Approximately 20% of pregnant women report elevated depressive symptoms.1 In low-income, African American women that percentage was doubled (42%).2 Women with elevated prenatal depressive symptoms are more likely to have smaller fetuses, premature births, and low birth weight infants.3,4 In 2012, 16.5% of African American women experienced a premature birth compared to the national average of 11.5%.5 Predictors for prenatal depressive symptoms include young age, single marital status, unemployment, smoking, poor overall health, low education, history of depression,6 history of childhood sexual abuse,7 lower levels of support,8 and elevated levels of anxiety.9 Neonates whose mothers have elevated prenatal depressive symptoms are at risk for neurobehavioral dysregulation as reflected by less positive affect, lower vagal tone, and elevated cortisol levels.3 Prenatal depressive symptoms contribute to altered patterns of mother-infant relationships, such as the mother’s expressed lack of desire for close contact with her infant, lack of satisfaction with being a mother, hostility, and feelings of resentment toward her infant.10
The biological mechanisms underlying this health disparity in pregnant African American women are poorly understood. In order to identify women at risk for prenatal depression, providers screen pregnant women using interview techniques or validated screening questionnaires.11 The American College of Obstetrics and Gynecology, however, does not recommend universal screening of pregnant or postpartum women for depression but recommends screening per the providers’ discretion and preference.12 Understanding the biological pathway for prenatal depression in African American women may increase the capacity for identification of women at risk before symptoms are present. Understanding this biological pathway may also enhance opportunities for providing needed treatment of vulnerable mothers. Thus improving infant outcomes. Previous reports have suggested oxytocin may be part of the potential biological pathways involved in perinatal depression.13–21 Accordingly, we hypothesized that oxytocin levels may predict perinatal depression.
Oxytocin is a nonapeptide hormone synthesized primarily in the brain, but also in peripheral organs (uterus, placenta, amnion, corpus luteum, testis, and heart). Oxytocin is released in response to various stimuli including social interactions, vaginal stimulation, labor progression, and lactation.15 Oxytocin release promotes actions relating to the development of social attachments.22,23 Oxytocin promotes a desire to touch and be touched, bonding between two people such as husband and wife or mother and child, and initiation of maternal behaviors.22–26 Low levels of oxytocin have been linked to altered patterns of mother-infant interactions including less touch,14 whereas high levels of oxytocin have been associated with greater mother-newborn interactions including increased gaze, vocalization, positive affect, and touch.13,18 Finally, low oxytocin levels in pregnancy may be predictive of postpartum depressive symptoms.21 However, oxytocin has not been explored during pregnancy in an urban African American sample. In a sample of urban African American women, we examined factors potentially associated with prenatal depressive symptoms including plasma oxytocin levels, birth weight/prematurity, demographics, anxiety, support from the infant’s father, and maternal health history.
Materials and Method
Design
The data for this secondary analysis were obtained from a larger longitudinal study of pregnant African American women. The larger study was designed to examine stress, pregnancy, and birth outcomes. Data were collected twice during pregnancy (15 – 22 and 25 – 37 weeks). Birth data were obtained from medical records.
Setting
Participants were recruited from a large medical center in Chicago, Illinois. Women were recruited after their standard prenatal care appointment.
Subjects
Subjects included 57 pregnant African American women at a prenatal care appointment between 15 – 22 weeks gestation. Women were invited into the study if they were 18 years of age or older, had a singleton pregnancy, and were able to read and write English. Per the larger longitudinal study, subjects with hypertension, pre-gestational diabetes, human immunodeficiency virus (HIV), autoimmune disorders, a multiple gestation pregnancy, or receiving medications such as dexamethasone, betamethasone, or inhaled steroid were excluded from the study. These characteristics may affect the larger study outcomes of maternal stress and pregnancy.
Measures
Data collected included medical record review for birth outcomes (e.g. gestational age at birth and birth weight), blood samples for plasma oxytocin analysis, and self-reported questionnaires.
Plasma oxytocin was analyzed in duplicate using enzyme-linked immunoassay (ELISA) (Enzo Life Sciences). Oxytocin detection levels were 15.6 pg/ml to 1000 pg/ml.27 The intra-assay coefficient of variability was 9.7% to 30% and an inter-assay coefficient of variability was 4.6% to 8.6%.
Self-reported data included a socio-demographic questionnaire in which women indicated their age, marital status, education, household income, and family involvement that included support of the infant’s father during pregnancy. Additionally, participants completed previously validated instruments to assess state anxiety and prenatal depressive symptoms,
State anxiety was measured by the State-Trait Anxiety Inventory (STAI).28 The state subscale of the STAI consists of 20 items (e.g., feeling happy, calm, jittery, upset, confused) rated on a 4-point Likert scale (1 = not at all and 4 = very much so) with scores ranging from 20 – 80. A higher score is associated with higher levels of anxiety.28 The Cronbach’s alpha for this study was 0.91.
Prenatal depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CESD).29,30 The CESD consists of 20 depressive symptoms (e.g., lonely, sad, restless) rated on a 4-point Likert scale (0 = rarely or none of the time to 3 = most or all of the time) with scores ranging from 0 – 60. A score of 16 or greater indicates clinically elevated depressive symptoms.31 For this study, the Cronbach’s alpha was 0.84.
Procedures
The Institutional Review Board at the University of Illinois at Chicago approved this study. Pregnant African American women between 15 – 22 weeks gestation were invited to participate. After giving informed consent, participants completed the packet of questionnaires in a private room after their prenatal visit. No stimuli were present that may affect oxytocin levels (such as pictures of loved ones, baby toys/dolls, or pregnancy/baby related magazines). On the same day, the participant’s blood was drawn into an ethylenediaminetetracetic acid (EDTA) sterile tube (10 ml) and placed on ice. This was completed in the afternoon (1:00 p.m. – 5:00 p.m.) for consistency. The samples were transported on ice, centrifuged (1500 × g, 15 minutes), and aliquoted within 3 hours of withdrawal. Once plasma was aliquoted, the samples were stored at −80°C for batch analysis. All assays were conducted according to manufacturer specifications, but samples were not extracted. Participants were given a $25 gift card for their participation and time. Three participants did not return to the clinic between 25 – 37 weeks and two additional participants delivered prior to the second data collection time. A member of the research team completed a maternal medical record review prenatally and postpartum (for birth data). The participants were not contacted after birth.
Data Analysis
Descriptive statistics were generated to describe sample characteristics. Paired sample t-tests were computed to analyze changes in depressive symptoms and oxytocin between the first and second data collection. Independent t-tests were computed to examine the relationship between prenatal depressive symptoms and gravida status (primigravida versus multigravida). Spearman’s correlation coefficient was computed to examine the relationships among depressive symptoms, raw oxytocin levels, birth weight, gestational age at birth, and anxiety. Analysis of variance was computed to examine depressive symptoms and support of the infant’s father during the pregnancy.
Due to the wide range of oxytocin values previously reported in the literature,18 after continuous data analysis, raw oxytocin values were categorized as low, normal, and high. These category distinctions were based on previous reports17 and graphical cut points. A one-way analysis of variance (ANOVA) was computed to examine the relationships between oxytocin categories and prenatal depressive symptoms, birth weight, and gestational age at birth.
Results
Sample Characteristics
The average age of women in our sample was 23.4 (SD = 4.8) years, and ages ranged between 18 and 36 years. Subjects’ predominately delivered their infants at term with an average gestational age at birth of 38.6 (SD = 2.5) weeks, with ranges from 25 – 41 weeks. The majority of women were multigravidas (75%). Most subjects did not have a college degree (86%) and had an annual income less than $30,000 (85%). A majority of women (70%) reported the baby’s father was fully involved with the pregnancy. At the first time point subjects reported moderate levels of anxiety (STAI = 35, SD = 11), and 35% had elevated depressive symptoms (score ≥ 16 on CESD). No significant differences were found in state anxiety or depressive symptoms between the first and second time point (see Table 1).
Table 1.
Sample Characteristics
| Mean (SD) | |
|---|---|
| Maternal age (years) | 23.4 (4.8) |
| Gestational age (weeks) | |
| First data point | 19.5 (2.5) |
| Second data point | 29.7 (3.1) |
| Birth | 38.6 (2.5) |
| Infant birth weight (grams) | 3105 (690) |
| Multigravida | 75% |
| Education | |
| % Less than High School | 21% |
| % High School Graduate | 24% |
| % Some College | 44% |
| % College Graduate | 11% |
| Marital Status | |
| Single | 84% |
| Married | 14% |
| Separated | 2% |
| Income less than 30,000 | 79% |
| Baby’s father involvement | |
| Not involved | 11% |
| Moderately involved | 19% |
| Fully involved | 70% |
| State Anxiety (STAI) | |
| First data point | 34.7 (11) |
| Second data point | 32.8 (10) |
| Depressive Symptoms (CESD) | |
| First data point | 13 (8.6) |
| Second data point | 12 (9.0) |
| CESD score 16 or greater | |
| First data point | 35% |
| Second data point | 33% |
Note. STAI = State Trait Anxiety Inventory. CESD = Center for Epidemiology Studies – Depressive Scale
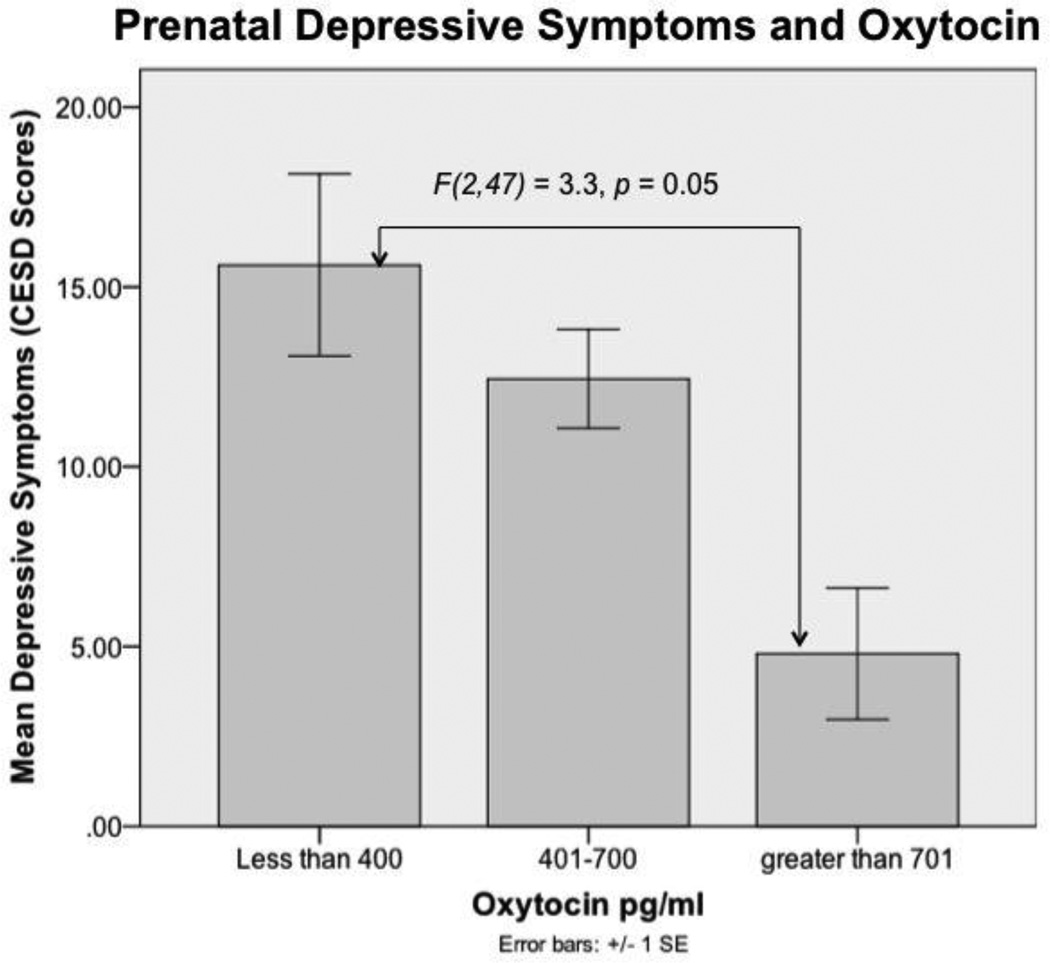
The average oxytocin value was 504 pg/ml, SD = 284.2, with a range between 170 and 2056 pg/ml. No significant differences in oxytocin levels were found between the first and second time point. Therefore, we conducted the remaining analysis only with the first data point. Oxytocin data was divided into low (<400 pg/ml), medium (401–700 pg/ml), and high (>701 pg/ml) categories, based on the distribution of the data and corroborated by previous reports on oxytocin.17,18,32 The low category represented the lower 35%, while the high category represented the upper 10%. The mean depressive symptoms in the low, normal, and high oxytocin categories were 15.6, 12.4, and 5.3 respectively (see Table 2).
Table 2.
Mean (SD) of the Oxytocin Values in the total Sample and by the Oxytocin Categories
| Oxytocin (pg/ml) |
Depressive symptoms (CESD scores) |
Birth Weight (grams) |
N | |
|---|---|---|---|---|
| Sample Means | 504 (284) | 13.1 (8.6) | 3105 (690) | 50 |
| Categories of Oxytocin | ||||
| Low Oxytocin (<400) | 304 (70) | 15.6 (10.7) | 2769 (811) | 18 |
| Medium Oxytocin (401–700) | 508 (73) | 12.4 (7.1) | 3223 (542) | 27 |
| High Oxytocin (>701) | 1030 (475) | 5.3 (4.6) | 3350 (791) | 5 |
Factors Associated with Prenatal Depressive Symptoms, Oxytocin and Birth Weights
Depressive symptoms were higher in multigravidas (t(51) = −2.37, p = 0.02), women with higher anxiety (r(47) = 0.71, p = 0.001), women who delivered their infants at an earlier gestational age (r(51) = −0.29, p = 0.04), and those without the support of the infant’s father (F(4,48) = 2.676, p = 0.04). There was no statistical relationship between depressive symptoms and birth weight (r(50) = −0.21, p = 0.15) or raw oxytocin levels(r(51) = −0.10, p = 0.53). After depressive symptoms were dichotomized into elevated and non-elevated (CESD score ≥ 16), there continued to be no statistical relationship between elevated depressive symptoms and birth weight (t(48) = 1.4, p = 0.17), or raw oxytocin levels (t(48) = 0.60, p = 0.55). In addition there was no relationship between the group of participants with elevated depressive symptoms (CESD score ≥ 16) and oxytocin category (low, medium, or high) (X2(2, N = 50) = 3.5, p = 0.18).
Oxytocin categories (low, medium, or high) and depressive symptoms (CESD scores) were related. Depressive symptoms were higher in women with low levels of oxytocin (n = 17) compared to women with high levels of oxytocin (n = 5) (F(2,47) = 3.3, p = 0.05, Fisher’s Least Significant Difference (LSD) = 0.015) (see Figure 1).
Figure 1.
Prenatal Depressive Symptoms and Oxytocin. Women with low oxytocin levels had more depressive symptoms compared to women with high oxytocin levels. Error bars represent one standard error from the mean. CESD: Center for Epidemiologic Studies Depression scale. Sample size for the groups is as follows: less than 400, n = 18; 401–700, n = 27; greater than 701, n = 5.
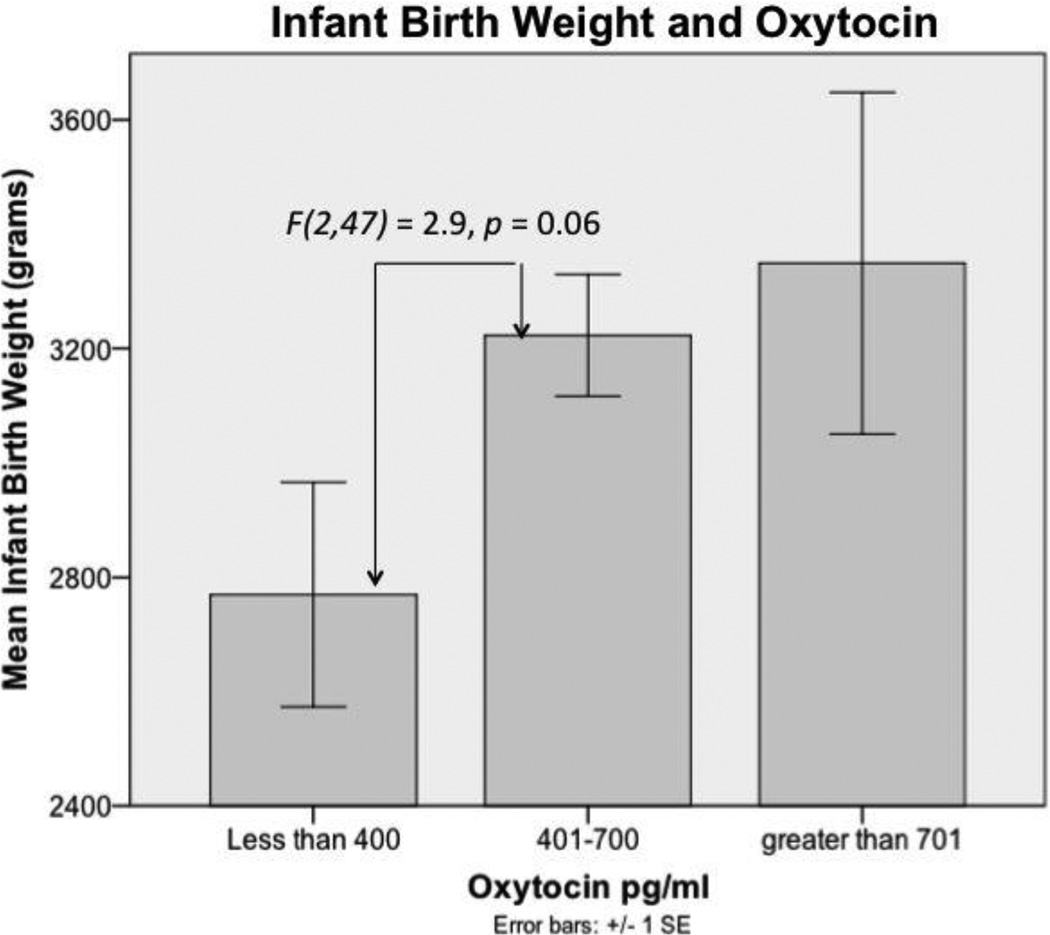
In addition, women with low levels of oxytocin also tended to be more likely to have infants with lower birth weights compared to women categorized as having medium and high levels of oxytocin (F(2,47) = 2.9, p = 0.06, LSD = 0.037, 0.063 respectively); although this difference in birth weights is not clinically significant (see Figure 2). There was no relationship between oxytocin categories (low, medium, or high) and gestational age at birth.
Figure 2.
Infant Birth Weight and Oxytocin. The relationship between prenatal oxytocin category and infant birth weight trended toward significance. The relationship between infant birth weight and raw prenatal oxytocin values was not significant. Error bars represent one standard error from the mean. Sample size for the groups is as follows: less than 400, n = 17; 401–700, n = 27; greater than 701, n = 5.
Discussion
In pregnant African American, primarily low-income women, a large percentage (35%) experienced elevated prenatal depressive symptoms. Depressive symptoms were higher in multigravidas, women who reported greater anxiety, and in women without involvement from the baby’s father. In addition, women with elevated prenatal depressive symptoms more frequently had low levels of prenatal oxytocin and earlier gestational ages at birth.
The prevalence of elevated prenatal depressive symptoms is consistent with other studies of pregnant African American women33,34 and low income mothers.35,36 Previous reports also identified anxiety and depression as comorbid conditions.37 In addition, we found the mean anxiety levels in our sample (STAI mean = 34) were similar to other reports from low–income, predominately African American pregnant women (STAI mean = 39).38 Other researchers have found that women with low levels of social support also reported higher levels of prenatal depressive symptoms.39,40 Specifically, the social support of the infant’s father has been identified as a protective factor for elevated postpartum depressive symptoms.41–43
The wide range (170 – 2056 pg/ml) of raw oxytocin levels reported in this investigation is consistent with previous studies using a similar methodology.13,44 The range of possible oxytocin values between our sample and other reports are comparable with oxytocin values in some cases exceeding 2000 pg/ml. The mean value of oxytocin from our sample was 504 pg/ml, compared to values of approximately 300 pg/ml, which are typical of other populations using identical measures.13,44 The comparatively high levels of oxytocin measured in this study may reflect physiological coping mechanisms associated with lifestyle in this vulnerable low-income pregnant African American population.
We identified a relationship between low oxytocin category and increased prenatal depressive symptoms. Prior reports have identified lower prenatal oxytocin values as a risk factor for postpartum depressive symptoms.21 In addition, the literature provides support that higher prenatal oxytocin values are related to positive postpartum maternal behaviors such as gaze, affect, touch, and vocalization during the first month postpartum.13 Higher postpartum oxytocin levels have been associated with positive affectionate parenting including vocalization, positive affect, and touch.16 In a non-pregnant population, lower oxytocin has been identified in individuals with greater depressive symptoms.19,20
Women with low prenatal oxytocin levels delivered infants who tended to have lower birth weights when compared to mothers with medium and high oxytocin levels. The mean difference in birth weight was not clinically significant, but it raises the question of the potential relationship between these factors.
Study Limitations
Limitations of this study include 1) small sample size, 2) limited variables, and 3) inclusion of only African American women. The sample size of 57 women included 6 women who had a premature birth. Further analysis with premature birth, prenatal depressive symptoms, and oxytocin were limited based on the small number of premature births. Second, because this study was a secondary analysis of existing data, we were limited by its design and the data collected. For example, longitudinal data from the postpartum period may have provided a more complete understanding of the ways these factors are associated. The third limitation is based on the homogeneity of the sample. The urban, low income, African American women in our sample may have experienced unique life events affecting their oxytocin levels and their self-report of prenatal depressive symptoms. This sample was chosen due to the known increased risk of prenatal depression in African American women, yet this limits the generalizability of these results.
Conclusions
A large percentage of pregnant African American urban women, report elevated prenatal depressive symptoms. There are many factors related to elevated prenatal depressive symptoms and oxytocin may be one of the biological pathways explaining this increased risk. Understanding the biologic pathway of prenatal depressive symptoms may also explain other pregnancy health disparities in African American women such as premature birth. Identifying the biological pathway for depressive symptoms might allow for improved identification and future treatment. Early identification of pregnant women at risk for depressive symptoms may improve known sequelae such as maternal health, mother-infant interactions, and infant development.45,46
Acknowledgements
Thank you to the women who participated in this study.
Source of Support:
The National Institutes of Health (NR 010176, Dr. Garfield and NR 010608, Dr. Giurgescu) and the Irving Harris Foundation (postdoctoral fellowship, Dr. Garfield) supported this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Disclosure Statement
The authors have reported that they have no relationships relevant to the contents of this paper to disclose and no competing financial interests exist.
References
- 1.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstetrics & Gynecology. 2005;106(5):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 2.Dailey DE, Humphreys JC. Social stressors associated with antepartum depressive symptoms in low-income African American women. Public Health Nursing. 2011 May-Jun;28(3):203–212. doi: 10.1111/j.1525-1446.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- 3.Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behavior and Development. 2006 Jul;29(3):445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Archives of Womens Mental Health. 2012 Feb;15(1):1–14. doi: 10.1007/s00737-011-0251-1. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton BE, Martal JA, Ventura MA. Births: Preliminary Data for 2012. National Vital Statistics Report. 2013;62(3) [PubMed] [Google Scholar]
- 6.Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women's Health. 2003 May;12(4):373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers CS, Lang AJ, Twamley EW, Stein MB. Sexual trauma and pregnancy: a conceptual framework. Journal of Women's Health. 2003 Dec;12(10):961–970. doi: 10.1089/154099903322643884. [DOI] [PubMed] [Google Scholar]
- 8.Myers ER, Aubuchon-Endsley N, Bastian LA, et al. Efficacy and Safety of Screenign for Postpartum Depression. Rockville (MD): 2013. [PubMed] [Google Scholar]
- 9.Field T, Diego M, Hernandez-Reif M, et al. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depression and Anxiety. 2003;17(3):140–151. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- 10.Perry DF, Ettinger AK, Mendelson T, Le HN. Prenatal depression predicts postpartum maternal attachment in low-income Latina mothers with infants. Infant Behavior and Development. 2011 Apr;34(2):339–350. doi: 10.1016/j.infbeh.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Boyd RC, Le HN, Somberg R. Review of screening instruments for postpartum depression. Archives of Womens Mental Health. 2005 Sep;8(3):141–153. doi: 10.1007/s00737-005-0096-6. [DOI] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 453: Screening for depression during and after pregnancy. Obstetrics and Gynecology. 2010 Feb;115(2 Pt 1):394–395. doi: 10.1097/AOG.0b013e3181d035aa. [DOI] [PubMed] [Google Scholar]
- 13.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007 Nov;18(11):965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 14.Feldman R, Zagoory-Sharon O, Weisman O, et al. Sensitive Parenting is Associated with Plasma Oxytocin and Polymorphisms in the OXTR and CD38 Genes. Biological Psychiatry. 2012 Feb 13;72(3):175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiology Review. 2001;81(2) doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 16.Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010 Aug 15;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouin JP, Carter CS, Pournajafi-Nazarloo H, et al. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010 Aug;35(7):1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007 Jun;28(6):1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Ozsoy S, Esel E, Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Research. 2009 Oct 30;169(3):249–252. doi: 10.1016/j.psychres.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 20.Scantamburlo G, Hansenne M, Fuchs S, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007 May;32(4):407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011 Aug;36(9):1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uvnas-Moberg K. The Oxytocin Factor. Cambridge: A Merloyd Lawrence Book; 2003. [Google Scholar]
- 23.Uvnas-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: The role of the oxytocinergic system. International Journal of Behavioral Medicine. 2005;12(2):59–65. doi: 10.1207/s15327558ijbm1202_3. [DOI] [PubMed] [Google Scholar]
- 24.Uvnas-Moberg K, Carter CS. Introduction to psychoneuroendocrinology volume: Is there a neurobiology of love? Psychoneuroendocrinology. 1998;23(8):749–750. [Google Scholar]
- 25.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 26.Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Progress in Brain Research. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 27.AssayDesigns. Oxytocin enzyme immunoassay kit. Ann Arbor, MI: Assay Designs, Inc; 2006. [Google Scholar]
- 28.Spielberger CD. Consulting Psychologists Press, Inc. Mind Garden, Inc; 1983. State-Trait Anxiety Inventory for adults: Manual and sample. [Google Scholar]
- 29.Canady RB, Stommel M, Holzman C. Measurement properties of the centers for epidemiological studies depression scale (CES-D) in a sample of African American and non-Hispanic White pregnant women. Journal of Nursing Measurement. 2009;17(2):91–104. doi: 10.1891/1061-3749.17.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radloff L. The CESD scale: A sel-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 31.Radloff L. The CESD scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 32.Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia Research. 2010 Dec;124(1–3):13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tandon SD, Cluxton-Keller F, Leis J, Le HN, Perry DF. A comparison of three screening tools to identify perinatal depression among low-income African American women. Journal of Affective Disorders. 2012 Jan;136(1–2):155–162. doi: 10.1016/j.jad.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holditch-Davis D, Miles MS, Weaver MA, et al. Patterns of distress in African- American mothers of preterm infants. Journal of Developmental and Behavioral Pediatrics. 2009 Jun;30(3):193–205. doi: 10.1097/DBP.0b013e3181a7ee53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segre LS, O'Hara MW, Arndt S, Stuart S. The prevalence of postpartum depression: the relative significance of three social status indices. Social Psychiatry and Psychiatric Epidemiology. 2007 Apr;42(4):316–321. doi: 10.1007/s00127-007-0168-1. [DOI] [PubMed] [Google Scholar]
- 36.Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010 Mar;125(3):609–617. doi: 10.1542/peds.2008-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinsella MT, Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clinical Obstetrics and Gynecology. 2009 Sep;52(3):425–440. doi: 10.1097/GRF.0b013e3181b52df1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zachariah R. Social support, life stress, and anxiety as predictors of pregnancy complications in low-income women. Research in Nursing & Health. 2009;32(4):391–404. doi: 10.1002/nur.20335. [DOI] [PubMed] [Google Scholar]
- 39.Aktan NM. Social support and anxiety in pregnant and postpartum women: a secondary analysis. Clinical Nursing Research. 2012 May;21(2):183–194. doi: 10.1177/1054773811426350. [DOI] [PubMed] [Google Scholar]
- 40.Webster J, Linnane JW, Dibley LM, Hinson JK, Starrenburg SE, Roberts JA. Measuring social support in pregnancy: can it be simple and meaningful? Birth. 2000 Jun;27(2):97–101. doi: 10.1046/j.1523-536x.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 41.Mezulis AH, Hyde JS, Clark R. Father involvement moderates the effect of maternal depression during a child's infancy on child behavior problems in kindergarten. Journal of Family Psychology. 2004 Dec;18(4):575–588. doi: 10.1037/0893-3200.18.4.575. [DOI] [PubMed] [Google Scholar]
- 42.Smith LE, Howard KS. Continuity of paternal social support and depressive symptoms among new mothers. Journal of Family Psychology. 2008 Oct;22(5):763–773. doi: 10.1037/a0013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fagan J, Lee Y. Perceptions and satisfaction with father involvement and adolescent mothers' postpartum depressive symptoms. Journal of Youth and Adolescents. 2010 Sep;39(9):1109–1121. doi: 10.1007/s10964-009-9444-6. [DOI] [PubMed] [Google Scholar]
- 44.Prevost M, Zelkowitz P, Tulandi T, et al. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Frontier in Public Health. 2014;2:1. doi: 10.3389/fpubh.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisman O, Granat A, Gilboa-Schechtman E, et al. The experience of labor, maternal perception of the infant, and the mother's postpartum mood in a low-risk community cohort. Archives of Womens Mental Health. 2010 Dec;13(6):505–513. doi: 10.1007/s00737-010-0169-z. [DOI] [PubMed] [Google Scholar]
- 46.Beck CT. Postpartum mood and anxiety disorders: Case studies, resarch, and nursing care. 2nd ed. Washington, D.C.: Association of Women's Health, Obstetric and Neonatal Nurses; 2008. [Google Scholar]