measurements were skewed. Therefore log transformation was performed prior to analysis
Correlations between microparticle levels and APLA serologies
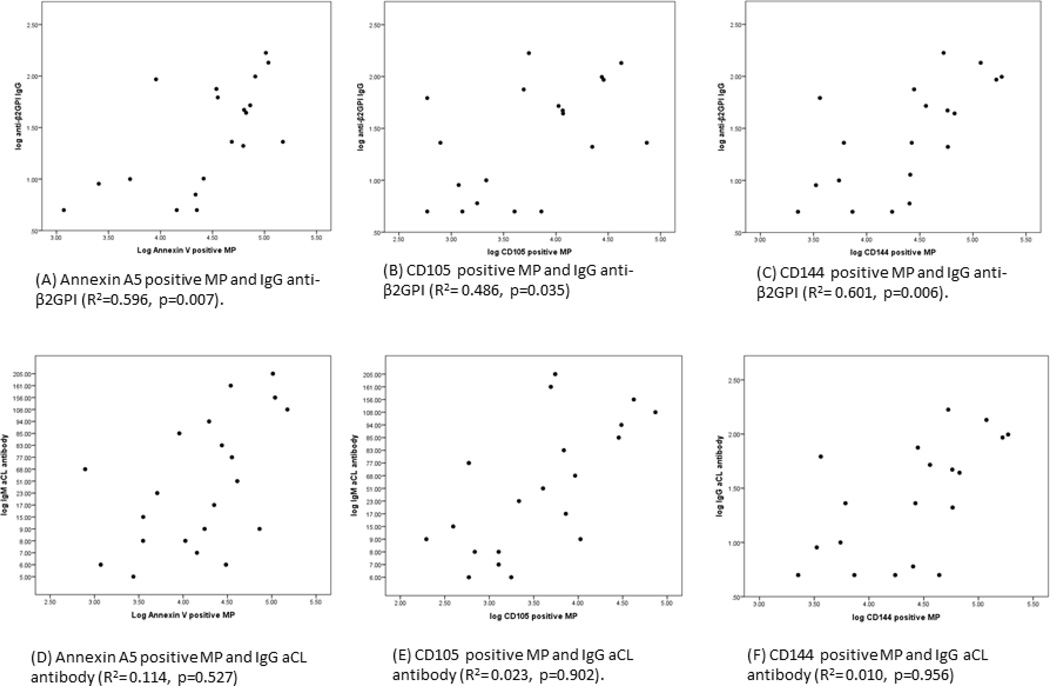
We next defined the association between circulating microparticle levels and anti-β2GPI and anticardiolipin antibodies. A strong positive correlation was observed between endothelial cell derived microparticles (CD105 and CD144 positive) and IgG anti-β2GPI antibodies antibodies (Figure 2). Levels of IgM anti-β2GPI antibodies also correlated strongly with levels of CD105 positive (R=0.63, p=0.003) and CD144 positive microparticles (R=0.58, p=0.006) (not shown). Levels of annexin V microparticles were also associated with elevated levels of anti-β2GPI IgG (R=0.60, p = 0.007) and IgM (R=0.57, p=0.007) antibodies. In contrast, there were no significant correlations between CD41 positive (platelet derived) and CD14 positive (monocyte derived) microparticles and anti-β2GPI antibodies. Anticardiolipin antibodies did not correlate with microparticle levels derived from any cell type.
Figure 2. Correlations between endothelial cell-derived microparticles and anti-β2GPI and anti-cardiolipin antibody levels.
The correlations between endothelial cell-derived microparticles and IgG anti-β2GPI or anticardiolipin antibody levels, or annexin V staining, were determined. Significant correlations were seen between microparticle levels and anti-β2GPI antibodies, and microparticle levels and annexin V staining. Similar correlations between microparticle levels and IgM anti-β2GPI antibodies were observed (see text).
There was no association between microparticle levels and a history of thrombosis, stroke or pregnancy loss (Table 4). The current use of anti-platelet agents or anticoagulants also did not affect microparticle levels.
Table 4.
Association between microparticle levels and clinical characteristics (venous thrombosis and pregnancy loss)
| Microparticle population |
DVT | P value (Wilcoxon) |
Pregnancy loss | P value (Wilcoxon) |
||
|---|---|---|---|---|---|---|
| Yes (n=29) | No (n=17) | Yes (n=6) | No (n=18) | |||
| Annexin V | 23887 (8712,73495) |
22367 (8443,74600) |
0.859 | 72790 (12503,131524) |
22367 (9137,65051) |
0.466 |
| CD105 | 5513 (1474,17523) |
3544 (491,11655) |
0.349 | 10595 (6383,51193) |
5513 (1474,11655) |
0.063 |
| CD144 | 25946 (7284,58627) |
17364 (4267,97280) |
0.856 | 36101 (19099,146121) |
25310 (7667,55330) |
0.400 |
| CD14 | 884 (318,1722) |
787 (393,1418) |
0.494 | 787 (442,3248) |
883 (392,1723) |
0.298 |
| CD41 | 36755 (12355,15890) |
49835 (27415,235268) |
0.454 | 35245 (31257,472826) |
25597 (11124,67787) |
0.211 |
| TF | 2257 (442,11388) |
1083 (196,7659) |
0.156 | 2264 (836,17071) |
2752 (492,21993) |
0.660 |
Origin of tissue factor positive microparticles
As tissue factor positive microparticles may play an important role in the development of thrombosis, and since APLA stimulate the expression of tissue factor by endothelial cells and monocytes, we assessed the contribution of microparticles derived from endothelial cells, monocytes and platelets to the total pool of tissue factor positive microparticles. Levels of tissue factor positive microparticles were significantly increased in patients compared to controls, though differences were seen among individual patients (Figure 3A). In controls, microparticles derived from endothelial cells, monocytes and platelets expressed a basal level of tissue factor, with the greatest number of tissue factor positive microparticles derived from platelets (Figure 3B). In patients, however, levels of tissue factor-expressing monocyte and endothelial cell-derived microparticles were both significantly increased, with the most marked increases observed in the latter (Figure 3B). Of the tissue factor positive microparticle pool in patients with APS, microparticles contributed by endothelial cells, platelet and monocytes accounted for 51.7%, 31% and 17.3% of the total microparticles, respectively.
Figure 3.
Levels of tissue factor positive microparticles. (A) Microparticles from patients and controls were stained with an antibody to tissue factor and analyzed by flow cytometry. This figure depicts the tissue factor positivity of microparticles from individual patients and controls. (B) Levels of tissue factor microparticles by cell type. Microparticles were double stained with a cell-specific antibody and antibody against tissue factor, as described in Materials and Methods, and analyzed by flow cytometry. This figure depicts the total as well as the percentages of tissue factor positive microparticles derived from endothelial cells, platelets or monocytes. (C) Confocal micrograph (× 63) of a single microparticle in patient plasma staining positively for tissue factor (green, FITC), CD14 (red-PE) or overlay after double staining. For size comparison, two fluorescent 1 µM flow cytometry beads are depicted.
DISCUSSION
This study demonstrates that plasma of patients with APLA contains elevated levels of microparticles derived from endothelial cells and platelets. Although absolute numbers of monocyte-derived microparticles were not elevated, monocyte derived microparticles nevertheless contributed substantially to the total tissue factor positive microparticle pool. We did not detect any significant differences in microparticle number or distribution in patients with APLA who had a history of thrombosis or pregnancy loss, though the majority of our patients had experienced such events and our study was not powered to detect such differences. Anti-platelet and anticoagulant therapy was not associated with differences in microparticle levels, as previously reported [40]. This observation is consistent with the hypothesis that anticoagulants do not directly inhibit an underlying mechanism of APS—vascular cell activation, and thus may simply mask the clinical symptoms of APS.
Evidence suggests that APS results, at least in part, from antibody-mediated activation of vascular cells by antiphospholipid antibodies. Perhaps most extensively studied are endothelial cell-reactive antibodies in these individuals, which activate endothelial cells in a β2GPI-dependent manner [15,24,46]. APLA have also been shown to induce platelet [20–22,47,47] and monocyte activation [18,19]. In light of the latter finding it is somewhat surprising that we did not detect significantly increased levels of monocyte derived microparticles in patients with APLA. However, the mechanisms of microparticle release in response to APLA, as well as the nature of the microparticles released may differ among various types of cells, and it is possible, for example, that monocyte-derived extracellular vesicles may be smaller than those released by endothelial cells and platelets, and not detectable by flow cytometry. Additional studies will be required to assess the release of microparticles from isolated monocytes in response to APLA in order to better define this process.
Previous studies have examined levels of microparticles in patients with APS. Combes et al analyzed endothelial cell-derived microparticles in sera of 30 patients with lupus anticoagulants and 30 healthy controls and found an approximately two-fold elevation of endothelial cell-derived microparticles in the former; mean endothelial cell microparticle levels were also higher in patients with thrombosis [40]. Dignat-George et al measured endothelial cell-derived microparticle levels in 111 patients (23 primary APS, 14 SLE associated APS, 28 with antiphospholipid antibodies but no SLE or history of thrombosis, 23 with SLE but no antiphospholipid antibodies, and 25 with thrombosis but without SLE) and 25 healthy controls [45]. These investigators observed higher levels of endothelial microparticles in patients with primary or secondary APS, or with thrombosis not related to antiphospholipid antibodies compared to SLE patients without APLA. They did not, however, quantify leukocyte or platelet derived microparticles. Vikerfors et al [48] and Jy et al [49] also observed elevated levels of endothelial cell microparticles in patients with APS but did not find an association between microparticle levels and a history of thrombosis. In contrast to our study, they reported no difference in platelet derived microparticles in patients with APS versus healthy controls. Inconsistencies such as these may reflect differences in patient selection, or technical differences in sample processing (including differences in time from collection to analysis, centrifugation speeds and times, and in particular whether microparticles were pelleted and resuspended before analysis), and whether the analysis was performed on fresh or frozen samples (Table 5). In our studies, we analyzed microparticles in platelet free plasma within 60 minutes of blood collection and did not subject samples to high centrifugation speeds, thereby attempting to minimize the potential for mechanical microparticle disruption that could lead to variance in measurements.
Table 5.
Studies of microparticles in patients with antiphospholipid antibodies
| Study | Subjects | Methods | Total MP |
Platelet MP | Endothelial MP | Tissue factor |
|---|---|---|---|---|---|---|
| This study | 47 aPL + patients, 144 controls |
Fresh samples, no pelleting, direct analysis of platelet poor plasma |
↑ in aPL + |
↑ in aPL + | ↑ EMP in aPL + No difference with a history of thrombosis or pregnancy loss. No difference with anticoagulation. EMP correlate with anti-B2GPI. |
↑ TF+ MP |
| Combes, JCI 1999[40] | 30 patients with LA, 30 healthy controls |
Fresh samples, not pelleted |
NS | NS | EMP ↑ in patients with LA. Higher levels in patients with thrombosis. No effect of anticoagulation. |
NS |
| Vikerfors, Lupus 2012[48] | 52 patients with APS, 52 healthy controls |
Frozen samples. Processing not described. |
↑ in APS |
No difference |
EMP ↑ in APS. No difference in total MP or EMP in patients with and without thrombosis or pregnancy loss. |
↑ TF+ MP |
| Dignat-George, Thromb Haemost 2004[45] | 35 APS 28 SLE aPL+ 23 SLE aPL − 25 thromb aPL− 25 controls |
Frozen samples. Not pelleted. |
NS | NS | EMP ↑ in APS or aPL + and was associated with DRVVT positivity. No effect of a history of thrombosis or anticoagulation. |
NS |
| Jy, Thromb Res 2007[49] | 88 APS patients (60 with thrombosis), 39 healthy controls |
Fresh samples. Not pelleted |
NS | No difference between APS and controls PMP ↑ in patients with thrombosis |
EMP ↑ in APS. No difference with a history of thrombosis |
NS |
| Periera, Lupus 2007[58] | 30 patients with SLE, 20 healthy controls |
Frozen samples. Pelleted |
↑ MP, mostly PMP |
PMP have ↑ potential to generate thrombin |
NS | |
| Willemze, Thromb Res 2014[57] | 30 APS, 72 asymptomatic aPL+ |
Frozen samples. Pelleted. MP-TF activity measured by FXa generation. |
NS | NS | NS | Higher MP-TF activity in APS than asympto matic aPL |
NS = not studied
Microparticles are released in response to diverse stimuli, particularly cellular apoptosis or activation. Our results, in which elevated levels of endothelial cell and platelet microparticles as well as monocyte-derived, tissue factor positive microparticles were elevated in patients with APS argues for a pathogenesis that involves activation of each of these cell types, ultimately leading to thrombosis formation. Since we selected patients who were at least 3 months from a thrombotic event, this chronic, subclinical activation state appears to be present even in the absence of overt thrombosis.
Mechanisms of cellular activation by antiphospholipid antibodies remain controversial. For example, in endothelial cells, several different receptors, including annexin A2, TLR4, TLR2, TLR7 and apoER2 have been reported to promote cellular activation by APLA [1,4], although most reports suggest an important role for NF-κB activation. Antiphospholipid antibodies have also been reported to stimulate platelet p38 MAPK phosphorylation in the presence of sub-threshold thrombin concentrations [22], and complexes of β2GPI and monoclonal anti-β2GPI antibodies promote platelet adhesion under flow [21]. Monocyte activation may be induced by interactions of β2GPI and anti-β2GPI antibodies with lipid raft structures containing annexin A2 [18], and circulating monocytes may be activated in patients with APS [50]. Additional work to better define the mechanisms of activation of different types of cells and whether common pathways exist are needed to better understand the pathogenesis of APS.
Our study is the first to demonstrate that levels of endothelial microparticles correlate with those of anti-β2GPI, but not anticardiolipin antibodies. Several studies have reported that the presence of LAC and anti-β2GPI antibodies correlate more closely with thrombosis than ACL [51–53], perhaps reflecting the higher specificity of anti-β2GPI antibody assays in light of the prevalence of β2GPI-independent ACL in the population [54]. The association of endothelial cell-derived microparticles and anti-β2GPI antibodies also supports the importance of β2GPI as an important autoantigen in activation of endothelial cells, and is consistent with in vitro studies demonstrating that APLA activate endothelial cells in a β2GPI-dependent manner [15].
Our studies are the first to attempt to define the sources of microparticle tissue factor in patients with antiphospholipid antibodies, and an intriguing observation was the prevalence of tissue factor positive, platelet derived microparticles. Expression of tissue factor by platelet-derived microparticles has been demonstrated in other disorders, such diabetes mellitus [55]. Though platelets contain and process tissue factor mRNA, synthesis of tissue factor protein by platelets has not been demonstrated. Therefore, it is likely that most of the tissue factor expressed by platelet-derived microparticles is contributed by monocyte-derived, tissue factor positive microparticles that fuse with platelets [56], though our studies imply that similar contributions from endothelial cell-derived microparticles may contribute as well.
In conclusion, our studies demonstrate elevated levels of endothelial cell and platelet-derived microparticles in fresh plasma from patients with antiphospholipid antibodies. We also provide evidence that tissue factor positive microparticles are elevated in these individuals. Although we did not demonstrate an increase in total monocyte-derived microparticles in patient plasma, monocyte microparticles nevertheless constituted a significant fraction of the total tissue factor positive microparticle pool. Since approximately 90% of our study population had a history of either thrombosis or pregnancy loss, our study was underpowered to detect a correlation of microparticle numbers with a history of APS-related clinical events. Moreover, though we did not measure tissue factor activity, our results are consistent with a recent report from Willemze et al in which elevated microparticle tissue factor activity was associated with thrombosis in patients with antiphospholipid antibodies [57]. Finally, our studies demonstrate a correlation between anti-β2GPI antibodies and circulating microparticle levels, consistent with the clinical importance of such antibodies and their role in cellular activation. While additional work will be required to better define the mechanisms by which different types of vascular cells are activated in patients with antiphospholipid antibodies, these studies suggest the importance of this process in humans, and suggest that additional work to determine whether microparticle levels may predict an increased risk of thrombosis in patients with antiphospholipid antibodies may be warranted.
Highlights.
Endothelial cell and platelet microparticles are elevated in patients with APLA
Tissue factor (TF)-positive microparticles are elevated in patients with APLA
Levels of endothelial microparticles correlate with levels of anti-β2GPI antibodies
Most TF-positive microparticles in patients with APLA are endothelial derived
Acknowledgements
This work was supported by P50HL081011 from the National Heart, Lung and Blood Institute, and by a bridge grant from the American Society of Hematology.
Abbreviations
- APS
Antiphospholipid syndrome
- APLA
Antiphospholipid antibody
- β2GPI
Beta-2-glycoprotein-I
- TF
Tissue factor
- SLE
Systemic Lupus Erythematosus
- aCL
anticardiolipin antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giannakopoulos B, Passam F, Rahgozar S, Krilis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007;109:422–429. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- 2.Rand JH. The antiphospholipid syndrome. Ann Rev Med. 2003;54:409–424. doi: 10.1146/annurev.med.54.101601.152412. [DOI] [PubMed] [Google Scholar]
- 3.Thiagarajan P, Shapiro SS. Lupus anticoagulants and antiphospholipid antibodies. Hematol Oncol Clin N A. 1998;12:1167–1192. doi: 10.1016/s0889-8588(05)70048-4. [DOI] [PubMed] [Google Scholar]
- 4.Pierangeli SS, Chen PP, Raschi E, Scurati S, Grossi C, Borghi MO, Palomo I, Harris EN, Meroni PL. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008;34:236–250. doi: 10.1055/s-0028-1082267. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376:1498–1509. doi: 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- 6.Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RFA. Lupus anticoagulant IgG's (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thrombosis and Haemostasis. 1991;66:629–632. [PubMed] [Google Scholar]
- 7.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Koike T. Anticardiolipin cofacto(s) and differential diagnosis of autoimmune disease. Lancet. 1990;335:177–178. doi: 10.1016/0140-6736(90)91697-9. [DOI] [PubMed] [Google Scholar]
- 8.Arad A, Proulle V, Furie RA, Furie BC, Furie B. {beta}2 glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood. 2011;24:3453–3459. doi: 10.1182/blood-2010-08-300715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannou Y, Romay-Penabad Z, Pericleous C, Giles I, Papalardo E, Vargas G, Shilagard T, Latchman DS, Isenberg DA, Rahman A, Pierangeli S. In vivo inhibition of antiphospholipid antibody-induced pathogenicity utilizing the antigenic target peptide domain I of beta2-glycoprotein I: proof of concept. J Thromb Haemost. 2009;7:833–842. doi: 10.1111/j.1538-7836.2009.03316.x. [DOI] [PubMed] [Google Scholar]
- 10.Esmon NL, Safa O, Smirnov MD, Esmon CT. Antiphospholipid antibodies and the protein C pathway. J Autoimmun. 2000;15:221–225. doi: 10.1006/jaut.2000.0407. [DOI] [PubMed] [Google Scholar]
- 11.Urbanus RT, de LB. Antiphospholipid antibodies and the protein C pathway. Lupus. 2010;19:394–399. doi: 10.1177/0961203309360841. [DOI] [PubMed] [Google Scholar]
- 12.de LB, Eckmann CM, van SM, Meijer AB, Mertens K, Van Mourik JA. Correlation between the potency of a beta2-glycoprotein I-dependent lupus anticoagulant and the level of resistance to activated protein C. Blood Coagul Fibrinolysis. 2008;19:757–764. doi: 10.1097/MBC.0b013e32830f1b85. [DOI] [PubMed] [Google Scholar]
- 13.Rand JH. Molecular pathogenesis of the antiphospholipid syndrome. Circ Res. 2002;90:29–37. doi: 10.1161/hh0102.102795. [DOI] [PubMed] [Google Scholar]
- 14.Rand JH, Wu XX, Quinn AS, Chen PP, McCrae KR, Bovill EG, Taatjes DJ. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: evidence from atomic force microscopy and functional assay. Am J Pathol. 2003;163:1193–1200. doi: 10.1016/S0002-9440(10)63479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simantov R, LaSala JM, Lo SK, Gharavi AE, Sammaritano LR, Salmon JE, Silverstein RL. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Papa N, Guidala L, Sala A, Buccellati C, Khamashta MA, Ichikawa K, Koike T, Balestrieri G, Tincani A, Hughes GRV, Meroni PL. Endothelial cells as targets for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis and Rheumatism. 1997;40:551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Lieske KA, McCrae BA, McCrae KR. Activation of endothelial cells by b2GPI/anti-b2GPI antibodies is mediated by annexin II cross-linking, and may involve TLR4. 2004:83a. [Google Scholar]
- 18.Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, Conti F, Buttari B, Riganò R, Ortona E, Valesini G. Anti-b2-Glycoprotein I antibodies induce monocyte release of tumor necrosis factor a and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 19.Lambrianides A, Carroll CJ, Pierangeli SS, Pericleous C, Branch W, Rice J, Latchman DS, Townsend P, Isenberg DA, Rahman A, Giles IP. Effects of polyclonal IgG derived from patients with different clinical types of the antiphospholipid syndrome on monocyte signaling pathways. J Immunol. 2010;184:6622–6628. doi: 10.4049/jimmunol.0902765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi T, Giannakopoulos B, Yan X, Yu P, Berndt MC, Andrews RK, Rivera J, Iverson GM, Cockerill KA, Linnik MD, Krilis SA. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54:2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 21.Pennings MT, Derksen RH, van LM, Adelmeijer J, VanHoorelbeke K, Urbanus RT, Lisman T, De Groot PG. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2'. J Thromb Haemost. 2007;5:369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 22.Vega-Ostertag M, Harris EN, Pierangeli SS. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 2004;50:2911–2919. doi: 10.1002/art.20434. [DOI] [PubMed] [Google Scholar]
- 23.Espinola RG, Liu X, Colden-Stanfield M, Hall J, Harris EN, Pierangeli SS. E-Selectin mediates pathogenic effects of antiphospholipid antibodies. J Thromb Haemost. 2003;1:843–848. doi: 10.1046/j.1538-7836.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, McCrae KR. Annexin II mediates endothelial cell activation by antiphospholipid/anti-β2-glycoprotein I antibodies. Blood. 2005;105:1964–1969. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- 25.Branch DW, Rodgers GM. Induction of endothelial cell tissue factor activity by sera from patients with antiphospholipid syndrome: A possible mechanism of thrombosis. Am J Obstet Gynecol. 1993;168:206–210. doi: 10.1016/s0002-9378(12)90915-1. [DOI] [PubMed] [Google Scholar]
- 26.McCrae KR, DeMichele A, Samuels P, Roth D, Kuo A, Meng Q, Rauch J, Cines DB. Detection of endothelial cell-reactive immunoglobulin in patients with anti-phospholipid antibodies. Br J Haematol. 1991;79:595–605. doi: 10.1111/j.1365-2141.1991.tb08087.x. [DOI] [PubMed] [Google Scholar]
- 27.Vismara A, Meroni PL, Tincani A, Harris EN, Barcellini W, Brucato A, Khamashta M, Hughes GRV, Zanussi C, Balestrieri G. Relationship between anti-cardiolipin and anti-endothelial cell antibodies in systemic lupus erythematosus. Clin Exp Immunol. 1988;74:247–253. [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ML, Williams KJ. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19:121–127. doi: 10.1097/MED.0b013e32835057e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond) 2013;124:423–441. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 30.Viera AJ, Mooberry M, Key NS. Microparticles in cardiovascular disease pathophysiology and outcomes. J Am Soc Hypertens. 2012;6:243–252. doi: 10.1016/j.jash.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, Dignat-George F. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010;104:456–463. doi: 10.1160/TH10-02-0111. [DOI] [PubMed] [Google Scholar]
- 32.Bucciarelli P, Martinelli I, Artoni A, Passamonti SM, Previtali E, Merati G, Tripodi A, Mannucci PM. Circulating microparticles and risk of venous thromboembolism. Thromb Res. 2012;129:591–597. doi: 10.1016/j.thromres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Ay C, Freyssinet JM, Sailer T, Vormittag R, Pabinger I. Circulating procoagulant microparticles in patients with venous thromboembolism. Thromb Res. 2009;123:724–726. doi: 10.1016/j.thromres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Rautou PE, Mackman N. Microvesicles as risk markers for venous thrombosis. Expert Rev Hematol. 2013;6:91–101. doi: 10.1586/ehm.12.74. [DOI] [PubMed] [Google Scholar]
- 35.Sellam J, Proulle V, Jungel A, Ittah M, Miceli RC, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM, Mariette X. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira J, Alfaro G, Goycoolea M, Quiroga T, Ocqueteau M, Massardo L, Perez C, Saez C, Panes O, Matus V, Mezzano D. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb Haemost. 2006;95:94–99. [PubMed] [Google Scholar]
- 37.Zwicker JI. Predictive value of tissue factor bearing microparticles in cancer associated thrombosis. Thromb Res. 2010;125(Suppl 2):S89–S91. doi: 10.1016/S0049-3848(10)70022-0. [DOI] [PubMed] [Google Scholar]
- 38.Zwicker JI, Liebman HA, Bauer KA, Caughey T, Campigotto F, Rosovsky R, Mantha S, Kessler CM, Eneman J, Raghavan V, Lenz HJ, Bullock A, Buchbinder E, Neuberg D, Furie B. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study) Br J Haematol. 2013;160:530–537. doi: 10.1111/bjh.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with the lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens AP, III, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton KK, Hattori R, Esmon CT, Simms PJ. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990;265:3809–3814. [PubMed] [Google Scholar]
- 43.Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood. 2011;118:2366–2374. doi: 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, Key NS, Mackman N. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129:80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N, Veit V, Combes V, Gentile S, Moal V, Sanmarco M, Sampol J. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thrombosis and Haemostasis. 2004;91:667–673. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 46.Del Papa N, Meroni PL, Tincani A, Harris EN, Pierangeli SS, Barcellini W, Borghi MO, Balestrieri G, Zanussi C. Relationship between anti-phospholipid and anti-endothelial cell antibodies: further characterization of the reactivity on resting and cytokine-activated endothelial cells. Clin Exp Rheum. 1992;10:37–42. [PubMed] [Google Scholar]
- 47.Lambrianides A, Carroll CJ, Pierangeli SS, Pericleous C, Branch W, Rice J, Latchman DS, Townsend P, Isenberg DA, Rahman A, Giles IP. Effects of polyclonal IgG derived from patients with different clinical types of the antiphospholipid syndrome on monocyte signaling pathways. J Immunol. 2010;184:6622–6628. doi: 10.4049/jimmunol.0902765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vikerfors A, Mobarrez F, Bremme K, Holmstrom M, Agren A, Eelde A, Bruzelius M, Antovic A, Wallen H, Svenungsson E. Studies of microparticles in patients with the antiphospholipid syndrome (APS) Lupus. 2012;21:802–805. doi: 10.1177/0961203312437809. [DOI] [PubMed] [Google Scholar]
- 49.Jy W, Tiede M, Bidot CJ, Horstman LL, Jimenez JJ, Chirinos J, Ahn YS. Platelet activation rather than endothelial injury identifies risk of thrombosis in subjects positive for antiphospholipid antibodies. Thromb Res. 2007;121:319–325. doi: 10.1016/j.thromres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Sanchez C, Barbarroja N, Messineo S, Ruiz-Limon P, Rodriguez-Ariza A, Jimenez-Gomez Y, Khamashta MA, Collantes-Estevez E, Cuadrado MJ, Aguirre MA, Lopez-Pedrera C. Gene profiling reveals specific molecular pathways in the pathogenesis of atherosclerosis and cardiovascular disease in antiphospholipid syndrome, systemic lupus erythematosus and antiphospholipid syndrome with lupus. Ann Rheum Dis. 2014;10:204–600. doi: 10.1136/annrheumdis-2013-204600. [DOI] [PubMed] [Google Scholar]
- 51.De Groot PG, Lutters B, Derksen RH, Lisman T, Meijers JC, Rosendaal FR. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost. 2005;3:1993–1997. doi: 10.1111/j.1538-7836.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 52.de Laat B, Pengo V, Pabinger I, Musial J, Voskuyl AE, Bultink IE, Ruffatti A, Rozman B, Kveder T, de MP, Boehlen F, Rand J, Ulcova-Gallova Z, Mertens K, De Groot PG. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: an international multicenter study. J Thromb Haemost. 2009;7:1767–1773. doi: 10.1111/j.1538-7836.2009.03588.x. [DOI] [PubMed] [Google Scholar]
- 53.Pengo V, Biasiolo A, Brocco T, Tonetto S, Ruffatti A. Autoantibodies to phospholipid binding plasma proteins in patients with thrombosis and phospholipid-reactive antibodies. Thrombosis and Haemostasis. 1996;75:721–724. [PubMed] [Google Scholar]
- 54.Hunt JE, McNeil HP, Morgan GJ, Crameri RM, Krilis SA. A phospholipid-b2-glycoprotein complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992;1:75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, McGeoch SC, Johnstone AM, Holtrop G, Sneddon AA, Macrury SM, Megson IL, Pearson DW, Abraham P, De RB, Lobley GE, O'Kennedy N. Platelet-derived microparticle count and surface molecule expression differ between subjects with and without type 2 diabetes, independently of obesity status. J Thromb Thrombolysis. 2014;37:455–463. doi: 10.1007/s11239-013-1000-2. [DOI] [PubMed] [Google Scholar]
- 56.Mackman N, Luther T. Platelet tissue factor: to be or not to be. Thromb Res. 2013;132:3–5. doi: 10.1016/j.thromres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Willemze R, Bradford RL, Mooberry MJ, Roubey RA, Key NS. Plasma microparticle tissue factor activity in patients with antiphospholipid antibodies with and without clinical complications. Thromb Res. 2014;133:187–189. doi: 10.1016/j.thromres.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira J, Alfaro G, Goycoolea M, Quiroga T, Ocqueteau M, Massardo L, Perez C, Saez C, Panes O, Matus V, Mezzano D. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb Haemost. 2006;95:94–99. [PubMed] [Google Scholar]