Abstract
Eosinophils are versatile cells that regulate innate and adaptive immunity, influence metabolism and tissue repair, and contribute to allergic lung disease. Within the context of immunity to parasitic worm infections, eosinophils are prominent yet highly varied in function. We have shown previously that when mice undergo primary infection with the parasitic nematode, Trichinella spiralis, eosinophils play an important, immune regulatory role that promotes larval growth and survival in skeletal muscle. In this study, we aimed to address the function of eosinophils in secondary infection with T. spiralis. By infecting eosinophil-ablated mice, we found that eosinophils are dispensable for immunity that clears adult worms or controls fecundity in secondary infection. In contrast, eosinophil ablation had a pronounced effect on secondary infection of skeletal muscle by migratory newborn larvae. Restoring eosinophils to previously infected, ablated mice caused them to limit muscle larvae burdens. Passive immunization of naïve, ablated mice with sera or immunoglobulin from infected donors, together with transfer of eosinophils, served to limit the number of newborn larvae that migrated in tissue and colonized skeletal muscle. Results from these in vivo studies are consistent with earlier findings that eosinophils bind to larvae in the presences of antibodies in vitro. Although our previous findings showed that eosinophils protect the parasite in primary infection, these new data show that eosinophils protect the host in secondary infection.
Introduction
Parasitic worms are estimated to infect two billion people worldwide and nearly 1 billion children live in areas of high transmission (1). Although drug therapy is often effective in clearing infections, reinfection rates are high (2), consistent with poorly sustained immunity. A relatively small number of anthelmintic drugs are available and the efficacy of anthelmintics is known to be limited by the emergence of drug resistant parasites (3-5). Advancing vaccination as a more sustainable alternative to chemotherapy requires understanding of immune mechanisms that are effective in preventing or clearing infection.
Clearance of worm infections occurs by mechanisms that vary among parasites, the hosts they infect, and the tissues they colonize (6). As is the case with other pathogens, mechanisms of immunity in primary worm infection often differ from those that are effective in preventing or clearing secondary infection. There is extensive evidence supporting key roles for Th2 cells in clearing primary, intestinal infections, although the effector mechanisms vary (7-9). Additional evidence documents important contributions of B cells and antibodies in controlling secondary infections and also in vaccine-induced protection (10-13). In the case of the parasitic nematode, Trichinella spiralis, T cell-mediated immunity that drives intestinal mastocytosis in primary infection is central to the mechanism of worm clearance in rats and mice (14-16); however, mast cell activation is neither necessary nor sufficient for antibody-mediated protection that clears larval stages from rats during a secondary intestinal infection (17-19). Mice do not demonstrate this same, antibody-mediated immunity to secondary infection but instead manifest worm expulsion that is accelerated but similar to that observed in primary infection (20).
Intestinal mastocytosis during primary T. spiralis infection occurs simultaneously with a pronounced tissue eosinophilia (21). In vitro studies have shown that eosinophils are capable of adhering to and killing several species of parasitic worms. In some species, including T. spiralis, killing occurs only in the presence of specific antibodies (22, 23). These findings served to set a paradigm for eosinophils as cytotoxic effector cells in worm infection. Early investigations of eosinophil effector function in vivo were performed by antibody-mediated depletion of eosinophils or manipulating IL-5 in mice. Findings from studies conducted with parasitic worms supported a role for IL-5 in immunity to some (24, 25) and not others (26). Subsequently, experiments with eosinophil-ablated mice have shown that eosinophils are dispensable in immunity to primary infections with intestinal worms (27-31). Secondary infections are less well studied, but challenge of eosinophil-ablated mice revealed that eosinophils contribute to protective immunity against Nippostrongylus brasiliensis by interfering with larval migration (30).
In contrast, results from infections with worms that colonize extraintestinal sites, including T. spiralis, show that eosinophils are beneficial to the parasite during primary infection (29, 32, 33). Trichinella completes its life cycle in a single host. Infective first-stage larvae are ingested and mature into adult worms in the intestine, where they reproduce and release newborn larvae (NBL). NBL enter the circulatory system and many transit the lung presumably en route to skeletal muscles (34). Larvae invade myotubes and establish chronic, intracellular infection. By infecting eosinophil-ablated mice, we have shown that in primary infections, eosinophils are required for efficient growth as well as survival of muscle larvae (29, 32). Survival is promoted via control of local nitric oxide (NO) -production by an eosinophil-driven IL-10 response (29, 32, 35, 36). The effect of eosinophil ablation on secondary infection has not been tested. Previous studies in IL-5-deficient or -depleted mice yielded contradictory results (26, 37). The unexpected properties of eosinophils in primary T. spiralis infection and the contradictory results between in vitro and in vivo studies of eosinophil function prompted us to test the contribution of eosinophils to secondary immunity to T. spiralis using eosinophil lineage-ablated mice.
We report here that eosinophils are required for control of secondary infection by T. spiralis. Although eosinophil ablation had no effect on intestinal immunity, it was associated with enhanced colonization of skeletal muscle. Transfer experiments showed that eosinophils interfered with migration of NBL, an effect that was dependent on the presence of specific antibodies. The results provide evidence in support of an antibody-dependent mechanism in which eosinophils limit secondary infection by T. spiralis.
Materials and Methods
Rats and mice
Adult Albino Oxford strain rats were produced and maintained in the Baker Institute vivarium. ΔdblGATA (eosinophil-ablated), MBP-/- (major basic protein deficient), EPO-/- (eosinophil peroxidase deficient) and IL-5-expressing transgenic (NJ.1638) (IL-5Tg+) mice were bred at Cornell Transgenic Mouse Core Facility and offspring were transferred to the Baker Institute. All strains were on a C57BL/6 background. C57BL/6 NHsd mice were purchased from Taconic as wild type (WT) control mice. Animal care was in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and experiments were performed with the approval of the Institutional Animal Care and Use Committee of Cornell University.
Parasite and Antigens
Trichinella spiralis first-stage larvae (L1) and NBL were recovered from rats and prepared as described previously (29). For oral infection, L1 were suspended in 2% nutrient broth (Difco), 0.6% gelatin (Fisher Scientific) and doses of 300 L1 were administered by gavage. For synchronous infection, 25,000 NBL were suspended in 0.25 ml serum-free DMEM (Mediatech, Inc.) and delivered by retro-orbital injection. Mice were euthanized by CO2 inhalation at the times indicated in each experiment. Whole body muscle larvae burdens were assessed 28 days postinfection (dpi), and intestinal worm burdens were estimated as described previously (17). To quantify migrating NBL, whole lung was disrupted on a tea strainer, tissue suspensions were centrifuged and resuspended in 10% formalin, and NBL were counted using a phase contrast microscope. Crude somatic antigens from L1 were prepared as previously described (29).
Challenge infection, sera collection, and passive immunization
ΔdblGATA mice were infected orally with 300 L1 and challenged with the same dose after 90 days. Immune sera were collected from WT or ΔdblGATA mice 28 days following re-infection. Normal sera were collected from naïve WT mice. In order to inactivate complement and IgE, all sera were heated to 56 °C for 30 minutes prior to use in experiments. For passive immunization, ΔdblGATA or WT mice were injected intraperitoneally with 0.2 ml serum from naïve or infected mice.
Preparation of immunoglobulin (Ig)
Immunoglobulins were precipitated from heat-treated serum of naïve or infected mice (20-28 days post challenge) with 35% saturated (NH4)2SO4. Precipitated Igs were resuspended in saline and dialyzed against saline. For passive immunization, mice were injected intraperitoneally with Ig equivalent to that recovered from 0.2 ml serum of naïve or infected mice.
Enzyme-linked immunosorbent assay (ELISA)
Cells from cervical lymph nodes (CLN) or mesenteric lymph nodes (MLN) of WT and ΔdblGATA mice were cultured, stimulated with antigen, and supernatants were assayed for IL-4, IL-5, IL-10, IL-13 and IFN-γ, as described previously (32). Serum antibodies specific for T. spiralis antigens were measured as described previously (17). Mouse sera were diluted 1:2000 for IgG1, or 1:100 for IgG2c. IgG1 was detected with rat anti-mouse IgG1 (BD PharMingen) and biotinylated mouse anti-rat IgG (BD PharMingen) followed by HRP-conjugated streptavidin (BD Biosciences). IgG2c was detected with goat anti-mouse IgG2c (Immunology Consultants Laboratory) and HRP-conjugated rabbit anti-goat IgG (Immunology Consultants Laboratory).
Eosinophil transfer experiments
Eosinophils were recovered from infected IL-5Tg+ mice 12-20 dpi. Cells were pooled from spleens and peritoneal lavage fluid and purified by positive selection on magnetic beads, as previously described (38). Briefly, eosinophils were labeled with PE-conjugated anti-SiglecF antibody (BD) and anti-PE microbeads (Miltenyi Biotec). Average purity of eosinophils from this procedure was >93%. After washing twice with PBS, 5 × 106 eosinophils were resuspended in 200 μl sterile PBS and injected i.v. into ΔdblGATA mice. Cells were transferred 4 times, on alternate days, for 8 days.
Cell preparation and Flow cytometry
Cells from individual diaphragms were recovered as described previously(38). After 15 min incubation with Fc block (eBioscience) and 10% normal mouse serum, cells were incubated for 15 min with PE conjugated anti-SiglecF (BD Pharmingen). Single cell suspensions of bone marrow cells and spleen cells were prepared from naïve or 90 dpi WT and ΔdblGATA mice. Cells were blocked as described above and incubated for 15 min with Brilliant Violet 421 conjugated anti-CD138 (Biolegend) and PE conjugated anti-B220 (eBioscience). Data were acquired using a Gallios flow cytometer (Beckman Coulter) and analyzed with FlowJo software (Tree Star).
Worm fecundity
Worm fecundity was determined as described (39). Briefly, 6 days following primary or secondary infection with 300 L1, female worms were recovered from the small intestines of C57BL/6 and ΔdblGATA mice. Individual female worms were placed in 100 μl of RPMI1640 containing 10% FCS in a single well of a 96-well plate and incubated at 37 °C for 24 hours in 5% CO2. The number of shed NBL was counted under a microscope. At least ten female worms were collected from each mouse, and the mean NBL value per female per mouse was used to calculate means for each treatment group.
ATP content
100 adult worms were collected from individual mice as described (40). Worms were washed three times and boiled for 15 min in PBS. Lysates were centrifuged (10,000 × g for 10 min) at 4 °C. ATP was measured in supernatants with an ENLITEN ATP Assay System Bioluminescence Detection Kit (FF2000, Promega, Madison, WI).
Statistical analysis
All experiments were performed twice with similar results. Means ± SD were calculated from data collected from individual mice unless otherwise indicated. Significant differences were determined using Student's t test or One-way ANOVA with Tukey's post hoc test for multiple means. Statistical analysis was performed with GraphPad Prism 5 software.
Results
Eosinophils are dispensable for intestinal immunity against secondary T. spiralis infection
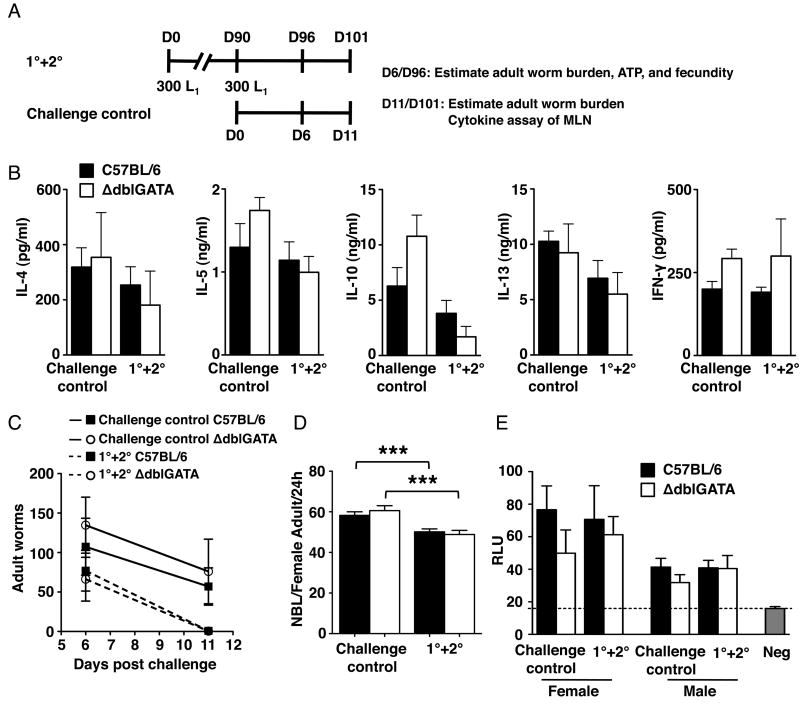
To investigate the role of eosinophils in secondary infection of T. spiralis, we first examined parameters of intestinal infection in eosinophil-ablated mice. We infected WT and ΔdblGATA mice with 300 L1 and then challenged them with the same dose 90 days later (1°+2°). A group of naïve mice received larvae at the 90 day time point in order to document the primary immune response (challenge control) (Fig. 1A). Cytokine production by antigen-stimulated MLN cultures was similar in WT and ΔdblGATA mice (Fig. 1B). Replicating the result obtained in eosinophil-ablated PHIL mice reported previously (29), the rate of intestinal worm expulsion was similar in the two strains in primary infection. Furthermore, expulsion was similarly accelerated in WT and ΔdblGATA in secondary infection (Fig. 1C). Worm fecundity and ATP content of male and female worms were not significantly different between strains in either infection (Fig. 1D and E). Taken together, the results indicate that eosinophil ablation did not influence the outcome of intestinal infection by T. spiralis. Therefore, any differences in either NBL migration or muscle colonization in eosinophil-ablated vs. WT mice that may be observed following intestinal infection would be attributed to extraintestinal effects of eosinophils and not to differences in ‘doses’ of NBL produced by intestinal worms.
Figure 1. Eosinophils are dispensable for intestinal immunity against T. spiralis infection.
(A) Design of experiments indicating timing of sample collections. D, day. (B) Cytokines detected in MLN cultures from WT and ΔdblGATA mice. (C) Adult worms in intestines of WT and ΔdblGATA mice during primary or secondary infection. (D) Fecundity of female adult worms and (E) ATP content of adult male and female worms recovered from the intestines of WT and ΔdblGATA mice during primary or secondary infection. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 4 mice. Significant differences were determined by ANOVA and Tukey's test. ***p < 0.0001.
Eosinophils prevent the accumulation of muscle larvae in secondary infection
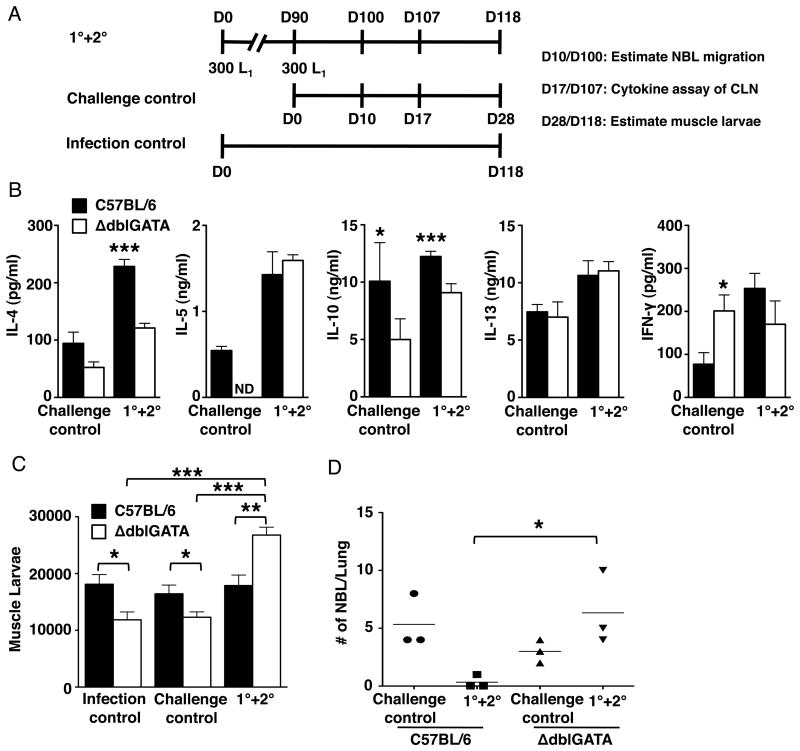
The influence of eosinophils on extraintestinal parasites, specifically migrating NBL and larvae that colonize skeletal muscle, was tested in experiments similar in design to those described above, but with an additional control group that received only the first infection (infection control) (Fig. 2A). Cytokines were assayed in CLN cultures, as these nodes drain the tongue and masseter, preferred sites of colonization by NBL. Antigen-stimulated CLN cultures prepared from ΔdblGATA mice 17 days after secondary infection produced less IL-4 than WT cells. This finding is similar to that observed in primary infection of PHIL and ΔdblGATA mice, reported previously (29, 32). IL-10 was reduced in both primary and secondary infection. No differences were observed in IL-5, IL-13 and IFN-γ (Fig. 2B) in secondary infection, although IFN-γ was increased in cultures from ablated mice undergoing primary infection, as reported previously (29, 32).
Figure 2. Eosinophils influence secondary immunity to muscle infection and limit the migration of NBL.
(A) Design of experiments indicating timing of sample collections. D, day. (B) Cytokines detected in CLN cultures from WT and ΔdblGATA mice. (C) Whole body muscle larval burdens in WT and ΔdblGATA mice. (D) Numbers of NBL in lungs recovered from WT and ΔdblGATA mice. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 5 mice. Significant differences were determined by Student's t test or ANOVA and Tukey's test. *p < 0.05, **p < 0.001, ***p < 0.0001.
WT mice prevented the accumulation of additional muscle stage larvae while ΔdblGATA mice did not show this resistance, i.e. their muscle burdens equaled the combined burdens of the two control groups (Fig. 2C). While few if any migrating NBL were recovered from lungs of WT mice ten days post-secondary infection, the number recovered from lungs of ΔdblGATA mice was significantly higher and was not different from that of the challenge control mice (Fig. 2D). Thus, the results support a mechanism in which eosinophils limit larval establishment in muscle by interfering with migration of NBL during re-infection.
Specific antibodies are required for eosinophil-mediated protection against secondary muscle infection
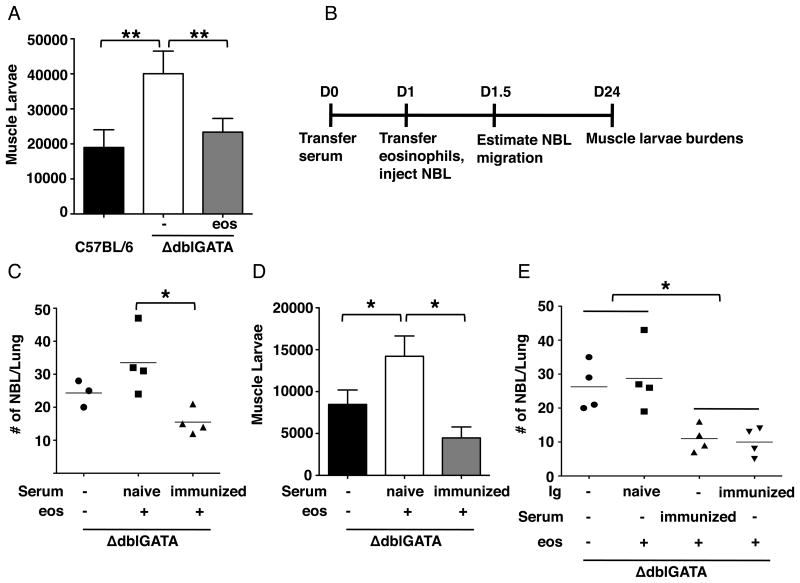
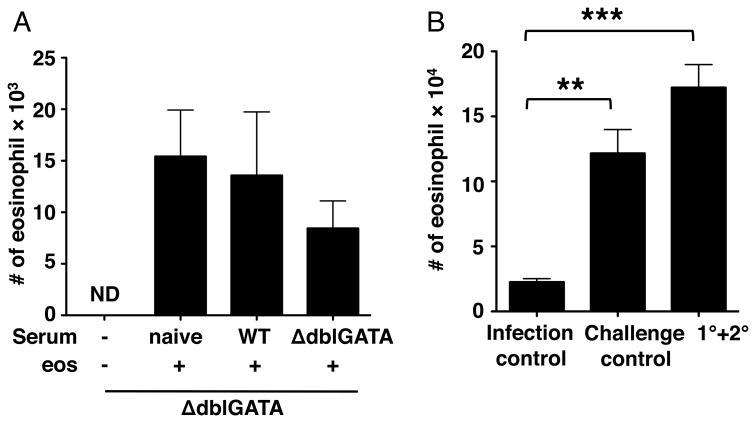
To confirm that eosinophils are required for the resistance to re-infection, we performed eosinophil transfer experiments. Restoring eosinophils to previously infected ΔdblGATA mice between days 4 and 10 following oral challenge infection resulted in fewer larvae maturing in skeletal muscle (Fig. 3A). [This result is the opposite of that observed when a nearly identical transfer protocol is applied to primary infection. In that case, larval burdens are increased in ΔdblGATA mice that receive eosinophils (32) (Fig. 3D, see naïve sera plus eosinophils).] With this result, we hypothesized the eosinophils clear migrating NBL from tissues throughout the body by working in concert with immune effectors that were induced by prior infection. Although it has not been documented in vivo, binding of antibodies to the body surfaces of NBL has been shown to promote eosinophil adhesion and degranulation in vitro (23). In order to test the protective effects of serum antibodies, we passively immunized naïve ΔdblGATA mice with sera from naïve or immune WT mice. The next day, eosinophils were restored to recipients and mice were infected intravenously with NBL (Fig. 3B). [Note that we used intravenous infection rather than oral infection in order to avoid any unknown effects of immune sera on intestinal infection that might have altered the release of NBL by adult worms.] Compared to ΔdblGATA mice that received naïve serum and eosinophils, there were fewer NBL in lungs of ΔdblGATA mice that received both immune serum and eosinophils (Fig. 3C). Consistent with previous findings, eosinophils improved muscle larval burdens in ΔdblGATA mice that received naïve serum; however, transfer of eosinophils to ΔdblGATA mice that received immune serum did not improve burdens (Fig. 3D). We next questioned whether specific antibodies are main mediators of this effect. Naïve ΔdblGATA mice were passively immunized with Ig from naïve or immune WT mice. Compared to ΔdblGATA mice that received eosinophils with Ig from naïve mice, mice that received both eosinophils and immune serum or Ig from immune mice showed reduced numbers of NBL in lungs, indicating that transfer of specific antibodies was as effective as immune serum in eosinophil-mediated protection (Fig. 3E). Taken together, the results support a mechanism in which specific antibodies enable eosinophils to defend against muscle infection by limiting the migration of NBL during re-infection. Furthermore, eosinophils and immune sera were sufficient to limit larval colonization of skeletal muscle in otherwise naïve mice, indicating that other immune effectors are not required.
Figure 3. Eosinophils cooperate with specific antibodies.
(A) Whole body muscle larval burdens in WT and ΔdblGATA mice, 28 days post-challenge. ΔdblGATA mice received 5×106 eosinophils or PBS every 48 hrs between 4 and 10 days post-challenge. (B) Design of passive immunization experiments indicating timing of sample collections. D, day. (C) Numbers of NBL in lungs recovered from ΔdblGATA mice, 12 hrs post-injection of NBL. (D) Whole body muscle larval burdens in ΔdblGATA mice, 24 days post-injection of NBL. (E) Numbers of NBL in lungs recovered from ΔdblGATA mice, 12 hrs post infection. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 4 mice. Significant differences were determined by ANOVA and Tukey's test. *p < 0.05, **p < 0.001.
Effect of eosinophils on plasma cells and generation of specific antibodies
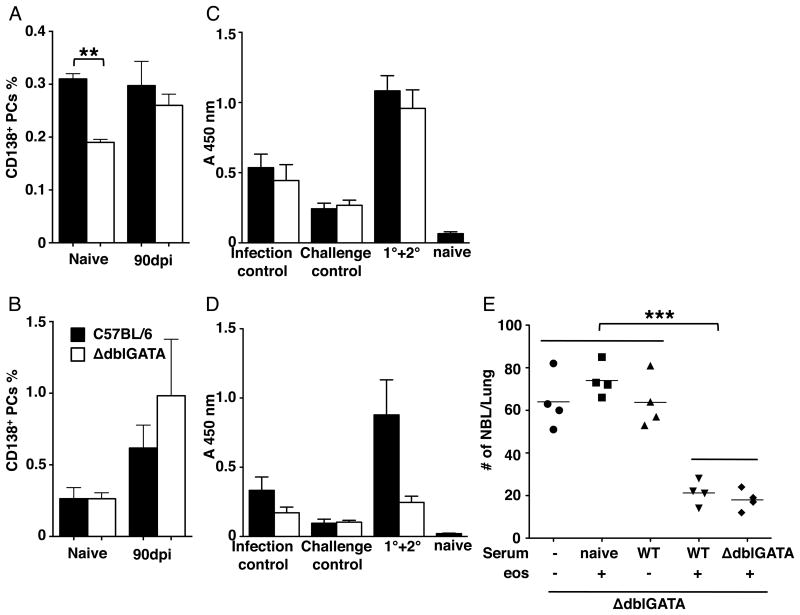
Eosinophils have been demonstrated to be required for long-term maintenance of plasma cells in the bone marrow (41, 42). We tested whether eosinophils influence numbers of plasma cells or the quantity and qualities of antibodies produced during T. spiralis infection. Consistent with published findings, there were fewer CD138+ plasma cells in the bone marrow in naïve, eosinophil-ablated mice (Fig. 4A); however, the difference was not evident after 90 days of infection (Fig. 4A). The number of plasma cells in spleen was not influenced by eosinophil ablation (Fig. 4B). Similar results were obtained in eosinophil-ablated PHIL mice (data not shown). We next compared the IgG1 and IgG2c responses mounted against parasite antigens by WT and ΔdblGATA mice. Serum IgG1 was similar between the two strains (Fig. 4C); however, serum IgG2c was significantly reduced in eosinophil-ablated mice that were infected and challenged (Fig. 4D). To test the significance of the difference in isotype composition, we passively immunized naïve ΔdblGATA mice with sera from naïve WT, immune WT, or immune ΔdblGATA mice, then restored eosinophils and infected mice with NBL (Fig. 3B). Immune sera from WT or ΔdblGATA mice were equally effective in limiting NBL migration to the lung (Fig. 4E). Thus, the difference in antibody isotype between strains did not influence the protection afforded by immune sera. Furthermore, immune sera alone did not confer protection to eosinophil-ablated mice.
Figure 4. Isotype response is influenced by eosinophils.
Percentage of CD138+ plasma cells in (A) bone marrow and (B) spleen of naive or 90 dpi WT and ΔdblGATA mice. Level of T. spiralis crude antigen specific (C) IgG1 and (D) IgG2c in serum collected from WT and ΔdblGATA mice. 1° + 2°, mice were infected on day 0 (1°), reinfected on day 90 (2°), and serum collected 28 days after 2° infection. Infection ctrl, serum collected 118 dpi. Challenge ctrl, serum collected 28 dpi. (E) Numbers of NBL in lungs recovered from ΔdblGATA mice, 12 hrs post-injection of NBL. ΔdblGATA mice were passively immunized with sera from naïve or immune WT or ΔdblGATA mice, intravenously infected with 25000 NBL following eosinophil transfer. Experimental design is diagrammed in Fig. 3B. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3-4 mice. Significant differences were determined by Student t test or ANOVA and Tukey's test. *p < 0.05, **p < 0.001, ***p < 0.0001.
Eosinophils are recruited to sites of infection in secondary infection
Our previous studies have demonstrated that eosinophils promote muscle larvae survival in primary infection by limiting production of toxic NO (29, 32). When eosinophils are absent, NO is produced and growing larvae are killed. We tested the hypothesis that during secondary infection, eosinophils may become encumbered in tissues by binding immune complexes formed by antibodies and NBL antigens, and that this would prevent eosinophil traffic to muscle. Failure of eosinophils to reach muscle would allow the development of NO-dominated local responses that would kill growing larvae (29, 32, 36). By recovering cells from skeletal muscle following passive immunization and eosinophil transfer, we found that recruitment of eosinophils to muscle after NBL injection was normal in ΔdblGATA mice (Fig. 5A). In a different experiment, eosinophils infiltrated muscle normally in WT mice following oral challenge (Fig. 5B). Both experiments documented that eosinophils access skeletal muscle efficiently in secondary infection and support the conclusion that the effector mechanism that limits colonization of muscle involves antibody-dependent, eosinophil-mediated interference with NBL migration.
Figure 5. Eosinophils are recruited to sites of infection in secondary infection.
(A) Numbers of eosinophils in diaphragms recovered from ΔdblGATA mice, 12 hrs post-infection with NBL. Experimental design is diagrammed in Fig. 3B. (B) Numbers of eosinophils in diaphragms recovered from WT mice. Experimental design is diagrammed in Fig. 2A. Note persistence of modest tissue eosinophilia 90 days post-infection (infection control). Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 4 mice. Significant differences were determined by Student t test or ANOVA and Tukey's test. **p < 0.001, ***p < 0.0001.
EPO and MBP are not required for immunity against secondary infection
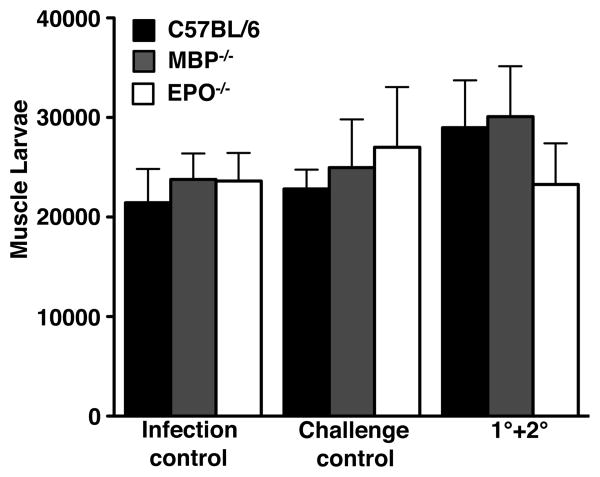
EPO and MBP are the two most abundant granule proteins in eosinophils (43). Both have been shown to be toxic for NBL in vitro (44, 45). We tested whether EPO and MBP are the effectors for eosinophil-mediated protection against secondary infection by challenging infected EPO-/- and MBP-/- mice (experimental design is shown in Fig. 2A). In contrast with ΔdblGATA mice, both EPO-/- and MBP-/- mice resisted secondary infection (Fig. 6).
Figure 6. Deficiency of EPO or MBP does not affect immunity against secondary infection.
Whole body muscle larval burdens in WT, EPO-/- and MBP-/- mice. Data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 5 mice. Significant differences were determined by ANOVA and Tukey's test
Discussion
Parasitic worms deploy a variety of strategies to evade or co-opt the immune response. Trichinella spiralis is a natural parasite of rodents, providing an accessible and readily manipulated life-cycle for the study of these mechanisms. When combined with passive transfer and adoptive transfer methods, eosinophil-ablated mice have proven to be powerful tools for investigating the role of eosinophils at the host/parasite interface. We observed that when re-infected with T. spiralis, ablated mice failed to manifest immunologic memory that normally limits colonization of skeletal muscle by larvae. This observation supports a key role for eosinophils in protection against super-infection.
Three life stages of T. spiralis encounter eosinophils during the course of infection: larval and adult worms in the intestine, migrating NBL that enter a variety of tissues, and muscle larvae that mature intracellularly in skeletal muscle. Despite the abundance and the prominence of eosinophils in the intestinal immune response, they do not influence adult worm clearance in primary infection (29). Similarly, eosinophils fail to influence either worm expulsion or Th2 immunity in primary infection by the closely related nematode, Trichuris muris (28). In secondary infection by T. spiralis, intestinal worm expulsion was accelerated; however, the absence of eosinophils did not affect clearance of worms, fecundity of female adult worms, or ATP content of male and female adults. Although the mechanism of secondary intestinal immunity against T. spiralis has not been thoroughly investigated, memory T cells are important for expulsion of worms (46, 47) and IL-4R signaling is crucial to worm expulsion in primary infection (7). Cytokine production in MLN of ablated and WT mice supported the conclusion that antigen-induced IL-4 production by intestinal cells was not altered by eosinophil ablation. Our results indicate that eosinophils are not required for the expression of intestinal immunity during secondary infection by T. spiralis.
The failure of eosinophil-ablated mice to prevent the accumulation of muscle larvae during secondary infection suggested that immunologic memory might be compromised. Th2 memory cells express IL-25 receptor and eosinophils are capable of influencing Th2 memory cell function by secreting IL-25 (48). Although we did not assay IL-25 directly, during secondary infection by T. spiralis, IL-4 and IL-10 were significantly reduced in CLN of ΔdblGATA mice, consistent with memory Th2 responses being impacted in muscle. Despite this deficiency of Th2 memory function, transfer of eosinophils to previously infected ΔdblGATA mice conferred protection against the secondary infection. Furthermore, transfer of eosinophils and immune serum to naïve mice was protective. Moreover, sera from ΔdblGATA and WT mice were equally effective in conferring immunity. Therefore, although T cell responses and development of memory T cells was promoted by eosinophils, activation of memory T cells was not required for eosinophils to limit migration of NBL.
In accordance with early in vitro studies (23, 44, 49, 50), the influence of eosinophils on NBL in vivo was dependent on the presence of immune serum or Ig. Mouse eosinophils express Fcα and Fcγ receptors (51, 52), but do not express Fcε receptors (53). Thus, a role for an eosinophil-IgE interaction in protection is unlikely. Additional evidence that IgE is not required for protection derives from results of passive transfer experiments in which heat-treated sera or Ig precipitated by 35% (NH4)2SO4 were protective. IgE is denatured by heat and excluded from precipitates prepared by salt precipitation at 35% saturation. Complement has been shown to be important for immunity to parasitic infections (54-56), and T. spiralis NBL are potent activators of complement (57). Furthermore, mouse eosinophils express functional complement receptors (58). The effect of immune serum may be mediated via complement receptors, Fcγ receptors, or some combination, and requires further investigation.
Eosinophil granule proteins have been implicated as being key to effector function. The two most abundant granule proteins in mouse eosinophils, major basic protein (MBP) and eosinophil peroxidase (EPO) (43), are required for protective immunity against Strongyloides stercoralis and Litomosoides sigmodontis in mice (31, 59). Both MBP and EPO are capable of killing T. spiralis NBL in vitro (44, 45); however, deletion of the genes encoding either MBP or EPO does not affect the capacity of mice to control primary T. spiralis infection (32). In secondary infection, our results indicate that infected MBP-/- and EPO-/- mice were similar to WT mice in resisting secondary infection. It is possible that MBP and EPO are functionally redundant, so that deleting both genes would be required to document their action against NBL in vivo; however, testing this hypothesis directly is not possible, because MBP and EPO double knockout mice lack eosinophils (60). A recent report documented a requirement for eosinophils in protection against primary, but not secondary infection by Brugia malayi. Similar to our findings in primary or secondary infection by T. spiralis his protection is independent of either MBP or EPO (61). Other granule proteins, such as eosinophil cationic protein (ECP), appear to be potent toxins and may be important (50). Alternatively, eosinophils may act through a granule protein-independent mechanism, for example, by adhering to and entrapping NBL as they move through tissue. This may be a two steps process, in which antibodies impede the movement of highly motile NBL such that the much less motile eosinophil can approach and adhere. Recent reports suggest that antibodies are capable of cooperating with basophils or alternatively activated macrophages to impede the mobility of larvae (62, 63). The potential association of eosinophils with Ig complexed with NBL, and their effect on NBL migration in tissue, require further investigation.
Our results show that the longstanding paradigm of defensive function for eosinophils against helminth infection is valid and highly significant in T. spiralis infection. Previously, we have reported that eosinophils support rather than limit colonization of skeletal muscle during primary infection by T. spiralis (29, 32). In this context, the mechanism by which eosinophils support larval growth in muscle is distinct from the mechanism of immune regulation that limits local NO synthesis (36). Our evidence supports the conclusion that these two, distinct eosinophil-mediated effects cooperate to promote survival of larvae in muscle. The host-protective function of eosinophils in the response to challenge represents a third role for eosinophils in T. spiralis infection. Understanding all of the contributions of eosinophils to immunity mounted in response to parasitic worms will be crucial to the development of effective vaccines and therapeutic interventions.
Acknowledgments
We thank Drs. Cynthia Leifer (Cornell University) and Dr. Eric Denkers (Cornell University) for helpful advice.
Abbreviations used in this paper
- CLN
cervical lymph node
- EPO
eosinophil peroxidase
- Ig
immunoglobulin
- MBP
major basic protein
- MLN
mesenteric lymph node
- NBL
newborn larvae
- NO
nitric oxide.
Footnotes
This work was supported by NIH grants AI081043 and AI097555 (J.A.) and HL065228 (J.L.).
Disclosures: The authors have no financial conflict of interest.
References
- 1.Organization, W. H. http://www.who.int/mediacentre/factsheets/fs366/en/
- 2.Jia TW, Melville S, Utzinger J, King CH, Zhou XN. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1621. doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207–222. doi: 10.1128/cmr.13.2.207-222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie RA, Williamson LH, Terrill TH, Kaplan RM. Efficacy of anthelmintics on South American camelid llama and alpaca farms in Georgia. Vet Parasitol. 2010;172:168–171. doi: 10.1016/j.vetpar.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Howell SB, Burke JM, Miller JE, Terrill TH, Valencia E, Williams MJ, Williamson LH, Zajac AM, Kaplan RM. Prevalence of anthelmintic resistance on sheep and goat farms in the southeastern United States. J Am Vet Med Assoc. 2008;233:1913–1919. doi: 10.2460/javma.233.12.1913. [DOI] [PubMed] [Google Scholar]
- 6.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban JF, Jr, Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, Gamble HR, Byrd C, Donaldson D, Else K, Finkelman FD. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 8.Turner JD, Faulkner H, Kamgno J, Cormont F, Van Snick J, Else KJ, Grencis RK, Behnke JM, Boussinesq M, Bradley JE. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J Infect Dis. 2003;188:1768–1775. doi: 10.1086/379370. [DOI] [PubMed] [Google Scholar]
- 9.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 10.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, Lund FE. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- 12.Wakelin D, Wilson MM. T and B cells in the transfer of immunity against Trichinella spiralis in mice. Immunology. 1979;37:103–109. [PMC free article] [PubMed] [Google Scholar]
- 13.Horsnell WG, Darby MG, Hoving JC, Nieuwenhuizen N, McSorley HJ, Ndlovu H, Bobat S, Kimberg M, Kirstein F, Cutler AJ, Dewals B, Cunningham AF, Brombacher F. IL-4Ralpha-associated antigen processing by B cells promotes immunity in Nippostrongylus brasiliensis infection. PLoS Pathog. 2013;9:e1003662. doi: 10.1371/journal.ppat.1003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alizadeh H, Wakelin D. Comparison of rapid expulsion of Trichinella spiralis in mice and rats. Int J Parasitol. 1982;12:65–73. doi: 10.1016/0020-7519(82)90097-2. [DOI] [PubMed] [Google Scholar]
- 15.Moqbel R, Wakelin D, MacDonald AJ, King SJ, Grencis RK, Kay AB. Release of leukotrienes during rapid expulsion of Trichinella spiralis from immune rats. Immunology. 1987;60:425–430. [PMC free article] [PubMed] [Google Scholar]
- 16.Tuohy M, Lammas DA, Wakelin D, Huntley JF, Newlands GF, Miller HR. Functional correlations between mucosal mast cell activity and immunity to Trichinella spiralis in high and low responder mice. Parasite Immunol. 1990;12:675–685. doi: 10.1111/j.1365-3024.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 17.Blum LK, Thrasher SM, Gagliardo LF, Fabre V, Appleton JA. Expulsion of secondary Trichinella spiralis infection in rats occurs independently of mucosal mast cell release of mast cell protease II. J Immunol. 2009;183:5816–5822. doi: 10.4049/jimmunol.0900944. [DOI] [PubMed] [Google Scholar]
- 18.Appleton JA, Schain LR, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988;65:487–492. [PMC free article] [PubMed] [Google Scholar]
- 19.Appleton JA, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats. Science. 1984;226:70–72. doi: 10.1126/science.6474191. [DOI] [PubMed] [Google Scholar]
- 20.Bell RG. Trichinella spiralis: evidence that mice do not express rapid expulsion. Exp Parasitol. 1992;74:417–430. doi: 10.1016/0014-4894(92)90204-n. [DOI] [PubMed] [Google Scholar]
- 21.Basten A, Boyer MH, Beeson PB. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970;131:1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capron M, Torpier G, Capron A. In vitro killing of S. mansoni schistosomula by eosinophils from infected rats: role of cytophilic antibodies. J Immunol. 1979;123:2220–2230. [PubMed] [Google Scholar]
- 23.Bass DA, Szejda P. Eosinophils versus neutrophils in host defense. Killing of newborn larvae of Trichinella spiralis by human granulocytes in vitro. J Clin Invest. 1979;64:1415–1422. doi: 10.1172/JCI109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Goff L, Loke P, Ali HF, Taylor DW, Allen JE. Interleukin-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infect Immun. 2000;68:2513–2517. doi: 10.1128/iai.68.5.2513-2517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2000;165:4544–4551. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 26.Herndon FJ, Kayes SG. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]
- 27.Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svensson M, Bell L, Little MC, DeSchoolmeester M, Locksley RM, Else KJ. Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection. Parasite Immunol. 2011;33:1–11. doi: 10.1111/j.1365-3024.2010.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37:1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.O'Connell AE, Hess JA, Santiago GA, Nolan TJ, Lok JB, Lee JJ, Abraham D. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun. 2011;79:2770–2778. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol. 2012;188:417–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babayan SA, Read AF, Lawrence RA, Bain O, Allen JE. Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy. PLoS Biol. 2010;8:e1000525. doi: 10.1371/journal.pbio.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruschi F, Solfanelli S, Binaghi RA. Trichinella spiralis: modifications of the cuticle of the newborn larva during passage through the lung. Exp Parasitol. 1992;75:1–9. doi: 10.1016/0014-4894(92)90116-r. [DOI] [PubMed] [Google Scholar]
- 35.Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 36.Huang L, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, Lee JJ, Appleton JA. Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol. 2014;193:4178–4187. doi: 10.4049/jimmunol.1400852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallance BA, Matthaei KI, Sanovic S, Young IG, Collins SM. Interleukin-5 deficient mice exhibit impaired host defence against challenge Trichinella spiralis infections. Parasite Immunol. 2000;22:487–492. doi: 10.1046/j.1365-3024.2000.00328.x. [DOI] [PubMed] [Google Scholar]
- 38.Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol. 2011;188:417–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.deVos T, Danell G, Dick TA. Trichinella spiralis: dose dependence and kinetics of the mucosal immune response in mice. Exp Parasitol. 1992;75:99–111. doi: 10.1016/0014-4894(92)90125-t. [DOI] [PubMed] [Google Scholar]
- 40.Scalfone LK, Nel HJ, Gagliardo LF, Cameron JL, Al-Shokri S, Leifer CA, Fallon PG, Appleton JA. Participation of MyD88 and interleukin-33 as innate drivers of Th2 immunity to Trichinella spiralis. Infect Immun. 2013;81:1354–1363. doi: 10.1128/IAI.01307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong TW, Doyle AD, Lee JJ, Jelinek DF. Eosinophils Regulate Peripheral B Cell Numbers in Both Mice and Humans. J Immunol. 2014 doi: 10.4049/jimmunol.1302241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 43.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buys J, Wever R, van Stigt R, Ruitenberg EJ. The killing of newborn larvae of Trichinella spiralis by eosinophil peroxidase in vitro. Eur J Immunol. 1981;11:843–845. doi: 10.1002/eji.1830111018. [DOI] [PubMed] [Google Scholar]
- 45.Wassom DL, Gleich GJ. Damage to Trichinella spiralis newborn larvae by eosinophil major basic protein. Am J Trop Med Hyg. 1979;28:860–863. [PubMed] [Google Scholar]
- 46.Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P, Artis D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venturiello SM, Giambartolomei GH, Costantino SN. Immune cytotoxic activity of human eosinophils against Trichinella spiralis newborn larvae. Parasite Immunol. 1995;17:555–559. doi: 10.1111/j.1365-3024.1995.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 50.Hamann KJ, Barker RL, Loegering DA, Gleich GJ. Comparative toxicity of purified human eosinophil granule proteins for newborn larvae of Trichinella spiralis. J Parasitol. 1987;73:523–529. [PubMed] [Google Scholar]
- 51.Decot V, Woerly G, Loyens M, Loiseau S, Quatannens B, Capron M, Dombrowicz D. Heterogeneity of expression of IgA receptors by human, mouse, and rat eosinophils. J Immunol. 2005;174:628–635. doi: 10.4049/jimmunol.174.2.628. [DOI] [PubMed] [Google Scholar]
- 52.de Andres B, Mueller AL, Blum A, Weinstock J, Verbeek S, Sandor M, Lynch RG. Fc gammaRII CD32 is linked to apoptotic pathways in murine granulocyte precursors and mature eosinophils. Blood. 1997;90:1267–1274. [PubMed] [Google Scholar]
- 53.de Andres B, Rakasz E, Hagen M, McCormik ML, Mueller AL, Elliot D, Metwali A, Sandor M, Britigan BE, Weinstock JV, Lynch RG. Lack of Fc-epsilon receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–3836. [PubMed] [Google Scholar]
- 54.Giacomin PR, Gordon DL, Botto M, Daha MR, Sanderson SD, Taylor SM, Dent LA. The role of complement in innate, adaptive and eosinophil-dependent immunity to the nematode Nippostrongylus brasiliensis. Mol Immunol. 2008;45:446–455. doi: 10.1016/j.molimm.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 55.Shaio MF, Hou SC, Chen JG, Wu CC, Yang KD, Chang FY. Immunoglobulin G-dependent classical complement pathway activation in neutrophil-mediated cytotoxicity to infective larvae of Angiostrongylus cantonensis. Ann Trop Med Parasitol. 1990;84:185–191. doi: 10.1080/00034983.1990.11812453. [DOI] [PubMed] [Google Scholar]
- 56.Giacomin PR, Wang H, Gordon DL, Botto M, Dent LA. Loss of complement activation and leukocyte adherence as Nippostrongylus brasiliensis develops within the murine host. Infect Immun. 2005;73:7442–7449. doi: 10.1128/IAI.73.11.7442-7449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong Y, Kim CW, Ghebrehiwet B. Trichinella spiralis: activation of complement by infective larvae, adults, and newborn larvae. Exp Parasitol. 1992;74:290–299. doi: 10.1016/0014-4894(92)90152-z. [DOI] [PubMed] [Google Scholar]
- 58.Lopez AF, Strath M, Sanderson CJ. IgG and complement receptors on purified mouse eosinophils and neutrophils. Immunology. 1981;43:779–786. [PMC free article] [PubMed] [Google Scholar]
- 59.Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, Lee JJ, Hoerauf A. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DT, Protheroe C, Colbert D, Kloeber J, Neely J, Shim KP, Dyer KD, Rosenberg HF, Lee JJ, Lee NA. Expression of the secondary granule proteins major basic protein 1 MBP-1 and eosinophil peroxidase EPX is required for eosinophilopoiesis in mice. Blood. 2013;122:781–790. doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cadman ET, Thysse KA, Bearder S, Cheung AY, Johnston AC, Lee JJ, Lawrence RA. Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog. 2014;10:e1003988. doi: 10.1371/journal.ppat.1003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, Minegishi Y, Ohta N, Watanabe N, Kanuka H, Karasuyama H. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med. 2013;210:2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esser-von Bieren J, Mosconi I, Guiet R, Piersgilli A, Volpe B, Chen F, Gause WC, Seitz A, Verbeek JS, Harris NL. Antibodies trap tissue migrating helminth larvae and prevent tissue damage by driving IL-4Ralpha-independent alternative differentiation of macrophages. PLoS Pathog. 2013;9:e1003771. doi: 10.1371/journal.ppat.1003771. [DOI] [PMC free article] [PubMed] [Google Scholar]