Abstract
A key question in both wound healing and fibrosis is the trigger for the initial formation of scar tissue. To help form scar tissue, circulating monocytes enter the tissue and differentiate into fibroblast-like cells called fibrocytes, but fibrocyte differentiation is strongly inhibited by the plasma protein Serum Amyloid P (SAP), and healthy tissues contain very few fibrocytes. In wounds and fibrotic lesions, mast cells degranulate to release tryptase, and in early wounds thrombin mediates blood clotting. Tryptase and thrombin are upregulated in wound healing and fibrotic lesions, and inhibition of these proteases attenuates fibrosis. Here we report that tryptase and thrombin potentiate human fibrocyte differentiation at biologically relevant concentrations and exposure times, even in the presence of concentrations of serum and SAP that normally completely inhibit fibrocyte differentiation. The fibrocyte potentiation by thrombin and tryptase is mediated by protease-activated receptors 1 and 2, respectively. Together, these results suggest that tryptase and thrombin may be an initial trigger to override SAP inhibition of fibrocyte differentiation to initiate scar tissue formation.
Introduction
Poorly-healing chronic wounds affect more than 6.5 million US patients per year (1). The opposite of poorly healing wounds is fibrosing diseases, where inappropriate scar tissue forms in an organ (2). Fibrosing diseases such as pulmonary fibrosis, congestive heart failure, liver cirrhosis, and kidney fibrosis are involved in 45% of deaths in the United States (3). Both wound healing and fibrosis involve scar tissue formation. A key component of scar tissue is the fibrocyte (4, 5). Monocytes are recruited to wounds or fibrotic lesions, and in response to unknown wound signals differentiate into fibrocytes (6, 7). Fibrocytes express collagen and other extracellular matrix proteins, secrete pro-angiogenic factors, and activate nearby fibroblasts to proliferate and secrete collagen (4–6, 8–10).
In serum-free cultures, monocytes differentiate into fibrocytes, but the presence of as little as 0.01% serum inhibits fibrocyte differentiation (11, 12). Fibrocyte differentiation can be inhibited by the plasma protein Serum Amyloid P (SAP), interferon-γ, and IL-12 (12–15). The SAP concentration in plasma is ~30 µg/ml (16). The IC50 for SAP inhibition of fibrocyte differentiation is 0.2 µg/ml (12, 17), and ~1 µg/ml SAP completely inhibits fibrocyte differentiation (12). In vivo, SAP slows wound healing, while removing SAP from a wound promotes healing (18, 19). Conversely, SAP injections that double the serum SAP concentration inhibit fibrosis in a variety of animal models (20–23).
Normal tissues contain very few fibrocytes (10). In humans, in addition to being present in plasma, a considerable amount of SAP appears to be present in the interstitial space (24). A key question in wound healing and fibrosis is thus the mechanism that overrides the inhibitory effect of SAP (and other fibrocyte differentiation inhibitors) to induce fibrocyte differentiation. One of the events preceding scar tissue formation in a healing wound is the clotting cascade, in which the protease thrombin cleaves fibrinogen to fibrin. Thrombin activity is upregulated in fibrotic lesions (25) and immediately after wounding (26). Thrombin causes inflammation when added to mouse lungs, increased concentrations of thrombin within lungs exacerbate fibrosis, and inhibition of thrombin attenuates fibrosis (27–30). Thrombin cleaves a six amino acid recognition site which is found on protease activated receptor-1 (PAR-1) (31, 32). This receptor is found on a variety of cell types including monocytes (33), and mediates the ability of thrombin to induce platelet aggregation (34).
Mast cells are found in both internal fibrotic lesions and sites of wound healing (35–37). Mast cells degranulate in response to external stimuli (37) to release, among other things, the protease tryptase (37–39). Tryptase is upregulated in areas of increased mast cell degranulation, including wounds and especially in fibrotic lung tissue (35, 36, 38–40). Extracellular tryptase is upregulated and associates with collagen increases in scar tissue in idiopathic pulmonary fibrosis (40). Tryptase cleaves at lysine and arginine residues, except when the following amino acid is proline (41). Tryptase activates protease activated receptor-2 (PAR-2) (37, 42). This receptor is found on a variety of cells including monocytes (33), and mediates the ability of tryptase to increase the proliferation of, and collagen production by, fibroblasts (37). Intratracheal administration of tryptase causes inflammation, and inhibition of tryptase attenuates this inflammation (43–46). Inhibition of PAR-2 receptors attenuates collagen deposition in a heart disease model (47).
In this report we show that relatively brief exposures of human mononuclear cells to levels of thrombin and tryptase that would be found in a wound potentiate human fibrocyte differentiation, overriding the SAP and serum inhibition, and could be a triggering mechanism for fibrocyte-mediated wound healing and fibrotic lesions.
Materials and Methods
Cell isolation and culture
Human blood was collected from volunteers who gave written consent and with specific approval from the Texas A&M University human subjects Institutional Review Board. Peripheral blood mononuclear cells (PBMC) and monocytes were isolated as previously described (13), and cultured as previously described (48), in either serum-free or protein-free media. Protein-free media (PFM) was Fibrolife basal media (Lifeline Cell Technology, Walkersville, MD) supplemented with 10 mM HEPES (Sigma, St. Louis, MO), 1× non-essential amino acids (Sigma), 1 mM sodium pyruvate (Sigma), 2 mM glutamine (Lonza, Basel, Switzerland), 100 U/ml penicillin and 100 µg/ml streptomycin (Lonza). Serum-free media (SFM) was PFM further supplemented with 10 µg/ml recombinant human insulin (Sigma), 5 µg/ml recombinant human transferrin (Sigma), and 550 µg/ml filter-sterilized human albumin (isolated and checked for purity as previously described (48)), fish skin gelatin (Sigma), or skim milk powder (EMD Millipore, Billerica, MD). Protein supplements were checked for concentration using absorbance at 280 nm. SFM made with each supplement was mixed with 500 ng/ml TPCK-treated bovine trypsin (Sigma) for 24 hours at 37°C, and assayed on 4–20% SDS gels (Bio-Rad, Hercules, California), which were silver stained to check for purity and the presence of breakdown products. Where indicated, PFM was supplemented to 2.5% (v/v) with sterile filtered non-blood type specific human serum, tested negative for hepatitis A and B and HIV I and II (Lonza and Gemini Bio-products, West Sacramento, California). Monocytes were purified, tested for purity, and cultured as previously described (48, 49). Fibrocyte counts, total cell counts, and collagen staining were performed as previously described (48).
Proteases, PAR agonists, and PAR inhibitors
PAR-1 agonists SFLLRN-NH2 (American Peptide Company, Sunnyvale, CA) and SFLLRNPNDKYEPF (Tocris, Minneapolis, MN), PAR-2 agonists 2f-LIGRL-NH2 (EMD Millipore, Billerica, MD) and AC 55541 (Tocris), TPCK-treated bovine trypsin (10,000 BAEE units/mg, Sigma) and human thrombin (1000 NIH units/mg, Sigma) were resuspended following the manufacturer’s instructions. Tryptase purified from human mast cells (70 BPVANA units/mg, Fitzgerald, Acton, MA) was mixed with 15 kDa heparin from porcine stomach (Sigma) in a 1:10 molar ratio of tryptase to heparin immediately after thawing (50). Trypsin, tryptase, thrombin, PAR-1 agonist, and PAR-2 agonist were incubated with PBMC as described previously (48). For timecourse experiments, protease-containing SFM was completely removed from PBMC after the indicated times (4, 12, or 24 hours), then replaced with fresh SFM, or SFM containing SAP or serum, as indicated.
PAR-1 inhibitors SCH 79797 hydrochloride (Axon Medchem, Reston, VA, added to cells at 5 µg/ml (51)) and vorapaxar (Axon Mechem, 25 µg/ml (52)), and PAR-2 inhibitors ENMD-1068 (Enzo Life Sciences, Farmingdale, NY, 50 µg/ml (37)) and FSLLRY (Tocris, 1 µg/ml (47)) were resuspended following the manufacturer’s instructions. After a 1-hour incubation on ice in PFM, PBMC were collected by centrifugation at 300 x g for 10 minutes, resuspended in ice-cold PBS, and washed at 300 x g for 10 minutes, twice. PBMC were then plated and allowed to differentiate under the indicated conditions as described previously (48).
SAP and interferon-γ
Human SAP was purified as previously described (12) with the following modifications. Human serum from blood donors was mixed 1:1 with PBS. This was mixed with gentle rolling in a 10:1 ratio with SP sepharose beads (GE Healthcare, Piscataway, NJ) in 20 mM Tris, 140 mM NaCl, 2 mM CaCl2 pH 8.0 and eluted as previously described (12). The SAP purity was checked by silver stain on an SDS-PAGE gel, and concentration was assessed by absorbance at 280 nm. Proteases at 40 ng/ml were co-incubated with purified SAP, and the reactions were analyzed by PAGE with 4–20% SDS gels and silver stained to assess cleavage of SAP. Recombinant human interferon-γ (IFN-γ, Peprotech, Rocky Hill, NJ) was resuspended according to the manufacturer’s instructions. SAP and IFN-γ were then added to SFM at the indicated concentrations.
Transwell migration
Transwell migration of monocytes was performed as previously described (53), with the following modifications: The filter size was 8 µm, SFM was used as media, cells were allowed to pass through the filter for 12 hours, and adherent cells that passed through the filter were imaged with an InCell 2000 microscope (GE Healthcare) and then counted by CellProfiler (54).
Statistics
Statistics were performed using Prism (Graphpad Software, San Diego, CA). Differences were assessed by two-tailed t-tests or Mann-Whitney tests. Significance was defined by p<0.05.
Results
Tryptase and thrombin potentiate fibrocyte differentiation
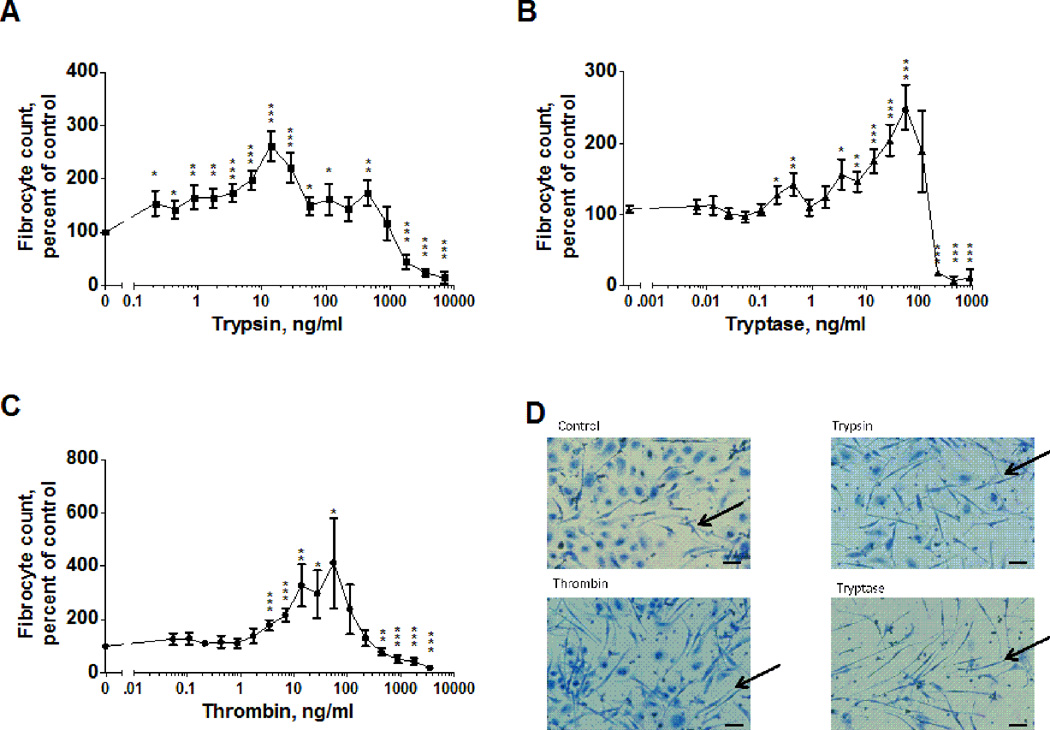
We previously observed that trypsin, but not chymotrypsin, pepsin, or endoprotease GluC, potentiates fibrocyte differentiation (48). Fibrocytes are involved in wound healing, and trypsin is used topically to speed wound healing (55–60). The extracellular levels of both tryptase and thrombin increase during the formation of scar tissue (26, 35). To determine whether tryptase and thrombin also potentiate fibrocyte differentiation, we examined the effect of these proteases on fibrocyte differentiation in culture. Our previous assays had been done in media with a supplement called ITS-3; due to difficulties in obtaining this, we substituted human albumin for ITS-3 (48). Human peripheral blood mononuclear cells (PBMC) were incubated with trypsin, tryptase or thrombin for 5 days in serum-free medium containing human albumin. The cells were then stained and scored for fibrocyte formation. In the absence of added proteases, we observed 388 to 1120 fibrocytes per 105 PBMCs from the different donors, similar to what we have previously observed (11, 13, 14, 48). Because of this variability, fibrocyte numbers were thus normalized to protease-free controls. In media with albumin as the supplement, 0.2 to 445 ng/ml trypsin potentiated fibrocyte differentiation (Figure 1A and D), encompassing the 20 to 200 ng/ml trypsin concentrations that we previously observed to increase fibrocyte differentiation in media with ITS-3 (48). Tryptase concentrations between 4 and 56 ng/ml and thrombin concentrations between 3 to 50 ng/ml also significantly increased the number of fibrocytes (Figures 1B–D). Tryptase concentrations above 100 ng/ml and thrombin concentrations above 400 ng/ml decreased the number of fibrocytes (Figure 1B and 1C). These effects were observed for all donors tested. In addition to having a unique morphology, fibrocytes express collagen, and the protease-induced increase in the number of visible fibrocytes was accompanied by an increase in the number of collagen-positive cells (Figure 2). The number of adherent cells following fixing and staining were not significantly affected by tryptase or thrombin (Supplementary Figure 1), suggesting that tryptase and thrombin specifically increase the number of differentiated fibrocytes, rather than increasing general cell viability or adhesion.
Figure 1. Trypsin, tryptase and thrombin potentiate fibrocyte differentiation.
PBMC were cultured in serum free media in the presence of the indicated concentrations of (A) trypsin (n=13), (B) tryptase (n=15), or (C) thrombin (n=14) for 5 days. Fibrocyte counts were normalized for each donor to the no-protease control. The no-protease controls developed 767 ± 90 fibrocytes per 105 PBMC. Values in A through C are mean ± SEM; the absence of error bars indicates that the error was smaller than the plot symbol. * indicates p < .05, ** p < .01, and *** p < .001 compared to the no-protease control (t-test). (D) Images of PBMC after 5 days with no protease (control), 12.5 ng/ml trypsin, 12.5 ng/ml thrombin, or 55 ng/ml tryptase. Arrows indicate fibrocytes. Bar is 40 µm.
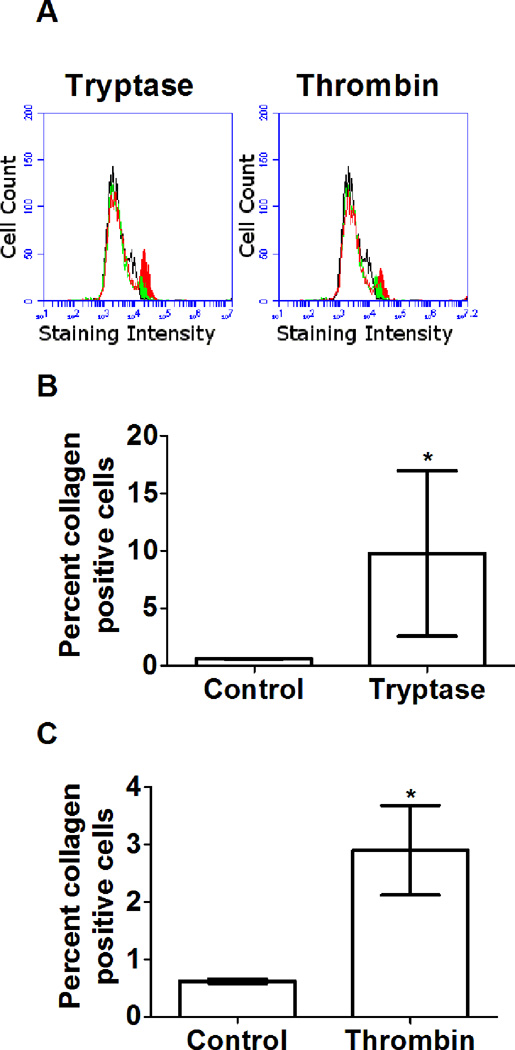
Figure 2. Tryptase and thrombin increase collagen secretion by PBMC.
(A) Representative flow plots of no-protease control PBMC stained with control rabbit IgG (black), and for collagen (green), and protease-treated PBMC stained for collagen (red). PBMC were exposed to 12.5 ng/ml (B) tryptase or (C) thrombin for 5 days, resuspended, stained for collagen, and assayed by flow cytometry. Values are mean ± SEM, n=5, * indicates p < .05 (t-test).
The plasma thrombin concentration in a clotting wound is ~37 nM (26). The molecular mass of thrombin is ~36 kDa, so this thrombin concentration is ~1.3 ×103 ng/ml. Far from a wound, the levels of active thrombin should be negligible, so assuming that a gradient of thrombin forms in the interstitial space, with the highest concentration in the clotting blood, at some point in the tissue near the wound the thrombin concentration would be on the order of the 3–50 ng/ml that potentiates fibrocyte differentiation in vitro (Figure 1). Tryptase requires stabilization by heparin to be enzymatically active (50). Tryptase (~35 kDa) signaling thus must be measured by its activity. In wound fluid, tryptase activity is ~0.30 mU/mg wound fluid protein, where a unit is the cleavage of 1 µmol/min of Z-gly-pro-arg-pNA (61). In the assay conditions used by (61) tryptase activity is ~1.75 × 108 mU/g tryptase, and 35 nM tryptase cleaves 30 pmol/sec of 0.2 mM Z-gly-pro-arg-pNA (62). Wound fluid contains ~31 mg/ml protein (63), and combining these indicates that wound fluid contains ~63 ng/ml tryptase, within the range of tryptase concentrations that promote fibrocyte differentiation in vitro (Figure 1). Together, these results suggest that physiological concentrations of thrombin and tryptase can potentiate fibrocyte differentiation.
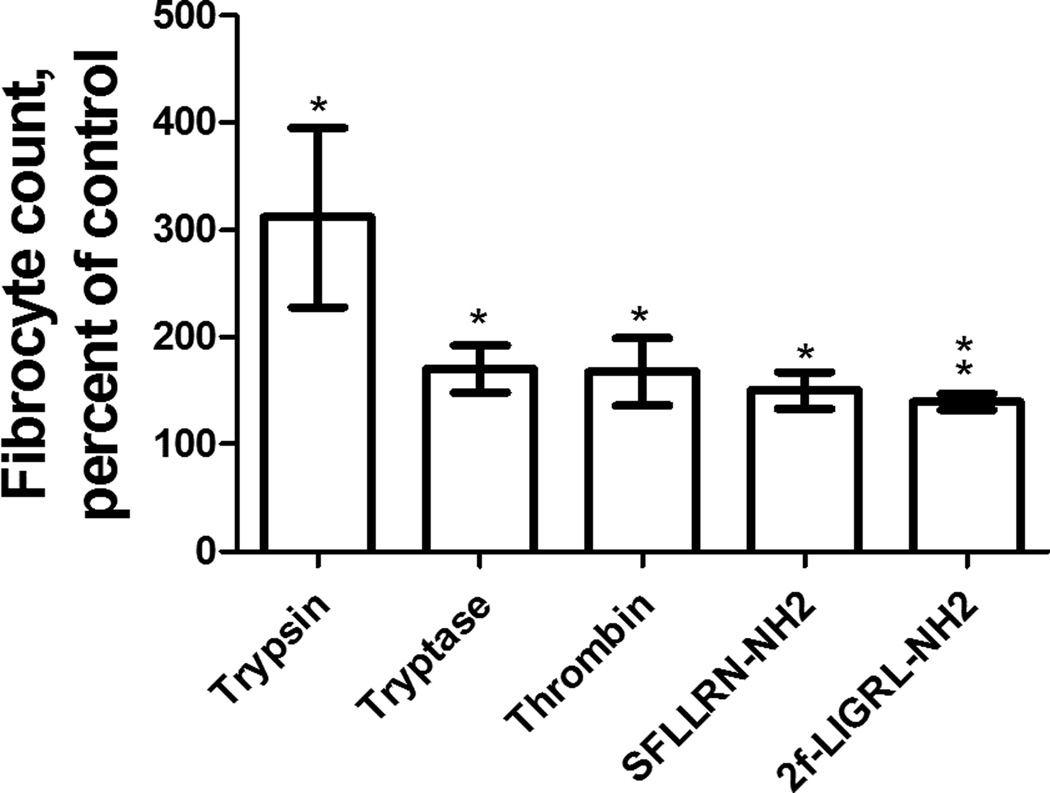
Fibrocytes differentiate from monocytes (4, 6, 11). Cells in the PBMC population include T cells, B cells, monocytes, and NK cells (12). To determine whether trypsin, tryptase, or thrombin act directly on monocytes to potentiate fibrocyte differentiation, we purified monocytes from PBMC by negative selection as previously described (48). Monocytes were 16% ± 9% (mean ± SEM, n=3) of the PBMCs and 92% ± 5% in the purified fraction. Trypsin, tryptase, and thrombin all potentiated the differentiation of fibrocytes from purified monocyte populations by a factor of ~2 (Figure 3), similar to the ~2-fold increase in fibrocyte these proteases caused in PBMC populations (Figure 1). Together, these results suggest that trypsin, tryptase, and thrombin directly affect monocytes to potentiate fibrocyte differentiation.
Figure 3. Trypsin, tryptase, thrombin, PAR-1 agonist, and PAR-2 agonist potentiate fibrocyte differentiation from purified monocytes.
Monocytes were cultured in SFM at 12.5 ng/ml protease or 10 µM agonist. After 5 days, fibrocytes were counted as in Figure 1. Values are mean ± SEM, n=3. * indicates p < .05, ** p < .01 compared to the SFM control (t-test).
Tryptase and thrombin potentiate fibrocyte differentiation in serum-containing media
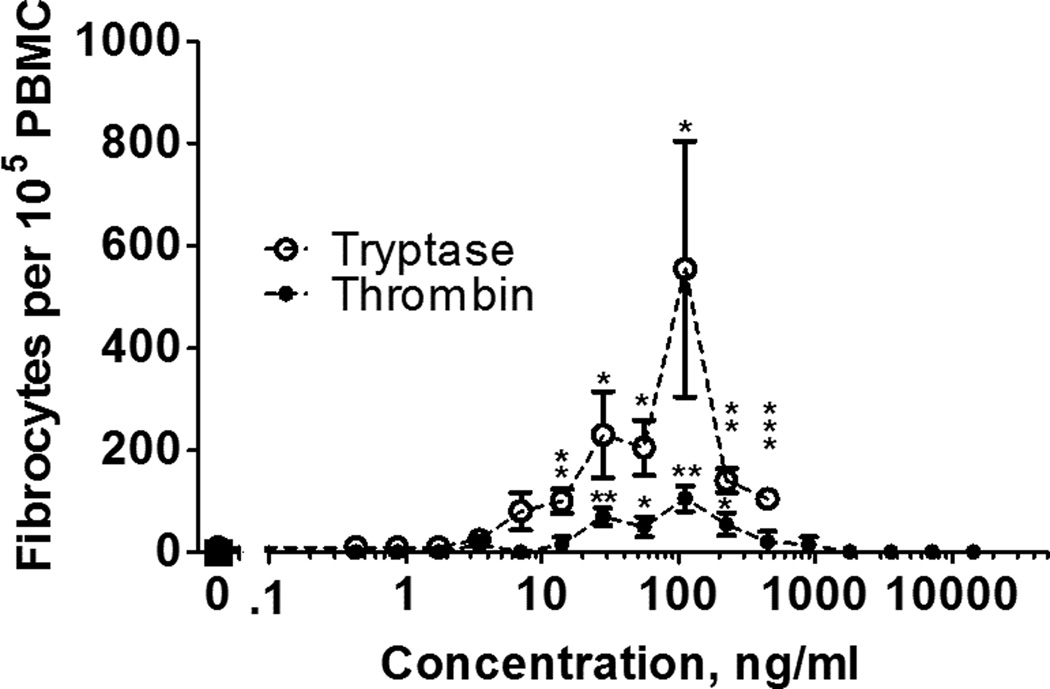
We previously observed that trypsin potentiates fibrocyte differentiation in media supplemented with human serum (48). Serum is present in a wound after blood clots (64). To determine whether tryptase and thrombin can potentiate fibrocyte differentiation in an environment containing serum, we incubated PBMC in serum-containing media for five days with tryptase and thrombin. As previously observed (12), serum inhibited fibrocyte differentiation (Figure 4). In the presence of serum, 14 to 446 ng/ml tryptase and 28 to 224 ng/ml thrombin potentiated fibrocyte differentiation (Figure 4), indicating that levels of these proteases that would be observed in a wound can override the inhibitory effect of serum.
Figure 4. Tryptase and thrombin potentiate fibrocyte differentiation in the presence of human serum.
PBMC were cultured in medium containing serum in the presence of the indicated concentrations of tryptase or thrombin for 5 days, and fibrocytes were counted as in Figure 1. Serum-containing media complete inhibited fibrocyte differentiation in the no-protease control. Values are mean ± SEM, n = 4. * indicates p < .05, ** p < .01, and *** p < .001 compared to the no-protease control (t-test).
Trypsin potentiates fibrocyte differentiation in the absence of albumin
The observation that three different proteases, in the presence of albumin, potentiate fibrocyte differentiation suggests that either the proteases potentiate fibrocyte differentiation, or a proteolytic fragment of albumin potentiates fibrocyte differentiation. To determine if fibrocyte potentiation is dependent on the protein composition of the defined media, we co-incubated PBMC with trypsin and fish gelatin or skim milk powder. Trypsin caused fibrocyte potentiation when mixed with fish gelatin or skim milk powder (Supplementary Figure 1). There is only a 15% sequence similarity between human albumin and fish gelatin, and the largest identical sequence is 3 amino acids. This suggests that after proteolytic digestion of the two proteins, there is no common peptide produced that could activate fibrocyte differentiation. The observation that trypsin potentiates fibrocyte differentiation in the presence of fish gelatin, and the absence of albumin, suggests that albumin is not necessary for the trypsin effect, and thus that trypsin may directly potentiate fibrocyte differentiation.
Trypsin, tryptase, and thrombin signal through protease-activated receptors
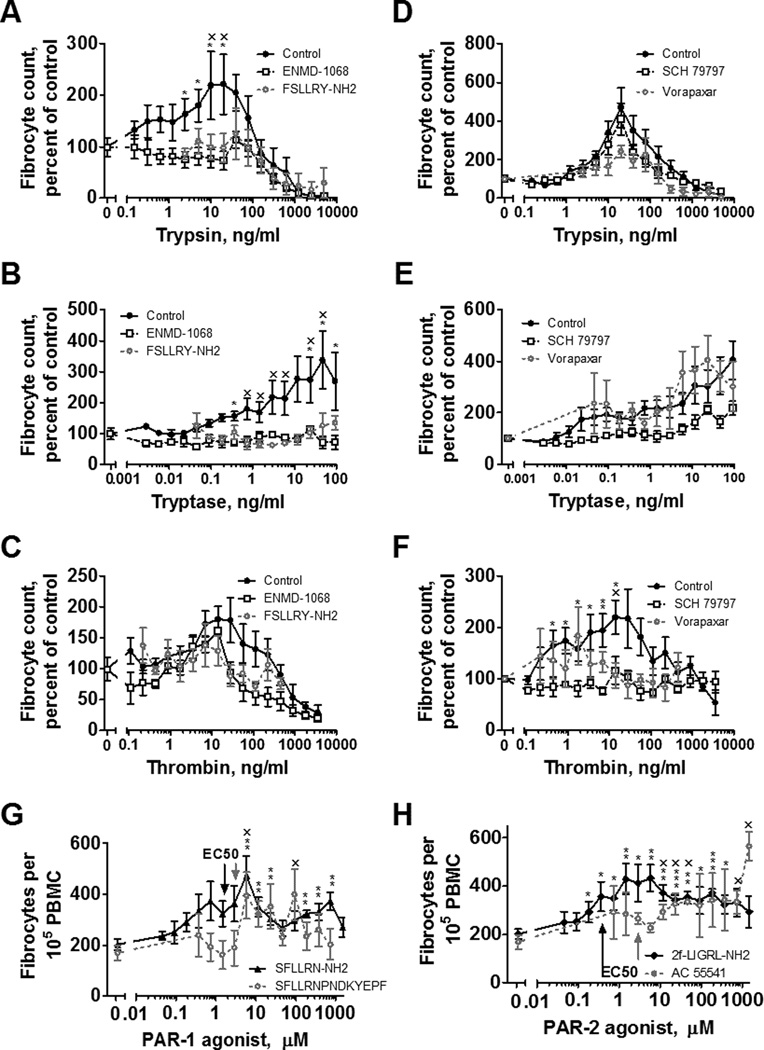
Since trypsin, rather than a proteolytic fragment of albumin, appears to potentiate fibrocyte differentiation, we examined the possibility that trypsin activates a cell surface receptor. Proteases can act as extracellular signals by cleaving the extracellular domains of protease-activated receptors (PARs) (65). PAR-1 and PAR-2 are expressed on human monocytes (33). To determine if trypsin, tryptase, or thrombin potentiate fibrocyte differentiation through PAR-1 or PAR-2, we examined the effect of these proteases on PBMC differentiation after exposure to PAR-1 and PAR-2 inhibitors. ENMD-1068 and FSLLYR-NH2 are peptides which selectively block PAR-2 activation but do not interfere with the activities of PAR-1, 3, or 4 (37, 47). ENMD-1068 at 26 nM and FSLLRY-NH2 at 12 nM inhibit PAR-2 function as measured by proliferation and collagen production in fibroblasts (37, 47). 26 nM ENMD-1068 and FSLLRY-NH2 decreased trypsin and tryptase potentiation of fibrocyte differentiation (Figure 5A and B), but thrombin potentiation of fibrocyte differentiation was not significantly affected (Figure 5C).
Figure 5. PAR-1 and PAR-2 affect fibrocyte differentiation.
PBMC were incubated with (A–C) inhibitors of protease activated receptor-2 (PAR-2) or (D–F) inhibitors of protease activated receptor-1 (PAR-1) before mixing with (A, D) trypsin, (B, E) tryptase, or (C, F) thrombin at the indicated concentrations. (G) PAR-1 agonists or (H) PAR-2 agonists were added to PBMC at the indicated concentrations. After 5 days, fibrocytes were counted as in Figure 1. Values are mean ± SEM, n=6. * indicates p < .05, ** p < .01, and *** p < .001 compared to the agonist-free or protease-free control (t-test) for ENMD-1068, SCH 79797, SFLLRN-NH2, and 2f–LIGRL-NH2. x indicates p < .05 for FSLLRY-NH2, Vorapaxar, SFLLRNDKYEPF, and AC 55541, each n=3. In G and H, arrows indicate the published EC50 concentrations.
SCH79797 and vorapaxar are inhibitors of PAR-1 that do not interfere with PAR-2 signaling (51, 52). SCH79797 at 70 nM and vorapaxar at 47 nM inhibit PAR-1 function in platelet aggregation assays (51, 52). SCH79797 and vorapaxar did not significantly affect trypsin’s or tryptase’s fibrocyte potentiation (Figure 5D and 5E), but blocked the ability of thrombin to potentiate fibrocyte differentiation (Figure 5F). Conversely, PAR-1 and PAR-2 agonists potentiated fibrocyte differentiation at concentrations similar to previously observed effective concentrations (66–69) when added to PBMCs (Figure 5G and 5H) or to purified monocytes (Figure 3). These data suggest that tryptase and trypsin signal through PAR-2, and thrombin signals through PAR-1, to potentiate fibrocyte differentiation.
Tryptase and thrombin compete with SAP to potentiate fibrocyte differentiation
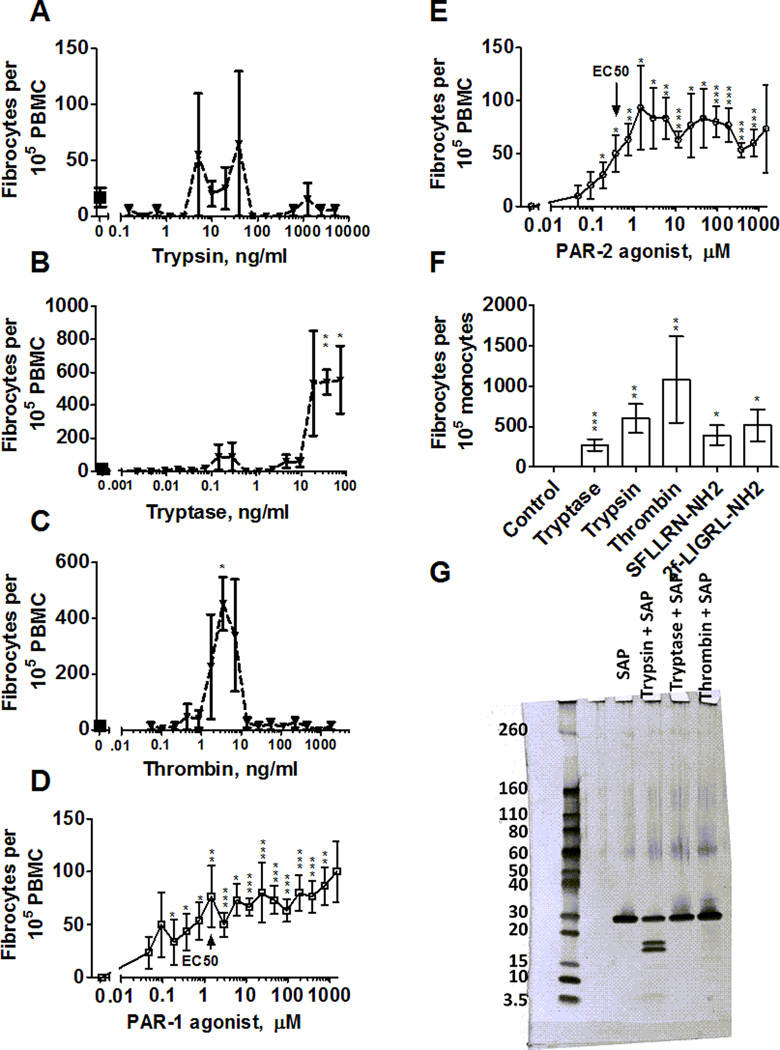
SAP inhibits the differentiation of monocytes into fibrocytes, while trypsin, tryptase and thrombin potentiate this differentiation. Tryptase and thrombin are present in wounds along with SAP (26, 35). To determine how these signals might compete with each other, we co-incubated SAP with proteases. As previously observed (12), 10 µg/ml SAP completely inhibited fibrocyte differentiation (Figure 6 A-F). In the presence of 10 µg/ml SAP, trypsin showed no significant potentiation of fibrocytes from PBMC (Figure 6A) but did potentiate fibrocyte differentiation from monocytes (Figure 6F). Levels of tryptase and thrombin that would be observed in a wound, as well as PAR-1 and PAR-2 agonists, competed with 10 µg/ml SAP to potentiate fibrocyte differentiation from PBMC (Figure 6B–E) and purified monocytes (Figure 6F). This potentiation was not caused by protease digestion of SAP, as only trypsin caused any measurable cleavage of SAP (Figure 6G).
Figure 6. Tryptase and thrombin compete with SAP to potentiate fibrocyte differentiation, but do not obviously digest SAP.
PBMC mixed with SAP at 10 µg/ml were incubated with the indicated concentrations of (A) trypsin, (B) tryptase, (C) thrombin, (D) PAR-1 agonist SFLLRN-NH2, or (E) PAR-2 agonist 2f–LIGRL-NH2 for 5 days. Fibrocytes were then counted as in Figure 1. Values are mean ± SEM, n=4. * indicates p < .05 compared to SAP with no protease or no agonist (t-test). (F) Monocytes in SFM were co-incubated with 10 µg/ml SAP, in the absence (control) or presence of 12.5 ng/ml protease, or 10 µM PAR-1 or PAR-2 agonist. After 5 days, fibrocytes were counted as in Figure 3. Values are mean ± SEM, n=3. * indicates p < .05, ** p < .01, and *** p < .001 compared to the SAP control (t-test). (G) Purified SAP was incubated with proteases at 40 ng/ml for 24 hours at 37° C, run on a 4–20% SDS-PAGE gel and silver stained. Molecular masses in kDa are indicated at left.
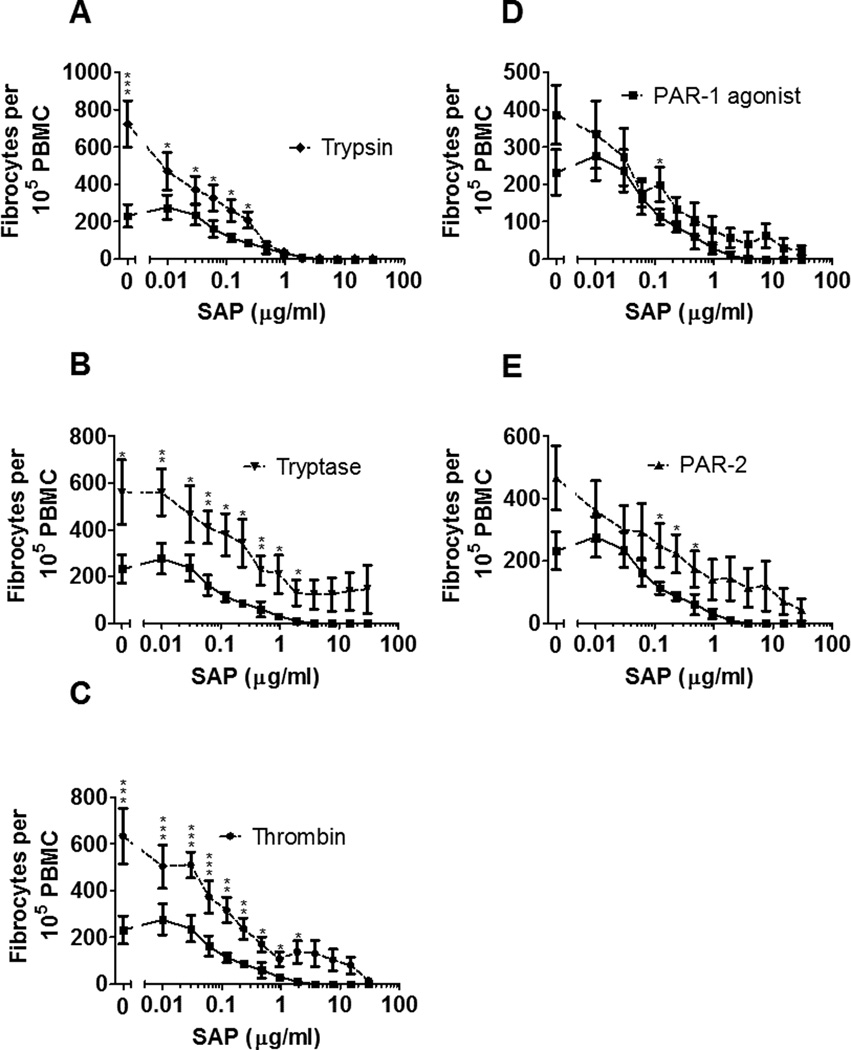
To determine if trypsin, tryptase or thrombin change the IC50 of SAP’s inhibition of fibrocyte differentiation, we co-incubated each protease with a series of SAP concentrations (Figure 7, Supplemental Figure 1E). Trypsin, tryptase, thrombin, PAR-1 agonist, and PAR-2 agonist significantly potentiated fibrocyte differentiation in the presence of SAP at some of the indicated SAP concentrations (Figure 7). SAP inhibited fibrocyte differentiation with an IC50 of 0.16 ± 0.05 µg/ml. SAP’s IC50 with trypsin was 0.23 ± 0.13 µg/ml; with tryptase 0.39 ± 0.13 µg/ml; with thrombin 1.03 ± 0.46 µg/ml; with PAR-1 agonist 0.44 ± 0.31 µg/ml; and with PAR-2 agonist 1.59 ± 0.77 µg/ml. The tryptase, thrombin, and PAR-2 agonist effects on the IC50 were significant (p<0.05; Mann-Whitney test). Together, these results indicate that tryptase and thrombin can compete with SAP to potentiate fibrocyte differentiation.
Figure 7. Tryptase and thrombin alter SAP’s IC50.
PBMC were co-incubated with the indicated concentrations of SAP and 12.5 ng/ml (A) trypsin, (B) tryptase, (C) thrombin, or 10 µM (D) PAR-1 agonist or (E) PAR-2 agonist. After 5 days, fibrocytes were counted as in Figure 1. In A-E, the lower curve is SAP alone. Values are mean ± SEM, n=6. * indicates p < .05, ** p < .01, and *** p < .001 compared to the no-protease or no-agonist control (Mann-Whitney test).
Trypsin, tryptase, thrombin, PAR-1 and PAR-2 agonist compete with interferon-gamma (IFN-γ) to potentiate fibrocyte differentiation
Like SAP, interferon-gamma (IFN-γ) is present in wounds and scar tissue (70, 71) and inhibits the differentiation of monocytes into fibrocytes (15). To determine how these signals might compete with each other, we co-incubated proteases with IFN-γ. As previously observed, 10 ng/ml IFN-γ inhibited fibrocyte differentiation (15). Each protease and receptor agonist potentiated fibrocyte differentiation in the presence of 10 ng/ml IFN-γ (Supplementary Figure 2).
To determine if proteases or PAR agonists act as monocyte chemoattracts or chemorepellents, we placed PBMC in the upper cup of a Boyden chamber and added each protease or agonist above, below, or on both sides of the filter. Each protease reduced PBMC migration through the filter, suggesting that these proteases may have a chemostatic effect on PBMC regardless of whether the protease was added above or below the filter (Supplementary Figure 2). Each agonist acted as a chemoattractant whether added above or below the filter (Supplementary Figure 2).
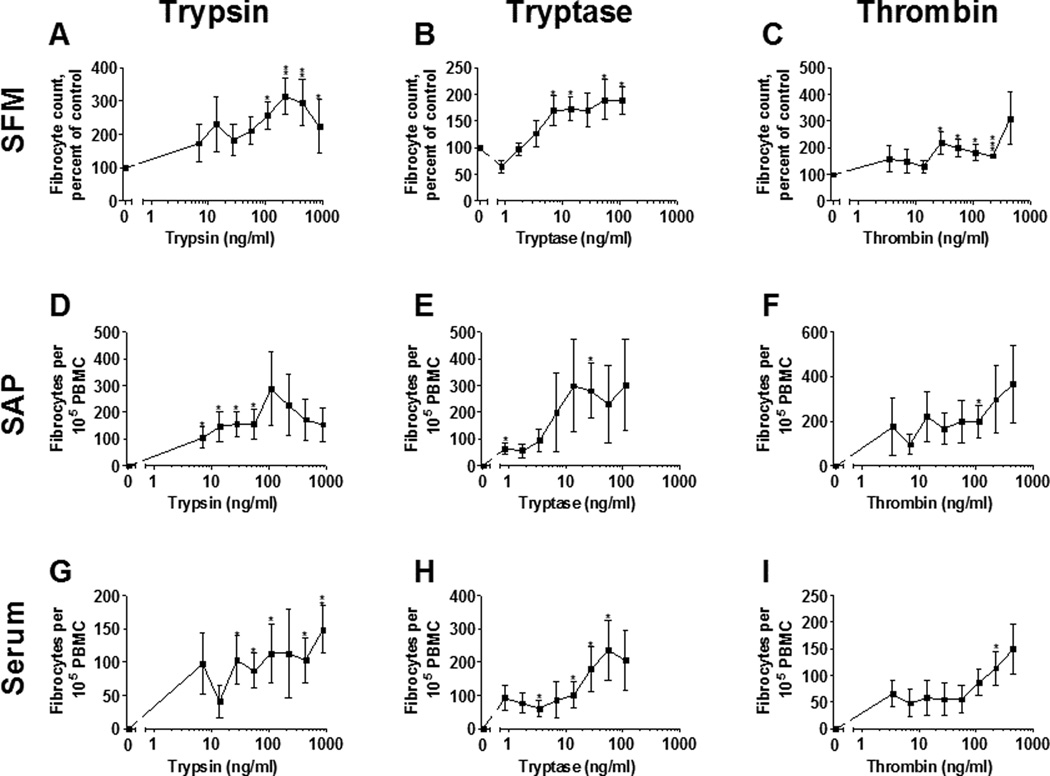
A brief exposure to trypsin, tryptase, and thrombin potentiates fibrocyte differentiation
Tryptase and thrombin are proteolytically active over short time frames in wounds and in scar tissue (26, 50). To determine if a brief exposure to these proteases is sufficient to potentiate fibrocyte differentiation, we allowed PBMC to adhere to plates and exposed them to tryptase, trypsin, or thrombin for 4 (Figure 8), 12 (Supplementary Figure 3), or 24 hours (Supplementary Figure 4), then completely removed the media and added fresh, protease-free media to the PBMC for the rest of the 5 day assay. These conditions approximate the bursts of time proteases would be active in a fibrotic lesion or a healing wound environment.
Figure 8. A 4 hour exposure to tryptase, trypsin, and thrombin potentiates fibrocyte differentiation.
PBMC were cultured in (A–C) SFM, (D–F) SFM with 2.5 µg/ml SAP, or (G–I) PFM with 2.5% (v/v) human serum in the presence of the indicated concentrations of (A,D,G) trypsin, (B,E,H) tryptase, or (C,F,I) thrombin for 4 hours, after which media was removed and replaced with (A–C) SFM , (D–F) SFM with 2.5 µg/ml SAP, or (G–I) PFM with 2.5% (v/v) human serum. Cells were then allowed to differentiate over the remainder of the five-day assay, and fibrocytes were counted as in Figure 1. Serum-containing and SAP-containing media completely inhibited fibrocyte differentiation in the protease-free control. Values are mean ± SEM, n = 5. * indicates p < .05, ** p < .01 and *** p < .001 compared to the protease-free control (t-test).
At each timepoint, tryptase, trypsin, and thrombin were able to potentiate fibrocyte differentiation over a broader range than had been previously established in by exposing PBMC to proteases for 5 days (Figure 1). Similarly, proteases at each timepoint were able to significantly potentiate fibrocyte differentiation in the presence of 2.5 µg/ml SAP or 2.5% (v/v) human serum. These data suggest that even a brief exposure to tryptase, trypsin, or thrombin over biologically relevant concentrations is sufficient to induce fibrocyte differentiation.
Discussion
In this report, we show that tryptase and thrombin potentiate fibrocyte differentiation and collagen production, and that this potentiation occurs even in the presence of levels of serum or SAP that completely inhibit fibrocyte differentiation. Tryptase and thrombin potentiation appears to act directly on monocytes, and is mediated by PAR-2 and PAR-1, respectively. Tryptase and thrombin potentiate fibrocyte differentiation at biologically relevant concentrations and exposure times. Not all proteases are pro-fibrotic, as pepsin and chymotrypsin do not activate PAR-1 or PAR-2 (72–74), and do not potentiate fibrocyte differentiation (48). These results suggest a triggering mechanism for fibrocyte-mediated wound healing and fibrotic lesions, where thrombin from clotting blood or tryptase from a sufficient amount of mast cell degranulation override SAP inhibition and initiate fibrocyte differentiation.
Tryptase, thrombin, PAR-1 signaling, and PAR-2 signaling have been implicated in the development of fibrosis through their effects on fibroblasts (25, 31, 32, 37, 40, 75–78). Both PAR-1 and −2 have been implicated in liver fibrosis in mice (79, 80), and both PAR-1 knockout mice and PAR-2 knockout mice are less susceptible to both induced heart disease and inflammation (81–83). Intratracheal administration of trypsin, tryptase, and thrombin cause inflammation, and inhibition of tryptase and thrombin attenuate this inflammation (27, 30, 43, 45). Inhibitors of tryptase (84–86) and thrombin (87), and antagonists of both PAR-1 (88) and PAR-2 (89) are patented for the treatment of fibrosis. Both tryptase (90) and thrombin (91) inhibitors are currently in clinical trials for the treatment of fibrosis. However, while PAR-1 and PAR-2 antagonists have been suggested as therapeutics (92, 93), neither is currently in clinical trials. PAR-1 and PAR-2 thus mediate both fibroblast proliferation and fibrocyte differentiation, two major components of scar tissue (49, 94–96). Our work thus strongly supports and expands the idea that tryptase, thrombin, PAR-1 signaling, and PAR-2 signaling potentiate wound healing and fibrosis.
Systemic mastocytosis involves the degranulation of mast cells throughout the body (97), and is associated with serious local, and moderate systemic, fibrosis (98, 99). Mast cell degranulation causes the release of tryptase. Serum tryptase in healthy patients is ~2 ng/ml, while mastocytosis patients have 20 to 100 ng/ml (100, 101). Our observation that 4 to 56 ng/ml tryptase potentiates fibrocyte differentiation suggests that the fibrosis seen in mastocytosis patients may be due to the released tryptase inducing fibrocyte differentiation.
That tryptase and thrombin compete with SAP suggests that the PAR-1 or PAR-2 pathway is capable of potentiating fibrocyte differentiation in the presence of SAP. Trypsin potentiates fibrocyte differentiation in the presence of SAP after a brief exposure (Figures 8–10), but not over the course of a 5-day differentiation (Figure 6A and Figure 7A). Tryptase and thrombin potentiated fibrocyte differentiation under all timecourse, SAP, and serum conditions. Tryptase and trypsin appear to signal through the same receptor (37). Whether our results imply that different PAR-2 isoforms exist, or that PAR-2 can differentiate between trypsin and tryptase signaling, is unclear.
A protein additive was necessary for fibrocyte potentiation. While albumin mixed with protease was the most effective media treatment for potentiating fibrocyte differentiation, both fish gelatin and milk powder also increased fibrocyte differentiation when mixed with protease. This suggests that the fibrocyte potentiation caused by proteases relies on a suitable protein additive, not solely albumin. Albumin is increased in fibrotic lesions (25) and healing wounds (102), and is decreased in chronic, non-healing wounds (103–105), implying that albumin’s presence may mediate the protease signaling that, by activating fibrocyte differentiation, may initiate key aspects of wound healing and fibrosis.
Supplementary Material
Acknowledgements
We thank the blood donors and the staff at Beutel Student Health Center for blood draws.
This work was supported by NIH grant HL118507.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- 6.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 7.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 8.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O’Byrne PM, Strieter RM, Kolb M. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 9.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8:145–150. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 11.Pilling D, Vakil V, Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods. 2009;351:62–70. doi: 10.1016/j.jim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox N, Pilling D, Gomer RH. NaCl Potentiates Human Fibrocyte Differentiation. PLoS One. 2012;7:e45674. doi: 10.1371/journal.pone.0045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One. 2011;6:e26078. doi: 10.1371/journal.pone.0026078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson SR, Tennent GA, Sethi D, Gower PE, Ballardie FW, Amatayakul-Chantler S, Pepys MB. Serum amyloid P component in chronic renal failure and dialysis. Clinica chimica acta; international journal of clinical chemistry. 1991;200:191–199. doi: 10.1016/0009-8981(91)90090-y. [DOI] [PubMed] [Google Scholar]
- 17.Crawford JR, Pilling D, Gomer RH. FcgammaRI mediates serum amyloid P inhibition of fibrocyte differentiation. J Leukoc Biol. 2012;92:699–711. doi: 10.1189/jlb.0112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomer RH, Pilling D, Kauvar LM, Ellsworth S, Ronkainen SD, Roife D, Davis SC. A serum amyloid P-binding hydrogel speeds healing of partial thickness wounds in pigs. Wound Repair Regen. 2009;17:397–404. doi: 10.1111/j.1524-475X.2009.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik-Mathuria B, Pilling D, Crawford JR, Gay AN, Smith CW, Gomer RH, Olutoye OO. Serum amyloid P inhibits dermal wound healing. Wound Repair Regen. 2008;16:266–273. doi: 10.1111/j.1524-475X.2008.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira AP, Cavassani KA, Hullinger R, Rosada RS, Fong DJ, Murray L, Hesson DP, Hogaboam CM. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. The Journal of allergy and clinical immunology. 2010;126:712–721. doi: 10.1016/j.jaci.2010.06.010. e717. [DOI] [PubMed] [Google Scholar]
- 21.Murray LA, Rosada R, Moreira AP, Joshi A, Kramer MS, Hesson DP, Argentieri RL, Mathai S, Gulati M, Herzog EL, Hogaboam CM. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5:e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, Elias JA, Hogaboam CM, Herzog EL. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43:154–162. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jager PL, Hazenberg BP, Franssen EJ, Limburg PC, van Rijswijk MH, Piers DA. Kinetic studies with iodine-123-labeled serum amyloid P component in patients with systemic AA and AL amyloidosis and assessment of clinical value. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998;39:699–706. [PubMed] [Google Scholar]
- 25.Duitman J, Ruela-de-Sousa RR, Shi K, De Boer OJ, Borensztajn KS, Florquin S, Peppelenbosch MP, Spek CA. Protease activated receptor-1 deficiency diminishes bleomycin-induced skin fibrosis. Mol Med. 2014 doi: 10.2119/molmed.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Blood coagulation at the site of microvascular injury: effects of low-dose aspirin. Blood. 2001;98:2423–2431. doi: 10.1182/blood.v98.8.2423. [DOI] [PubMed] [Google Scholar]
- 27.Moffatt JD, Lever R, Page CP. Effects of inhaled thrombin receptor agonists in mice. Br J Pharmacol. 2004;143:269–275. doi: 10.1038/sj.bjp.0705926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, Moffatt JD, Laurent GJ, Derian CK, Eickelberg O, Chambers RC. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119:2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell DC, Goldsack NR, Marshall RP, McAnulty RJ, Starke R, Purdy G, Laurent GJ, Chambers RC. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. The American journal of pathology. 2001;159:1383–1395. doi: 10.1016/S0002-9440(10)62525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogatkevich GS, Ludwicka-Bradley A, Nietert PJ, Akter T, van Ryn J, Silver RM. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheum. 2011;63:1416–1425. doi: 10.1002/art.30255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 32.Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci U S A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Pedrera C, Aguirre MA, Buendia P, Barbarroja N, Ruiz-Limon P, Collantes-Estevez E, Velasco F, Khamashta M, Cuadrado MJ. Differential expression of protease-activated receptors in monocytes from patients with primary antiphospholipid syndrome. Arthritis Rheum. 2010;62:869–877. doi: 10.1002/art.27299. [DOI] [PubMed] [Google Scholar]
- 34.Gallwitz M, Enoksson M, Thorpe M, Hellman L. The extended cleavage specificity of human thrombin. PLoS One. 2012;7:e31756. doi: 10.1371/journal.pone.0031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermes B, Feldmann-Boddeker I, Welker P, Algermissen B, Steckelings MU, Grabbe J, Henz BM. Altered expression of mast cell chymase and tryptase and of c-Kit in human cutaneous scar tissue. The Journal of investigative dermatology. 2000;114:51–55. doi: 10.1046/j.1523-1747.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 36.Pesci A, Majori M, Piccoli ML, Casalini A, Curti A, Franchini D, Gabrielli M. Mast cells in bronchiolitis obliterans organizing pneumonia. Mast cell hyperplasia and evidence for extracellular release of tryptase. Chest. 1996;110:383–391. doi: 10.1378/chest.110.2.383. [DOI] [PubMed] [Google Scholar]
- 37.Wygrecka M, Dahal BK, Kosanovic D, Petersen F, Taborski B, von Gerlach S, Didiasova M, Zakrzewicz D, Preissner KT, Schermuly RT, Markart P. Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: role of transmembrane SCF and the PAR-2/PKC-alpha/Raf-1/p44/42 signaling pathway. The American journal of pathology. 2013;182:2094–2108. doi: 10.1016/j.ajpath.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Eklund A, van Hage-Hamsten M, Skold CM, Johansson SG. Elevated levels of tryptase in bronchoalveolar lavage fluid from patients with sarcoidosis. Sarcoidosis. 1993;10:12–17. [PubMed] [Google Scholar]
- 39.Walls AF, Bennett AR, Godfrey RC, Holgate ST, Church MK. Mast cell tryptase and histamine concentrations in bronchoalveolar lavage fluid from patients with interstitial lung disease. Clinical science. 1991;81:183–188. doi: 10.1042/cs0810183. [DOI] [PubMed] [Google Scholar]
- 40.Andersson CK, Andersson-Sjoland A, Mori M, Hallgren O, Pardo A, Eriksson L, Bjermer L, Lofdahl CG, Selman M, Westergren-Thorsson G, Erjefalt JS. Activated MCTC mast cells infiltrate diseased lung areas in cystic fibrosis and idiopathic pulmonary fibrosis. Respiratory research. 2011;12:139. doi: 10.1186/1465-9921-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez J, Gupta N, Smith RD, Pevzner PA. Does trypsin cut before proline? J Proteome Res. 2008;7:300–305. doi: 10.1021/pr0705035. [DOI] [PubMed] [Google Scholar]
- 42.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 43.Huang C, De Sanctis GT, O’Brien PJ, Mizgerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL. Evaluation of the substrate specificity of human mast cell tryptase beta I and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 44.Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, Moon SH, Cao B, Ogbu C, Jeong KW, Kozu G, Nakanishi H, Kahn M, Chi EY, Henderson WR., Jr Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002;168:1992–2000. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- 45.Krishna MT, Chauhan A, Little L, Sampson K, Hawksworth R, Mant T, Djukanovic R, Lee T, Holgate S. Inhibition of mast cell tryptase by inhaled APC 366 attenuates allergen-induced late-phase airway obstruction in asthma. The Journal of allergy and clinical immunology. 2001;107:1039–1045. doi: 10.1067/mai.2001.115631. [DOI] [PubMed] [Google Scholar]
- 46.Saw S, Kale SL, Arora N. Serine protease inhibitor attenuates ovalbumin induced inflammation in mouse model of allergic airway disease. PLoS One. 2012;7:e41107. doi: 10.1371/journal.pone.0041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLarty JL, Melendez GC, Brower GL, Janicki JS, Levick SP. Tryptase/Protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58:264–270. doi: 10.1161/HYPERTENSIONAHA.111.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White MJ, Glenn M, Gomer RH. Trypsin potentiates human fibrocyte differentiation. PLoS One. 2013;8:e70795. doi: 10.1371/journal.pone.0070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz LB, Bradford TR. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986;261:7372–7379. [PubMed] [Google Scholar]
- 51.Ahn HS, Foster C, Boykow G, Stamford A, Manna M, Graziano M. Inhibition of cellular action of thrombin by N3-cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H–pyrrolo[3, 2-f]quinazoline-1,3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochemical pharmacology. 2000;60:1425–1434. doi: 10.1016/s0006-2952(00)00460-3. [DOI] [PubMed] [Google Scholar]
- 52.Chackalamannil S, Wang Y, Greenlee WJ, Hu Z, Xia Y, Ahn HS, Boykow G, Hsieh Y, Palamanda J, Agans-Fantuzzi J, Kurowski S, Graziano M, Chintala M. Discovery of a novel, orally active himbacine-based thrombin receptor antagonist (SCH 530348) with potent antiplatelet activity. Journal of medicinal chemistry. 2008;51:3061–3064. doi: 10.1021/jm800180e. [DOI] [PubMed] [Google Scholar]
- 53.Maharjan AS, Roife D, Brazill D, Gomer RH. Serum amyloid P inhibits granulocyte adhesion. Fibrogenesis Tissue Repair. 2013;6:2. doi: 10.1186/1755-1536-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamentsky L, Jones TR, Fraser A, Bray MA, Logan DJ, Madden KL, Ljosa V, Rueden C, Eliceiri KW, Carpenter AE. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics. 2011;27:1179–1180. doi: 10.1093/bioinformatics/btr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anigstein L. United States Patent Office, United States. 1959. Wound healing agent obtained from blood and method of preparation. [Google Scholar]
- 56.Fortney DZ. United States Patent Office. United States: W.R. Grace and Company; 1996. Compositions containing protease produced by vibrio and method of use in debridement and wound healing. [Google Scholar]
- 57.Martin GJ. United States Patent Office. United States: The National Drug Company; 1961. Wound healing composition. [Google Scholar]
- 58.Carson SN, Wiggins C, Overall K, Herbert J. Using a castor oil-balsam of Peru-trypsin ointment to assist in healing skin graft donor sites. Ostomy Wound Manage. 2003;49:60–64. [PubMed] [Google Scholar]
- 59.Cetrulo GI. Use of trypsin intravenously in a gunshot wound. J Am Med Assoc. 1953;152:605–606. doi: 10.1001/jama.1953.63690070001010. [DOI] [PubMed] [Google Scholar]
- 60.Pritchard DI. Treatment of wounds. In: U. S. P. Office, editor. United States Patent Office. United States: 2011. [Google Scholar]
- 61.Huttunen M, Harvima IT. Mast cell tryptase and chymase in chronic leg ulcers: chymase is potentially destructive to epithelium and is controlled by proteinase inhibitors. The British journal of dermatology. 2005;152:1149–1160. doi: 10.1111/j.1365-2133.2005.06428.x. [DOI] [PubMed] [Google Scholar]
- 62.Harvima IT, Harvima RJ, Eloranta TO, Fraki JE. The allosteric effect of salt on human mast cell tryptase. Biochimica et biophysica acta. 1988;956:133–139. doi: 10.1016/0167-4838(88)90259-2. [DOI] [PubMed] [Google Scholar]
- 63.Iizaka S, Sanada H, Nakagami G, Sekine R, Koyanagi H, Konya C, Sugama J. Estimation of protein loss from wound fluid in older patients with severe pressure ulcers. Nutrition. 2010;26:890–895. doi: 10.1016/j.nut.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Laiho K. Albumin as a marker of plasma transudation in experimental skin lesions. International journal of legal medicine. 2004;118:282–288. doi: 10.1007/s00414-004-0460-5. [DOI] [PubMed] [Google Scholar]
- 65.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiological reviews. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 66.Hoekstra WJ, Hulshizer BL, McComsey DF, Andrade-Gordon P, Kauffman JA, Addo MF, Oksenberg D, Scarborough RM, Maryanoff BE. Thrombin receptor (PAR-1) antagonists. Heterocycle-based peptidomimetics of the SFLLR agonist motif. Bioorganic & medicinal chemistry letters. 1998;8:1649–1654. doi: 10.1016/s0960-894x(98)00292-3. [DOI] [PubMed] [Google Scholar]
- 67.Kanke T, Ishiwata H, Kabeya M, Saka M, Doi T, Hattori Y, Kawabata A, Plevin R. Binding of a highly potent protease-activated receptor-2 (PAR2) activating peptide, [3H]2-furoyl-LIGRL-NH2, to human PAR2. Br J Pharmacol. 2005;145:255–263. doi: 10.1038/sj.bjp.0706189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vassallo RR, Jr, Kieber-Emmons T, Cichowski K, Brass LF. Structure-function relationships in the activation of platelet thrombin receptors by receptor-derived peptides. J Biol Chem. 1992;267:6081–6085. [PubMed] [Google Scholar]
- 69.Gardell LR, Ma JN, Seitzberg JG, Knapp AE, Schiffer HH, Tabatabaei A, Davis CN, Owens M, Clemons B, Wong KK, Lund B, Nash NR, Gao Y, Lameh J, Schmelzer K, Olsson R, Burstein ES. Identification and characterization of novel small-molecule protease-activated receptor 2 agonists. The Journal of pharmacology and experimental therapeutics. 2008;327:799–808. doi: 10.1124/jpet.108.142570. [DOI] [PubMed] [Google Scholar]
- 70.Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- 71.Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S, Okamura H, Kurimoto M, Hiraga Y, Tatsuno T, Abe S, Sato N. IL-12 and IL-18 are increased and stimulate IFN-gamma production in sarcoid lungs. J Immunol. 2001;166:642–649. doi: 10.4049/jimmunol.166.1.642. [DOI] [PubMed] [Google Scholar]
- 72.Kawao N, Sakaguchi Y, Tagome A, Kuroda R, Nishida S, Irimajiri K, Nishikawa H, Kawai K, Hollenberg MD, Kawabata A. Protease-activated receptor-2 (PAR-2) in the rat gastric mucosa: immunolocalization and facilitation of pepsin/pepsinogen secretion. Br J Pharmacol. 2002;135:1292–1296. doi: 10.1038/sj.bjp.0704562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Altrogge LM, Monard D. An assay for high-sensitivity detection of thrombin activity and determination of proteases activating or inactivating protease-activated receptors. Analytical biochemistry. 2000;277:33–45. doi: 10.1006/abio.1999.4356. [DOI] [PubMed] [Google Scholar]
- 74.Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dawes KE, Gray AJ, Laurent GJ. Thrombin stimulates fibroblast chemotaxis and replication. European journal of cell biology. 1993;61:126–130. [PubMed] [Google Scholar]
- 76.Pilcher BK, Kim DW, Carney DH, Tomasek JJ. Thrombin stimulates fibroblast-mediated collagen lattice contraction by its proteolytically activated receptor. Experimental cell research. 1994;211:368–373. doi: 10.1006/excr.1994.1100. [DOI] [PubMed] [Google Scholar]
- 77.Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude OP, Laurent GJ. Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. The Biochemical journal. 1998;333(Pt 1):121–127. doi: 10.1042/bj3330121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheel G, Rahfoth B, Franke J, Grau P. Acceleration of wound healing by local application of fibronectin. Archives of orthopaedic and trauma surgery. 1991;110:284–287. doi: 10.1007/BF00443459. [DOI] [PubMed] [Google Scholar]
- 79.Kallis YN, Scotton CJ, Mackinnon AC, Goldin RD, Wright NA, Iredale JP, Chambers RC, Forbes SJ. Proteinase activated receptor 1 mediated fibrosis in a mouse model of liver injury: a role for bone marrow derived macrophages. PLoS One. 2014;9:e86241. doi: 10.1371/journal.pone.0086241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knight V, Tchongue J, Lourensz D, Tipping P, Sievert W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology. 2012;55:879–887. doi: 10.1002/hep.24784. [DOI] [PubMed] [Google Scholar]
- 81.Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, Foy D, Hafezi-Moghadam A, Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- 82.Damiano BP, Cheung WM, Santulli RJ, Fung-Leung WP, Ngo K, Ye RD, Darrow AL, Derian CK, de Garavilla L, Andrade-Gordon P. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. The Journal of pharmacology and experimental therapeutics. 1999;288:671–678. [PubMed] [Google Scholar]
- 83.Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, Andrade-Gordon P, Kotzsch M, Spring D, Luther T, Abe J, Pohlman TH, Verrier ED, Blaxall BC, Mackman N. Protease-activated receptor-1 contributes to cardiac remodeling and hypertrophy. Circulation. 2007;116:2298–2306. doi: 10.1161/CIRCULATIONAHA.107.692764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Randall S, Alberte WPR., Jr . Tryptase enzyme inhibiting aminopyridines. In: U. States, editor. United States Patent Office. United States: 2010. [Google Scholar]
- 85.Hiroshi Kido HN. In: Tryptase inhibitor and novel guanidino derivatives. U. S. P. Office, editor. United States: 2002. [Google Scholar]
- 86.Yong Mi Choi-Sledeski, Patrick Wai-Kwok GL. In: Tropinone benzylamines as beta-tryptase inhibitors. U. S. P. Office, editor. United States: 2012. [Google Scholar]
- 87.Joanne Van Ryn PG-C, Solca Flavio. Combination therapy in treatment of cancer and fibrotic diseases. In: U. States, editor. United States Patent Office. 2014. [Google Scholar]
- 88.Steve Cohen MN. Antagonists of protease activated receptor-1 (par1) In: U. S. P. Office, editor. United States Patent Office. United States: 2008. [Google Scholar]
- 89.Joe William Boyd PM, Higginbottom Michael, Simpson Iain, Mark Mountford David, Daniel Savory Edward. In: PROTEASE ACTIVATED RECEPTOR 2 (PAR2) ANTAGONISTS. U. S. P. Office, editor. United States: 2012. [Google Scholar]
- 90.Cairns JA. Inhibitors of mast cell tryptase beta as therapeutics for the treatment of asthma and inflammatory disorders. Pulmonary pharmacology & therapeutics. 2005;18:55–66. doi: 10.1016/j.pupt.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 91.Utah Uo. Dabigatran’s Effect on Changes in Atrial Fibrosis in Patients With Atrial Fibrillation (DEPAF) 2013 [Google Scholar]
- 92.Lohman RJ, Cotterell AJ, Barry GD, Liu L, Suen JY, Vesey DA, Fairlie DP. An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:2877–2887. doi: 10.1096/fj.11-201004. [DOI] [PubMed] [Google Scholar]
- 93.Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011;163:141–172. doi: 10.1111/j.1476-5381.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. Journal of molecular and cellular cardiology. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cellular and molecular life sciences : CMLS. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovascular research. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 97.Akin C, Metcalfe DD. Systemic mastocytosis. Annual review of medicine. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 98.Chiu A, Nanaji NM, Czader M, Gheorghe G, Knowles DM, Chadburn A, Orazi A. The stromal composition of mast cell aggregates in systemic mastocytosis. Mod Pathol. 2009;22:857–865. doi: 10.1038/modpathol.2009.53. [DOI] [PubMed] [Google Scholar]
- 99.Li CY, Baek JY. Mastocytosis and fibrosis: role of cytokines. International archives of allergy and immunology. 2002;127:123–126. doi: 10.1159/000048182. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, Metcalfe DD. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. 1995;96:2702–2710. doi: 10.1172/JCI118337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matito A, Morgado JM, Alvarez-Twose I, Sanchez-Munoz L, Pedreira CE, Jara-Acevedo M, Teodosio C, Sanchez-Lopez P, Fernandez-Nunez E, Moreno-Borque R, Garcia-Montero A, Orfao A, Escribano L. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:e76116. doi: 10.1371/journal.pone.0076116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iizaka S, Sanada H, Matsui Y, Furue M, Tachibana T, Nakayama T, Sugama J, Furuta K, Tachi M, Tokunaga K, Miyachi Y. Serum albumin level is a limited nutritional marker for predicting wound healing in patients with pressure ulcer: two multicenter prospective cohort studies. Clin Nutr. 2011;30:738–745. doi: 10.1016/j.clnu.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Anthony D, Rafter L, Reynolds T, Aljezawi M. An evaluation of serum albumin and the sub-scores of the Waterlow score in pressure ulcer risk assessment. J Tissue Viability. 2011;20:89–99. doi: 10.1016/j.jtv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Williams DF, Stotts NA, Nelson K. Patients with existing pressure ulcers admitted to acute care. J Wound Ostomy Continence Nurs. 2000;27:216–226. doi: 10.1067/mjw.2000.107875. [DOI] [PubMed] [Google Scholar]
- 105.James TJ, Hughes MA, Cherry GW, Taylor RP. Simple biochemical markers to assess chronic wounds. Wound Repair Regen. 2000;8:264–269. doi: 10.1046/j.1524-475x.2000.00264.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.