Abstract
Following herpes simplex virus type 1 (HSV-1) corneal infection, CD4+ T cells are expanded in the draining lymph nodes (DLN) and re-stimulated in the infected cornea to regulate the destructive inflammatory disease herpes stromal keratitis (HSK). The contribution of cornea resident, cornea infiltrating, and DLN resident dendritic cells (DC) to CD4+ T cell expansion in DLN and re-stimulation in corneas is unknown. Cornea resident and infiltrating DCs were selectively depleted by timed local (subconjunctival) injection of diphtheria toxin (DT) into mice that express high affinity DT receptors (DTR) from the CD11c promoter. Corneal and DLN DC were depleted by systemic (intraperitoneal) DT treatment. We found that: a) DC that were resident in the cornea and DLN at the time of infection or that migrate into the tissues during the first 24 h after infection were not required for CD4+ T cell expansion; b) DC that infiltrated the cornea > 24 h after infection were responsible for most of the CD4+ T cell expansion measured in the DLN at 3 and 7 days post infection (dpi); c) non cornea-derived DC that infiltrate the DLN > 24 h after infection made a modest contribution to CD4+ T cell expansion at 3 dpi, but did not contribute at 7 dpi; and d) surprisingly HSK development between 7–21 dpi did not require corneal DC. DC-independent HSK development appears to reflect close interactions of CD4+ T cells with MHC class II positive corneal epithelial cells and macrophages in infected DC-depleted corneas.
Keywords: rodent, dendritic cells, T cells, viral, antigen presentation/processing, transgenic/knockout mice, mucosa, spleen and lymph nodes
Introduction
Corneal clarity is required for unobstructed vision, but can be compromised by inflammation and scar tissue formation. Therefore, herpes simplex virus type 1 (HSV-1) infection presents the cornea with the dilemma of eliminating the virus while minimizing inflammation and scarring. Indeed, the cornea has developed mechanisms to discourage inflammation that are collectively referred to as corneal immune privilege (1). The immune privilege of the cornea was previously thought to result in part from a lack of antigen presenting cells such as macrophages and dendritic cells (DC). However, recent studies have identified a stratified network of DC and macrophages that is nearly identically represented in mouse and human corneas, although the functional program of these cells remains poorly understood (2, 3). Moreover, HSV-1 infection of mouse corneas can lead to severe inflammation and scar tissue formation, depending on the strains of mice and virus employed. When this destructive inflammation called herpes stromal keratitis (HSK) does occur, it is typically regulated by CD4+ T cells that produce Th1 and Th17 cytokine patterns (4–9).
Tissue resident DC can subserve a number of important functions including: a) activating innate immunity (10–12); b) directly or indirectly (through antigen transfer for cross presentation) alerting the immune system to the presence of non-self antigens; c) fine-tuning the adaptive immune responses to particular pathogens, and d) re-stimulating effector T cells that infiltrate infected tissue. In addition, recent studies have demonstrated an ancillary role for resident or very early infiltrating corneal DC in promoting HSV-1 clearance from the cornea by directing the migration of natural killer cells and inflammatory monocytes to the site of HSV-1 lesions in the central cornea (13). However, the relative contribution of resident corneal DC, DC that infiltrate the cornea after infection, and DC that are resident in the draining lymph nodes (DLN) in expanding naive HSV-specific CD4+ T cells in DLN and re-stimulating the CD4+ T effector cells that infiltrate the cornea to mediate HSK remains unexplored.
In our HSV-1 corneal infection model, HSV-1 replication is largely eliminated by 4–6 days post infection (dpi), and the CD4+ T cells that mediate HSK infiltrate the cornea around 7 dpi (14, 15). Based on these kinetics, we hypothesized that free virus could enter the lymphatics for presentation to naïve CD4+ T cells at least up to 4 dpi, but beyond 4 dpi transport by resident corneal DC or infiltrating corneal DC might become increasingly important. We further hypothesized that if DC are required to re-stimulate infiltrating CD4+ T cells to mediate HSK as previously suggested (16–18), locally depleting corneal DC at the time of HSK development would reduce the incidence and severity of HSK. We show that expansion of CD4+ T cells in the DLN: 1) does not require DC that are resident in the cornea or DLN at the time of infection, nor those that infiltrate these tissues during the first 24hrs after infection; 2) at 3 dpi is completely DC-dependent and primarily induced by migratory DC that infiltrate the cornea > 24 hrs after infection, and to a lesser extent by DLN DC that are not derived from the cornea; and 3) at 7 dpi is partially DC-dependent with only cornea-derived DC contributing. We further show that development of HSK from 7–21 dpi does not require DC within the cornea.
Materials and Methods
Reagents
The following fluorochrome-labeled antibodies were used: FITC-conjugated anti-CD45 (30-F11), PerCP-conjugated anti-CD45 (30-F11), Pacific Blue-conjugated anti-CD4 (RM4-5), phycoerythrin-Cy7-conjugated anti-CD4 (RM4-5), phycoerythrin-conjugated anti-CD4 (RM4.5), and phycoerythrin-conjugated Ly-6G (1A8) were purchased from BD Pharmingen (San Diego, CA). eFluor 450-conjugated anti-CD3 (17A-2), eFluor780-conjugated anti-CD3 (17A-2), V450-conjugated anti-CD11b (M1/70), allophycocyanin-conjugated anti-CD11c (N418), allophycocyanin-conjugated MHC II (M5/114.15.2), eFluor 450-conjugated anti-F4/80, and eFluor780-conjugated anti-Gr-1 (RB6-8C5) were purchased from eBiosciences (San Diego, CA). The appropriate isotype control antibodies were purchased from their respective vendors.
Mice
Female wild-type (WT) BALB/cJ, C57BL/6, and CD11c-DTR [C.FVB-Tg(Itgax-DTR/EGFP)57Lan/J and B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J] mice, 6–8 wk old, were purchased from The Jackson Laboratory (Bar Harbor, ME). Bone marrow chimeras were created by transferring bone marrow from CD11c-DTR mice into lethally irradiated WT BALB/cJ or C57BL/6 mice to avoid lethality associated with multiple DT treatments of CD11c-DTR mice (19). Briefly, BALB/cJ or C57BL/6 hosts underwent two treatments of 500 rads in an animal γ-irradiator, and 2×106 bone marrow cells from CD11c-DTR donor mice were transferred intravenously. The resulting mice (referred to herein as CD11c-DTR chimeras) were housed under immunocompromised mouse conditions and treated continuously with 2 mg/ml neomycin from Sigma-Aldrich (St. Louis, MO) in their drinking water. The CD11c-DTR chimeras were fully reconstituted and ready for experimental use after 8 weeks (13). All experimental animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Ocular HSV-1 infection and tracking of surrogate antigen
HSV-1 strain RE was purified and mice were infected as previously described (13). In experiments using surrogate antigen, 20 ug of Alexa Fluor 594 conjugated to ovalbumin (OVA – AF594) in 3 uL PBS was applied topically to mouse corneas at various times after HSV-1 infection. All animal experiments were conducted in accordance with guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committee.
Flow cytometry for phenotypic analysis
At indicated times, DLN and corneas were excised from euthanized mice. DLN were minced and incubated with 500 µl DMEM (Lonza) containing 10% FCS (Atlanta Biologicals, Atlanta, GA) and 420 U/ml collagenase type I (Sigma-Aldrich) for 30 min at 37°C. Corneas were dissected, cut in quarters, incubated in 100 uL DMEM (Lonza) containing 10% FBS (Atlanta Biologicals, Atlanta, GA) and 840 U/mL collagenase type I (Sigma-Aldrich) for 1 hour at 37°C, and dispersed into single cell suspension by trituration through a p200 pipette tip. Data were collected on a FACSAria cytometer and analyzed by FACSDiva software (BD Biosciences, San Diego, CA). Dendritic cells were identified as CD45+ CD3− CD11c+. CD4+ T cells were identified as CD45+ CD3+ CD4+. Neutrophils were identified as CD45+ Ly6g+ CD11b+.
Measurement of CD4+ T cell proliferation
Infected mice received an i.p. injection of 1 mg BrdU per mouse to measure CD4+ T cell proliferation. DLN were excised 4 hours after BrdU injection, and dispersed cells were stained with anti-CD45, anti-CD3, and anti-CD4, for 30 minutes at 4°C. CD4+ T cells that divided over the 4 hour period were quantified by flow cytometry using a BrdU proliferation assay kit (catalog no. 559619; BD Pharmingen) according to manufacturers’ instructions.
Confocal microscopy
Whole corneas were excised, flattened by making radial incisions, washed in PBS 4% FBS, and then incubated in 1% paraformaldehyde for 2 hours. The corneas were then washed two more times in PBS 4% FBS, incubated with the appropriate antibodies overnight at 4°C, washed in PBS 4% FBS for 15 min at 4°C three more times, and then mounted. Draining lymph nodes were excised, halved and carefully washed in PBS 4% FBS; Fc receptors were blocked by incubation with anti-CD16/CD32 for 1 hour at 4°C, and DLN were then incubated with the appropriate antibodies overnight, followed by five 20 min washes in PBS 4% FBS. The DLN were then treated with 2% paraformaldehyde for 2 hours at 4°C, and then washed in PBS 4% FBS for 20 min three times before mounting. Images of corneas and DLN were acquired on an Olympus Fluoview 1000× confocal microscope with a 1.4 NA 60× oil objective, a 1.4 NA 40× oil objective, or a 0.85 NA 20× oil objective. Images were acquired by sequential scanning to avoid fluorescence crossover, and Z stacks were acquired at Nyquist sampling frequency through the tissue. All image reconstructions were made using Fiji (20).
Statistical analysis
All statistical analyses were computed with GraphPad Prism software, using unpaired t tests or one – way ANOVA with Bonferroni’s posttest. The p values < 0.05 were considered statistically significant.
Results
DC depletion of CD11c-DTR mice is selective and transient
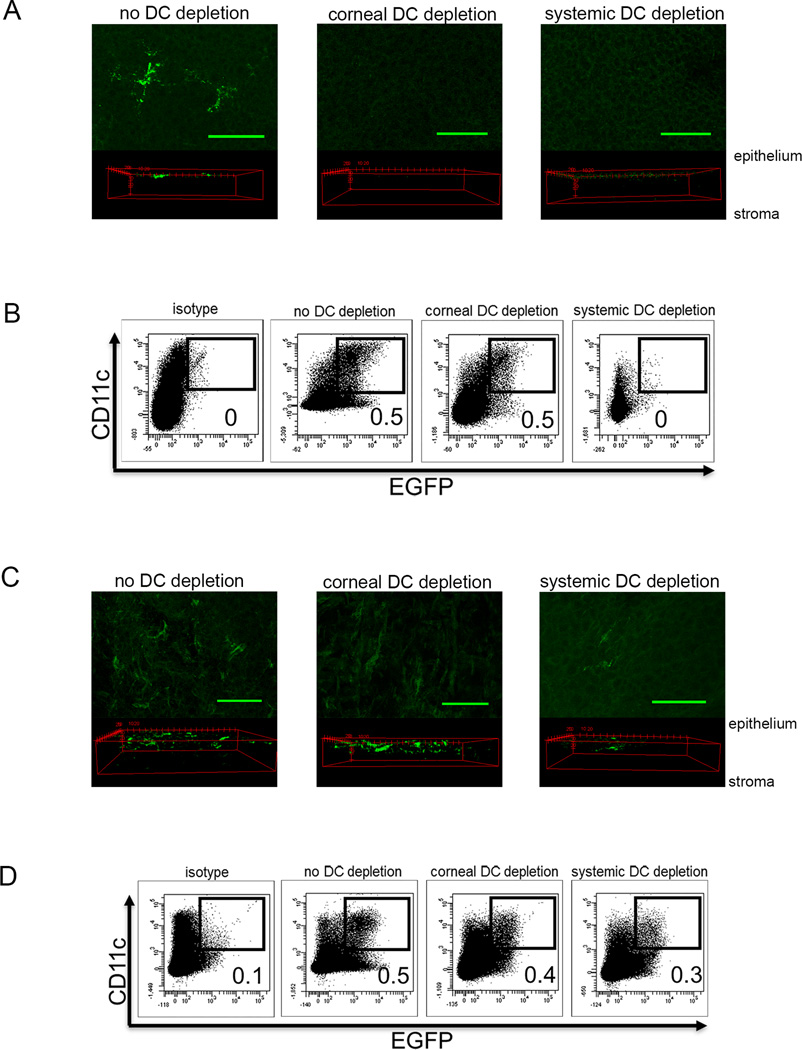
We employed protocols in which CD11c-DTR chimeric mice were selectively depleted of corneal DC by local subconjunctival (sconj) DT injection, or were systemically depleted of DC by i.p. DT treatment. The sconj DT treatment efficiently and selectively depleted DC from the cornea (Fig. 1A), whereas i.p. DT treatment depleted DC from both the cornea and the DLN (Fig. 1B). DC depletion from both tissues was transient, such that a single DT treatment 2 days before corneal HSV-1 infection depleted DC from the cornea and DLN (i.p. treatment) or selectively from the cornea (sconj treatment) through 0 dpi with initial recovery of DC observed at 1 dpi (Fig. 1C&D).
Figure 1. Selective depletion of DC populations.
BALB/c CD11c-DTR bone marrow chimeric mice received subconjunctival injections of 30 ng DT (corneal DC depletion), intraperitoneal injections of 150 ng DT (Systemic DC depletion), or similar injections of PBS two days before HSV-1 corneal infection. The corneas and DLN were excised at 0 and 1 day post infection (dpi). The corneas were fixed and mounted for confocal microscopic imaging. Representative z stack and 3d projections from the paracentral region of the cornea show that both DT treatments resulted in effective depletion of EGFP positive DC at 0 dpi (A) with partial recovery of DC at 1 dpi (C). DLN were dispersed with collagenase into single cell suspension, and stained for CD11c-APC, and analyzed for EGFP expression from the CD11c promoter and CD11c surface staining. Representative dot plots show efficient depletion of CD11c+ EGFP+ cells from the DLN following systemic, but not corneal DT treatment (B); with partial recovery of DC in the DLN at 1 dpi (D). Numbers on flow plots represent percentage of CD11c+ EGFP+ cells gated on CD45+ cells based on an APC isotype control and EGFP staining in DLN of wild type mice. Measuring bar denotes 100 um. Data are representative of three experiments, 3 – 5 mice per group.
DC that are resident in the cornea and DLN at the time of infection are not required for CD4+ T cell expansion
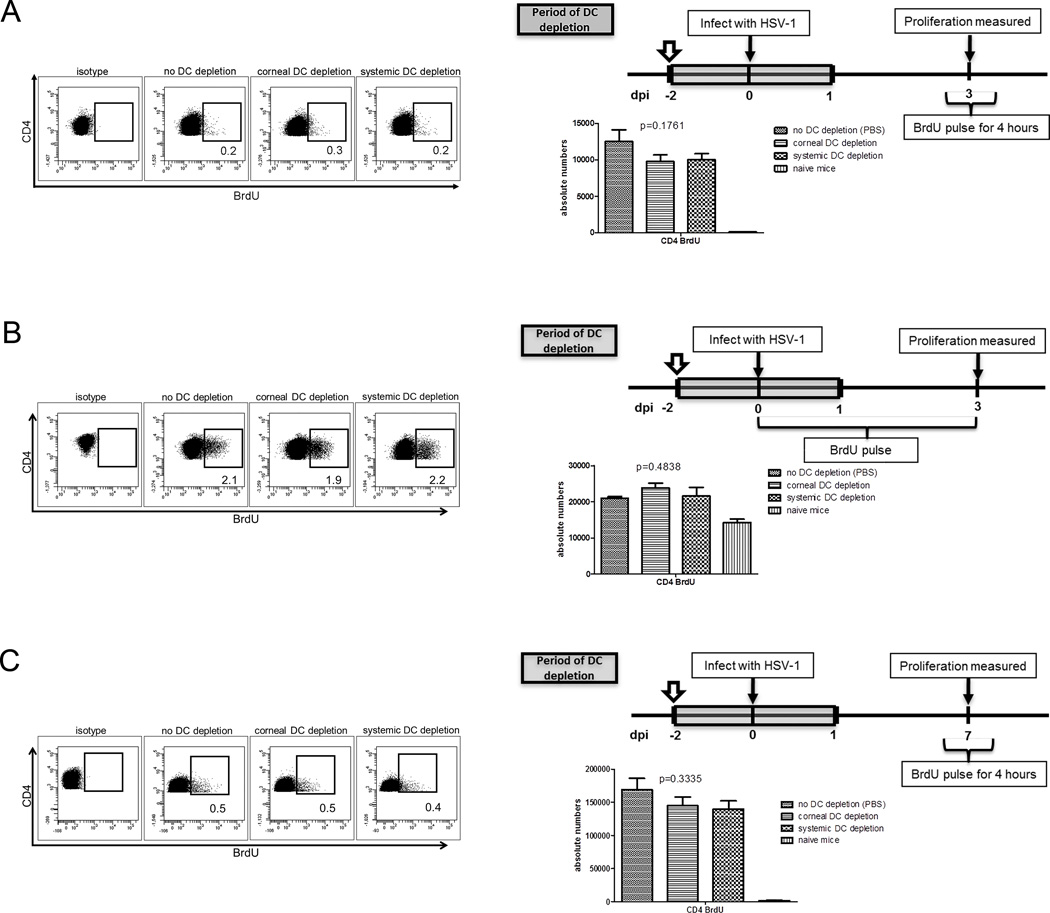
Depleting DC from the cornea or DLN up to 24 h after HSV-1 corneal infection had no impact on CD4+ T cell expansion in the DLN when measured at 3 dpi using a 4 h or a 0–3 day BrdU pulse (Fig. 2A&B) or measured at 7 dpi using a 4 h BrdU pulse (Fig. 2C). Thus, resident corneal DC or those that infiltrate the cornea during the first 24 h after infection are not required for optimal expansion of CD4+ T cells in the DLN.
Figure 2. Resident DCs are not essential for CD4+ T cell expansion in the DLN or for HSK.
BALB/c CD11c-DTR bone marrow chimeric mice received subconjunctival injections of 30 ng DT (corneal DC depletion), intraperitoneal injections of 150 ng DT (systemic DC depletion), or similar injections of PBS. A single DT treatment was administered 2 days before HSV-1 corneal infection to locally deplete cornea resident DC or systemically deplete both cornea-, and DLN-resident DC as well as those that migrated into the tissue up to 1 dpi. Mice received intraperitoneal injections of 1 mg BrdU at the indicated times. DLN were excised at 3 or 7 dpi. Single cell suspensions of the DLN were stained with antibodies to CD3, CD4, and BrdU to measure CD4+ T cell proliferation by flow cytometry. Depletion of tissue resident DC had no effect on CD4+ T cell proliferation when measured at 3 dpi using a 4 h (A) or 3-day (B) BrdU pulse; or when measured at 7 dpi (C) using a 4 h BrdU pulse. The p values for group differences were determined using a one-way ANOVA with Bonferroni post-tests (no DC depletion vs systemic DC depletion, no DC depletion vs corneal DC depletion, and systemic DC depletion vs corneal DC depletion: not significant). Data are representative of three experiments, with 5 mice per group.
Cornea-derived DC are primarily responsible for early CD4+ T cell expansion
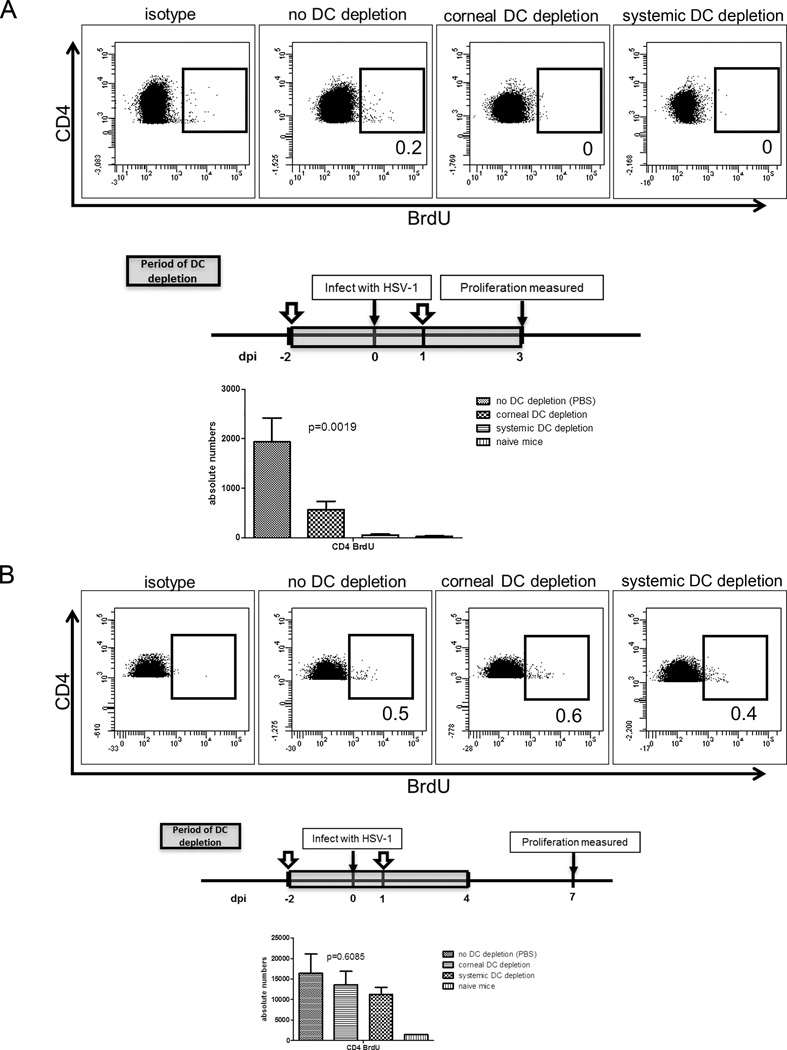
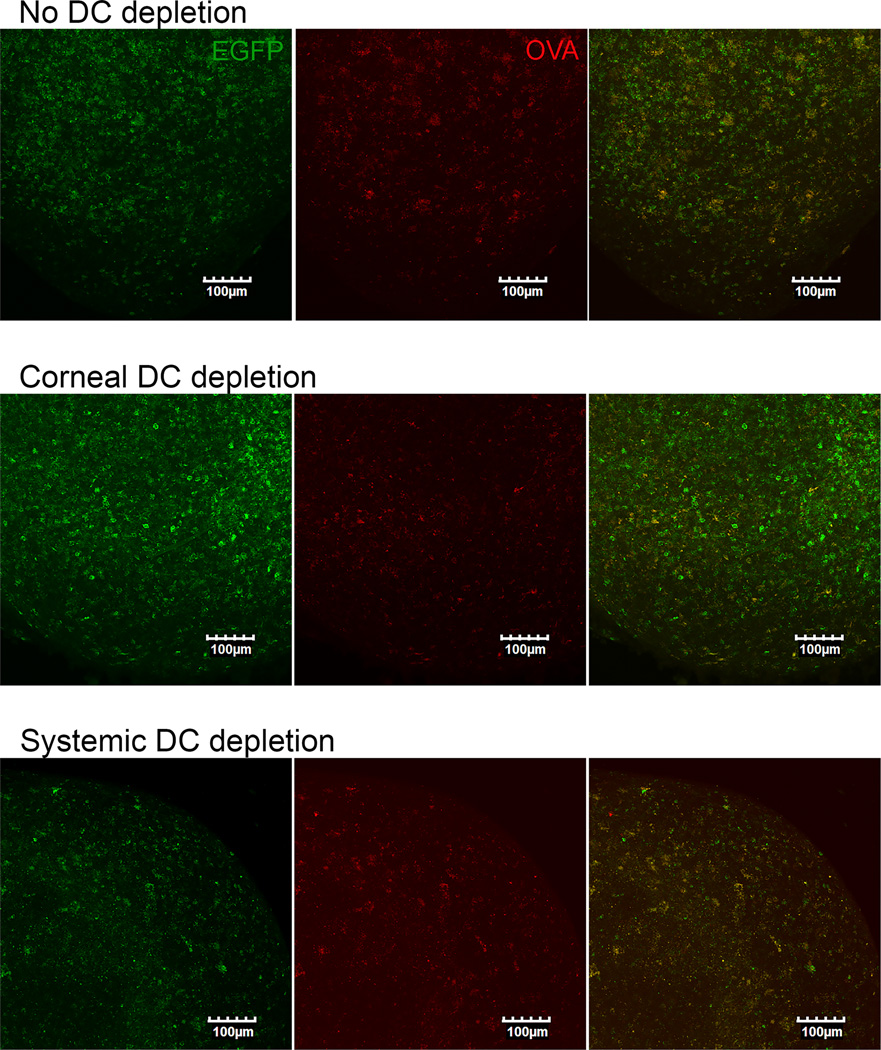
Mice were continually depleted of corneal DC only or corneal and DLN DC through 3 dpi, through serial DT treatments at −2 and 1 dpi (Fig. 3). Selective DC depletion from the cornea reduced CD4+ T cell expansion in the DLN at 3 dpi by 71%, with the remaining 29% of proliferation stimulated by DC that were not cornea-derived (Fig. 3A). However, CD4+ T cell expansion returned to control levels at 7 dpi when DC were permitted to recover from 4–7 dpi (Fig. 3B). A recent report demonstrated that local migratory DC are absolutely required for expansion of CD4+ T cells in DLN following HSV-2 infection of the vaginal mucosa, and suggested that the inability of DLN-resident DC to present viral antigens was due to failure of free antigen to access the lymphatics when topically applied to that mucosal surface (21). Since local migratory DC are not absolutely required for CD4+ T cell expansion following corneal infection, we determined if free antigen can reach the DLN when applied to the surface of the cornea. When fluorescein-conjugated ovalbumin was applied to the cornea as a surrogate antigen, fluorescein was readily detectable in the DLN within 24 h (Fig. 4) suggesting that free antigen has access to the DLN following application to the corneal surface. Note that in these experiments, fluorescein-conjugated ovalbumin and not free fluorescein was applied (22, 23).
Figure 3. CD4+ T cell expansion in DLN at 3 dpi is dependent on both cornea-derived and DLN resident DC.
BALB/c CD11c-DTR bone marrow chimeric mice received local (subconjunctival) or systemic (intraperitoneal) DT treatment at −2 and +1 days relative to HSV-1 corneal infection (dpi) to deplete cornea-derived (corneal DC depletion) or cornea-derived and DLN (systemic DC depletion) DC up to 4 dpi. Mice received intraperitoneal injections of 1 mg BrdU 4-h prior to DLN excision. Single cell suspensions of the DLN were stained with antibodies to CD3, CD4, and BrdU to measure CD4+ T cell proliferation by flow cytometry. Bar graphs depict mean ± SEM absolute number of BrdU+ (proliferated) CD4+ T cells/DLN. (A) CD4+ T cell proliferation was significantly reduced by corneal DC depletion, and abrogated by systemic DC depletion. (B) Corneal or systemic DC depletion up to 4 dpi had no impact on CD4+ T cell proliferation measured at 7 dpi. The p values for group differences were determined using a one-way ANOVA with Bonferroni post-tests (for 3A: no DC depletion vs corneal DC depletion, no DC depletion vs systemic DC depletion, and corneal DC depletion vs systemic DC depletion are p<0.05; for 3B: no depletion vs systemic DC depletion, no depletion vs corneal DC depletion, and systemic DC depletion vs corneal DC depletion: not significant). Data are representative of three experiments, with 5 mice per group.
Figure 4. Antigen applied to the cornea drains to the lymph nodes in the absence of cornea DCs.
BALB/c CD11c-DTR bone marrow chimeric mice received local (subconjunctival) or systemic (intraperitoneal) DT treatment at −2 dpi to deplete cornea-derived (corneal DC depletion) or cornea-derived and DLN (systemic DC depletion) DC up to 1 dpi or PBS (No DC Depletion). At 0 dpi corneas were infected topically with a suspension of HSV-1 containing AF594-conjugated ovalbumin (OVA-AF594). At 1 dpi, DLN were excised, cut in half, fixed with 1% paraformaldehyde, and mounted for confocal imaging of DC identified by EGFP expression from the CD11c promoter (green) and AF594 tagged Ova (red). Compressed images of z-stacks in the xy plane of lymph nodes are representative of three experiments, 3–5 mice per group.
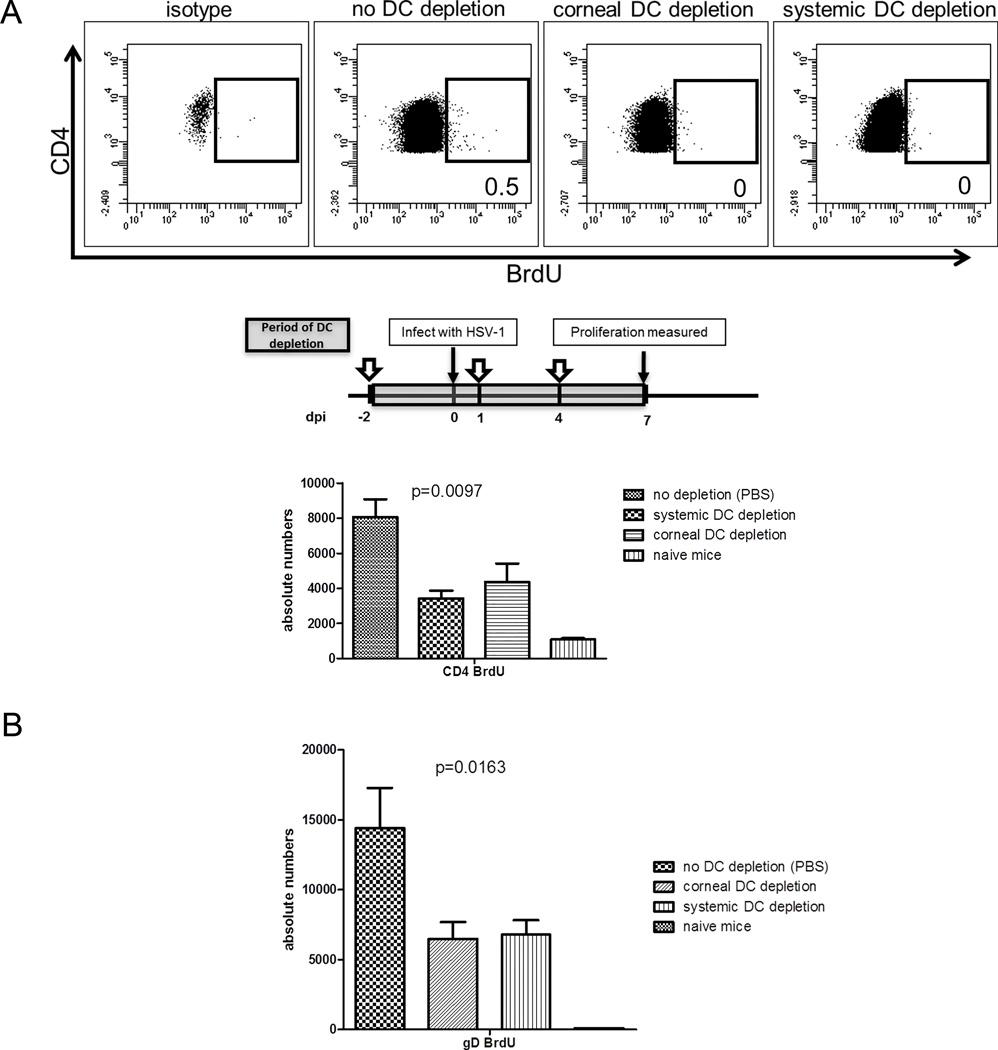
Only DC derived from the infected cornea can stimulate late HSV-specific CD4+ T cell expansion at 7 dpi
Data in Figure 3B were consistent with the notion that DC migration from the cornea between 4 and 7 dpi was responsible for the recovery of CD4+ T cell expansion at 7 dpi. This concept received further support from the observation that extending selective depletion of corneal DC through 7 dpi significantly reduced CD4+ T cell proliferation measured at 7 dpi, and no additional impairment of CD4+ T cell expansion was observed with continuous systemic DC depletion (Fig. 5A). Since a C57BL/6 CD4+ T cell epitope was recently identified on HSV-1 glycoprotein D (gD290–302) (24), we repeated the depletion of corneal DC in C57BL/6 CD11c-DTR bone marrow chimeric mice and quantified the expansion of HSV-specific CD4+ T cells in the DLN using MHC II multimers containing the gD epitope. As illustrated in Figure 5B, the HSV-specific CD4+ T cell expansion in C57BL/6 mice was reduced by 55%, comparable to the 53% reduction in expansion of the overall CD4+ T cells in BALB/c mice.
Figure 5. DC that stimulate CD4+ T cell proliferation at 7 dpi are entirely cornea derived.
BALB/c or C57BL/6 CD11c-DTR bone marrow chimeric mice received local (subconjunctival) or systemic (intraperitoneal) DT treatment at −2, +1, and +4 days relative to HSV-1 corneal infection (dpi) to deplete cornea-derived (corneal DC depletion) or cornea-derived and DLN (systemic DC depletion) DC up to 7 dpi. Mice received intraperitoneal injections of 1 mg BrdU 4 h prior to DLN excision. (A) Single cell suspensions of the BALB/c DLN were stained with antibodies to CD3, CD4, and BrdU to measure CD4+ T cell proliferation by flow cytometry. (B) Single cell suspensions of C57BL/6 DLN were stained with MHC class II multimers containing an HSV-1 gD290–302 epitope. Bar graphs depict mean ± SEM absolute number of BrdU+ (proliferated) BALB/c CD4+ T cells (A) or C57BL/6 gD-specific CD4+ T cells (B) per DLN. The p values for group differences were determined using a one-way ANOVA with Bonferroni post-tests (for 5A and B: no DC depletion vs corneal DC depletion and no DC depletion vs systemic DC depletion are p<0.05, corneal DC depletion vs systemic DC depletion is not significant). Data are representative of three experiments, with 5 mice per group.
Our previous study using UV-B irradiation to locally deplete corneal DC suggested a requisite role for corneal DC in re-stimulating the effector CD4+ T cells that mediate HSK in infected corneas (18). Since UV-B could affect cells other than DC we re-evaluated this role for corneal DC using DT treatment to deplete only DC and evaluated its effect on HSK progression. We used i.p. DT treatment to deplete corneal DC without the possible confounding mechanical effect of sconj injections.
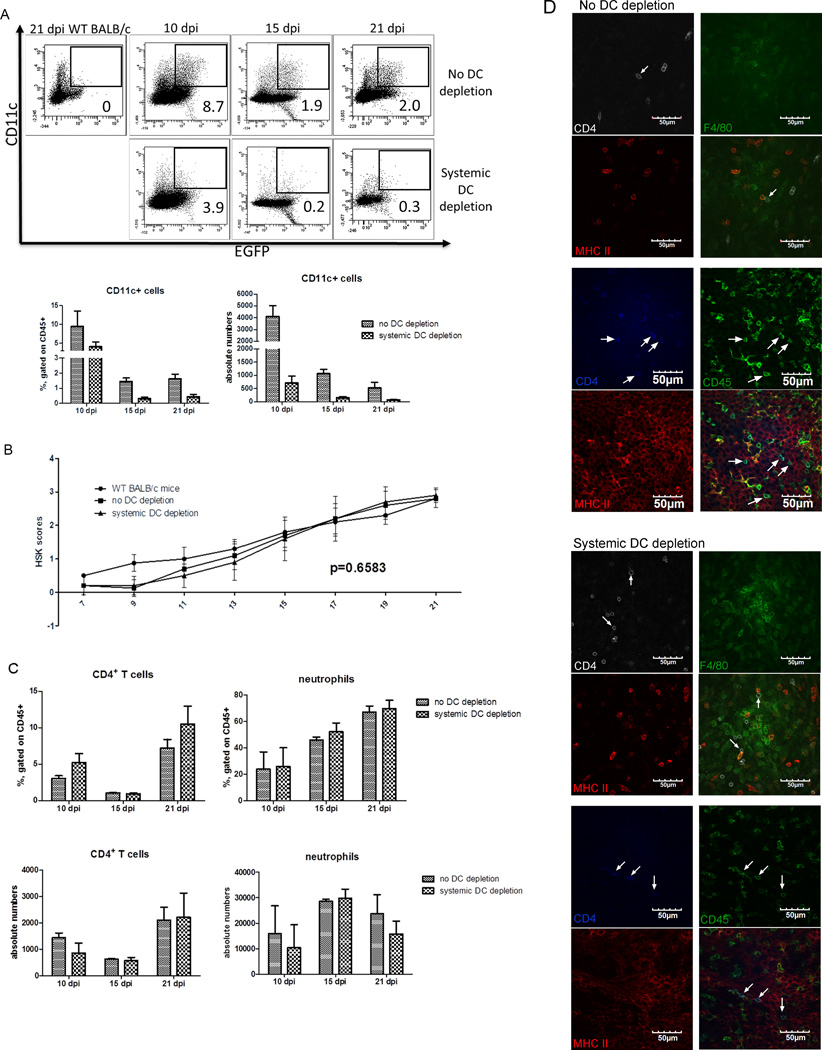
To assess the efficacy of i.p. DT treatment in maintaining DC depletion, corneas were excised from DT treated and mock treated mice at 10, 15, and 21 dpi, the cells were dispersed with collagenase, and analyzed by flow cytometry for expression of various cell lineage markers. DC depletion was effectively maintained at all three times as shown by the selective absence of CD11c+ and eGFP+ DC in corneas of DT treated mice (Fig. 6A). Surprisingly, depletion of corneal DC from 7–21 dpi had no effect on HSK progression (Fig. 6B). Consistent with the HSK data, DC depletion had no effect on the level of infiltrating CD4+ T cells or neutrophils (Fig. 6C). Many CD4+ T cells were found in close apposition to MHC class II positive F4/80 positive (presumed macrophages) and MHC class II positive-, CD45 negative cells with location and morphology consistent with epithelial cells in HSV-1 infected corneas of both DC-depleted and non-depleted mice (Fig. 6D). Taken together, these finds provide strong support for the concept that DC are not necessary to re-stimulate the CD4+ effector T cells that mediate HSK, and that their antigen presenting function can be provided by MHC class II positive macrophages and corneal epithelial within infected corneas.
Figure 6. DCs are not required for re-stimulation of CD4+ T cells within the cornea during HSK.
Wild type and CD11c-DTR bone marrow chimeric BALB/c mice received HSV-1 corneal infections, and chimeric mice received intraperitoneal injections of PBS or DT every other day from 7–21 dpi. (A) Corneas were excised at 10, 15, and 21 dpi, dispersed with collagenase, stained for surface CD45 and CD11c, and analyzed by flow cytometry for surface CD11c expression and EGFP expression from the CD11c promoter gated on CD45+ cells. Representative flow plots show CD11c+ EGFP+ cells. Numbers on flow plots represent percentage of CD11c+ EGFP+ cells gated on CD45+ cells. Bar graphs show mean ± SEM frequency and absolute number of CD11c+ EGFP+ cells. (B) HSK severity was monitored over time and recorded as the mean ± SEM HSK severity. (C) Corneas were excised at the indicated time, dispersed with collagenase, surface stained for CD4, CD11b, and Ly6g. (D) At 10 dpi whole mounts of corneas were stained for CD4 (white), MHC II (red), and F4/80 (green) (columns 1 & 2) or for CD45 (green), CD4 (blue), and MHC II (red) (columns 3 & 4) and examined with a confocal microscope. Images shown in the xy plane from the peripheral part of the cornea are shown with arrows identifying CD4+ T cells that are closely associated with MHC II+ macrophages and corneal epithelial cells. Bar graphs depict mean ± SEM frequency and absolute number of CD4+ T cells and neutrophils (CD11b+ Ly6g+). All data are representative of three experiments (mean ± SEM of five mice per group). The significance of group differences in number and frequency of leukocyte populations in DC depleted and non-depleted mice was analyzed by a Student’s t-test. The significance of group differences in HSK severity was determined by calculating the area under the curve for each mouse and then performing an ANOVA with Bonferroni post-tests (comparisons of all pairs of columns are not significant).
Discussion
Like other mucosal surfaces, the cornea is constantly exposed to the environment and can serve as the portal of entry for pathogens. However, the cornea has a unique requirement for translucence, and has developed a variety of mechanisms to inhibit inflammatory responses that can be antithetical to tissue clarity. Normal corneas are armed with stratified networks of resident DC and macrophages, but the functional characteristics of these cells remain largely undefined. For instance, the ability of the resident corneal DC to migrate from the cornea to the DLN remains controversial, particularly in the setting of corneal infection (25, 26). We and others have proposed that resident corneal DC take up viral antigens following HSV-1 corneal infections and transport them to the DLN for either direct presentation to naïve T cells or for cross presentation by DLN resident DC. However, our current findings cast doubt on that theory. We show that depleting the resident corneal DC as well as those that migrate into the cornea during the first 24 h after HSV-1 corneal infection has no impact on the expansion of CD4+ T cells in the DLN. This might result in part from the direct infection of DC that are present in the cornea in the presence of high levels of replicating virus, which inhibits maturation and produces a lytic infection in immature DC, and inhibits the antigen presenting function of mature DC (27–30). Thus, DC that infiltrate the cornea when the virus load is reduced might be better able to acquire viral antigens from infected epithelial cells without being directly infected by the virus. Alternatively, this might reflect reduced antigen presenting or migratory capability of DC that are resident in the immune privileged cornea.
There are certain features of our depletion model that enable us to specifically deplete corneal DC. First, unlike their counterparts in the skin, corneal DC appear to be radiation sensitive, and are fully depleted in irradiated bone marrow recipients. Thus, in the reconstituted CD11c-DTR chimeras all of the corneal DC are susceptible to DT depletion. Second, the dose of DT that is administered subconjunctivally effectively depletes DC from the cornea without affecting their counterparts in the DLN as illustrated in Figure 1B. Finally, a single sconj or i.p. injection of DT very reproducibly depletes corneal DC for 3 days, with rapid reconstitution of DC from the blood ensuing quickly thereafter. This protocol made it possible to transiently and selectively deplete DC in the cornea or transiently deplete DC systemically.
Following HSV-1 corneal infection the virus replicates in the corneal epithelium and is present in the tear film that bathes the cornea until around 4–7 dpi when replicating virus is eliminated from the cornea primarily by an innate immune response (13). Thus, it is likely that free virus can enter the lymphatics and be presented by DLN resident DC at least through 4 dpi. In support of this concept, we show that depletion of corneal DC beginning prior to HSV-1 corneal infection and continuing through 3 dpi dramatically reduced but did not eliminate CD4+ T cell expansion in the DLN at 3 dpi, whereas systemically depleting both corneal DC and DLN resident DC completely abrogated CD4+ T cell expansion at 3 dpi. These findings are consistent with those of Allenspach et al (31) who demonstrated that DLN resident DC are required for the initial trapping and activation of antigen specific CD4+ T cell precursors in the DLN, whereas migratory DC from tissue are responsible for most of the CD4+ T cell expansion. Our findings conflict with those of Lee et al (21) who showed migratory DC from the vaginal mucosa exclusively present HSV antigens to CD4+ T cells following HSV-2 infection. This difference seems to lie in the fact that Lee et al (21) found that fluorochrome conjugated antigen was completely confined to the mucosa of the vaginal vault following topical application to the vaginal mucosa. Therefore, free antigen could not be acquired from the lymph for presentation by DLN resident DC. In contrast, we find that free antigen is delivered to the lymph nodes draining the cornea following topical application to the mucosal surface of the cornea (Fig. 4).
In contrast to the apparent joint contribution of migratory corneal DC and DLN resident DC to CD4+ T cell expansion at 3 dpi, migratory corneal DC appear to be the only DC population required for CD4+ T cell expansion at 7 dpi. This selective requirement for corneal derived DC was observed both for expansion of the polyclonal CD4+ T cell population in DLN of BALB/c mice, and for selective expansion of HSV-specific CD4+ T cells in the DLN of C57BL/6 mice. The requisite role for migratory corneal DC after 4 dpi might be explained in two ways. First, reduced release of free HSV-1 in the cornea after 4 dpi might decrease the flow of free virus through lymphatic channels, necessitating transport of viral antigens acquired from infected corneal cells to the DLN by migratory DC. Second, a previous study demonstrated that all HSV-specific CD8+ T cell precursors are stimulated in the DLN by 3 dpi (32). If this is true for CD4+ T cells as well, it might obviate the need for the trapping and initial activation function of DLN-resident DC.
Systemic DC depletion did not completely abrogate the expansion at 7 dpi of the polyclonal CD4+ T cell population in BALB/c mice or the HSV gDT-II specific CD4+ T cell population in C57BL/6 mic (Fig. 5). This is in contrast to CD4+ T cell expansion in DLN at 3 dpi that was completely abrogated by DC depletion. Thus, a currently undefined antigen presenting cell population appears to modestly contribute to late CD4+ T cell expansion at a time when DLN resident DC are no longer able to do so.
Previous studies from our laboratory and others (18, 33–35) strongly suggested that DC in the infected cornea are required to re-stimulate CD4+ effector T cells that infiltrate the cornea at around 7 dpi, enabling them to regulate the inflammation associated with HSK. Indeed, DC depletion by UV-B irradiation of one cornea followed by bilateral HSV-1 infection of both corneas resulted in HSK development only in the cornea that was not depleted of DC. Since CD4+ T cells expanded in the DLN draining the cornea that was not DC ablated would have access to both corneas, these findings strongly suggested a requisite role for DC in re-stimulating CD4+ T cells within the infected cornea. However, our current data argue against that interpretation in that local selective DC depletion starting at 7 dpi and continuing through 21 dpi did not influence HSK progression. Indeed, CD4+ T cells are seen interacting closely with F4/80 positive cells in both DC depleted and non-depleted corneas. This observation is consistent with the notion that macrophages can supplant the need for antigen presentation by DC in infected corneas. We also observed CD4+ T cells interacting closely with MHC class II positive, CD45− cells with morphological characteristics of corneal epithelial cells in infected corneas. These MHC class II positive apparent epithelial cells were found in clusters in the corneal epithelium consistent with possible up-regulation of MHC II by a diffusible mediator such as IFN-γ. Whether these epithelial cells contribute to CD4+ T cell activation within infected corneas will require further experimentation. The observed lack of a requisite role for corneal DC in re-stimulating CD4+ effector T cells conflicts with findings of Iijima, N. et al (36) who demonstrated that CD11b+ CD11c+ cells are required to re-stimulate CD4+ T cells that infiltrate the HSV-2 infected vaginal mucosa. The reason for this difference in DC requirement in the two types of mucosal surface is not clear, but might relate to the availability of alternative class II positive cells in the infected cornea.
Acknowledgements
We thank Dr. Sophia Jeon and Moira Geary for technical assistance, Nancy Zurowski for flow cytometry acquisition, and Kira Lathrop for assistance with confocal microscopy.
This work was supported by National Eye Institute Grants R01-EY010359 (to R.L.H.), P30-EY08099 (to R.L.H.), an unrestricted research grant (to R.L.H.) from Research to Prevent Blindness; and a grant from the Eye and Ear Foundation of Pittsburgh (to R.L.H.).
Abbreviations used in this article
- DC
dendritic cell
- DLN
draining lymph nodes
- dpi
days post infection
- DT
diphtheria toxin
- EGFP
enhanced green fluorescent protein
- HSK
herpes stromal keratitis
- WT
wildtype
References
- 1.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 2.Knickelbein JE, Watkins SC, McMenamin PG, Hendricks RL. Stratification of Antigen-presenting Cells within the Normal Cornea. Ophthalmol Eye Dis. 2009;1:45–54. doi: 10.4137/oed.s2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knickelbein JE, Buela KA, Hendricks RL. Antigen-Presenting Cells Are Stratified Within Normal Human Corneas and Are Rapidly Mobilized During Ex Vivo Viral Infection. Investigative Ophthalmology & Visual Science. 2014;55:1118–1123. doi: 10.1167/iovs.13-13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Q, Hendricks RL. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J Exp Med. 1996;184:1435–1447. doi: 10.1084/jem.184.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumpf TH, Shimeld C, Easty DL, Hill TJ. Cytokine production in a murine model of recurrent herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2001;42:372–378. [PubMed] [Google Scholar]
- 6.Frank GM, Divito SJ, Maker DM, Xu M, Hendricks RL. A Novel p40-Independent Function of IL-12p35 Is Required for Progression and Maintenance of Herpes Stromal Keratitis. Investigative Ophthalmology & Visual Science. 2010;51:3591–3598. doi: 10.1167/iovs.09-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan R, Remeijer L, van Dun JM, Osterhaus AD, Verjans GM. Granulocyte macrophage colony-stimulating factor expression in human herpetic stromal keratitis: implications for the role of neutrophils in HSK. Invest Ophthalmol Vis Sci. 2007;48:277–284. doi: 10.1167/iovs.06-0053. [DOI] [PubMed] [Google Scholar]
- 8.Maertzdorf J, Osterhaus ADME, Verjans GMGM. IL-17 expression in human herpetic stromal keratitis: Modulatory effects on chemokine production by corneal fibroblasts(1) Journal of Immunology. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 9.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foti M, Granucci F, Ricciardi-Castagnoli P. A central role for tissue-resident dendritic cells in innate responses. Trends Immunol. 2004;25:650–654. doi: 10.1016/j.it.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nature Immunology. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 12.Foti M, Granucci F, Pelizzola M, Beretta O, Ricciardi-Castagnoli P. Dendritic cells in pathogen recognition and induction of immune responses: a functional genomics approach. J Leukoc Biol. 2006;79:913–916. doi: 10.1189/jlb.1005547. [DOI] [PubMed] [Google Scholar]
- 13.Frank GM, Buela KA, Maker DM, Harvey SA, Hendricks RL. Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea. J Immunol. 2012;188:1350–1359. doi: 10.4049/jimmunol.1101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepisto AJ, Frank GM, Xu M, Stuart PM, Hendricks RL. CD8 T cells mediate transient herpes stromal keratitis in CD4-deficient mice. Invest Ophthalmol Vis Sci. 2006;47:3400–3409. doi: 10.1167/iovs.05-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun H, Rowe AM, Lathrop KL, Harvey SA, Hendricks RL. Reversible nerve damage and cornea pathology in murine herpes simplex stromal keratitis. J Virol. 2014 doi: 10.1128/JVI.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendricks RL, Tumpey TM. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest Ophthalmol Vis Sci. 1990;31:1929–1939. [PubMed] [Google Scholar]
- 17.Divito SJ, Hendricks RL. Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis. Investigative Ophthalmology & Visual Science. 2008;49:1488–1495. doi: 10.1167/iovs.07-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendricks RL, Janowicz M, Tumpey TM. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992;148:2522–2529. [PubMed] [Google Scholar]
- 19.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, Iwasaki A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wikstrom ME, Stumbles PA. Mouse respiratory tract dendritic cell subsets and the immunological fate of inhaled antigens. Immunol Cell Biol. 2007;85:182–188. doi: 10.1038/sj.icb.7100039. [DOI] [PubMed] [Google Scholar]
- 23.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 25.Lee EJ, Rosenbaum JT, Planck SR. Epifluorescence intravital microscopy of murine corneal dendritic cells. Invest Ophthalmol Vis Sci. 2010;51:2101–2108. doi: 10.1167/iovs.08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikloska Z, Bosnjak L, Cunningham AL. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J Virol. 2001;75:5958–5964. doi: 10.1128/JVI.75.13.5958-5964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Samady L, Costigliola E, MacCormac L, McGrath Y, Cleverley S, Lilley CE, Smith J, Latchman DS, Chain B, Coffin RS. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: Potential of vhs(−) HSV vectors for dendritic cell-mediated immunotherapy. Journal of Virology. 2003;77:3768–3776. doi: 10.1128/JVI.77.6.3768-3776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller SN, Jones CM, Smith CM, Heath WR, Carbone FR. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J Exp Med. 2002;195:651–656. doi: 10.1084/jem.20012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Hendricks RL. B7 costimulatory requirements of T cells at an inflammatory site. J Immunol. 1998;160:5045–5052. [PubMed] [Google Scholar]
- 34.Seo SK, Park HY, Choi JH, Kim WY, Kim YH, Jung HW, Kwon B, Lee HW, Kwon BS. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J Immunol. 2003;171:576–583. doi: 10.4049/jimmunol.171.2.576. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Lepisto AJ, Hendricks RL. CD154 signaling regulates the Th1 response to herpes simplex virus-1 and inflammation in infected corneas. J Immunol. 2004;173:1232–1239. doi: 10.4049/jimmunol.173.2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci U S A. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]