Abstract
4-1BB ligand (4-1BBL) and its receptor, 4-1BB, are both induced on T cells after activation, however, little is known about the role of 4-1BBL. We now show that 4-1BBL can transmit signals that limit T cell effector activity under tolerogenic conditions. Cross-linking 4-1BBL inhibited IL-2 production in vitro, primarily with suboptimal TCR stimulation. Furthermore, naïve 4-1BBL-deficient OT-II transgenic T cells displayed a greater conversion to effector T cells in vivo when responding to soluble OVA peptide in WT hosts, whereas development of Foxp3+ Treg cells was not altered. A greater number of effector T cells also differentiated from naïve WT OT-II T cells when transferred into 4-1BB-deficient hosts, suggesting APC-derived 4-1BB is likely to trigger 4-1BBL. Indeed, effector T cells that could not express 4-1BBL accumulated in larger numbers in vitro when stimulated with 4-1BB-expressing mesenteric lymph node DCs. 4-1BBL was expressed on T cells when antigen presentation was limiting, and 4-1BBL was aberrantly expressed at very high levels on T cells that could not express 4-1BB. Trans-ligation, antibody-capture, and endocytosis experiments additionally showed that T cell intrinsic 4-1BB regulated internalization of membrane 4-1BBL, implying that the strong induction of 4-1BB on T cells may counteract the suppressive function of 4-1BBL by limiting its availability. These data suggest that 4-1BBL expressed on T cells can restrain effector T cell development, creating a more favorable Treg to effector cell balance under tolerogenic conditions, and this may be particularly active in mucosal barrier tissues where 4-1BB-expressing regulatory DCs present antigen.
Introduction
TNF and TNFR superfamily interactions play crucial roles in regulating pro-inflammatory as well as anti-inflammatory responses in autoimmune diseases (1, 2). Among these, the binding of 4-1BB to 4-1BBL has been documented to promote cell activation, survival, and differentiation, primarily through 4-1BB signaling activity in T cells, NK cells, and DCs. However, there have been reports that 4-1BB-deficient T cells and myeloid lineage cells hyper-proliferate (3, 4), suggesting that the interaction between 4-1BB and 4-1BBL might also be suppressive in certain situations. This suppressive action may be transmitted through 4-1BB itself, leading to production of modulatory molecules such as RALDH in DCs as recently described (5). However, such suppressive function may also be attributed in alternate scenarios to signals emanating from 4-1BBL. Although 4-1BBL was originally thought to be simply a ligand for 4-1BB (6), there has been accumulating evidence that it can transduce signals when interacting with 4-1BB (7, 8). Cross-linking 4-1BBL was shown to promote or suppress immune cell differentiation suggesting that the result of 4-1BBL signaling is likely to be cell-specific and/or context dependent (4, 9-11). In particular, ligation of 4-1BBL with an Fc fusion protein of 4-1BB promoted IL-10 production in bone marrow-derived macrophages, supporting its potential suppressive functionality (10), and several studies with 4-1BB.Fc or 4-1BB-expressing cells have reported inhibition of T cell responsiveness, implying a negative activity of 4-1BBL (3, 12, 13). Despite some reports finding evidence of expression of 4-1BBL on activated T cells (6, 14), 4-1BBL is usually barely detectable and thus the regulation and primary function of 4-1BBL on this cell type is still not clear.
Our current study demonstrates a T cell intrinsic regulation of 4-1BBL by 4-1BB itself when 4-1BB is strongly induced in a T cell. We find that 4-1BBL signaling can play a very early rate-limiting step in antigen-dependent T cell activation, under conditions of limiting antigen presentation and inflammation, when 4-1BB is not highly expressed, suppressing IL-2 production and effector T cell clonal expansion. We also show that the physiological relevance of 4-1BBL-mediated suppressive function in T cells may manifest in microenvironments such as the GALT where 4-1BB-expressing DCs may encounter recently activated T cells that express 4-1BBL, further adding to the ability of regulatory APCs to limit the differentiation or expansion of effector T cells.
Materials and Methods
Mice
8-10-wk-old C57BL/6 (B6) mice or Ly5.1 congenic B6 mice were purchased from the Jackson Laboratory. 4-1BB−/− or 4-1BBL−/− OT-II TCR transgenic mice were generated by crossing OTII mice with 4-1BB−/− mice (from B. Kwon (3)) or 4-1BBL−/− mice (from Amgen), respectively, and maintained at La Jolla Institute for Allergy and Immunology. All experiments were done in compliance with the regulations of the La Jolla Institute for Allergy and Immunology animal care committee in accordance with guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
Murine 4-1BB and 4-1BBL Constructs
Murine 4-1BB cDNA was synthesized by RT-PCR using total RNA from activated splenic CD4 T cells of B6 mice. Full-length (256 aa) and cytoplasmic tail-deleted (ΔC: 213 aa) 4-1BB expression constructs were generated in pBABE-puro backbone (a kind gift of Dr. Chris Benedict, La Jolla Institute for Allergy and Immunology) tagged with myc (GAGCAGAAGCTGATCAGCGAGGAAGACCTG: EQKLISEEDL) in the N-terminus and His6 (CACCATCACCATCACCAT: HHHHHH) in the C-terminus. Murine 4-1BBL cDNA was obtained from total RNA of splenic CD11c+ cells by RT-PCR. 4-1BBL (full length of 309 aa) was also inserted into pBABE-puro with an N-terminal FLAG tag (GACTACAAGGACGATGACGATAAG: MDYKDDDDK) and a C-terminal HA tag (TACCCTTATGACGTGCCAGATTACGCC: YPYDVPDYA).
Cells and in vitro cultures
CD4+ T cells were enriched from the spleen and lymph nodes of WT, 4-1BB−/−, or 4-1BBL−/−mice on the OT-II TCR transgenic background by using a CD4 T cell isolation kit with LS columns (Miltenyi Biotec), according to the manufacturer's instructions. Naïve (CD44lo CD62Lhi CD25−) CD4+ T cells were further purified by FACS. T cells (2 x 105/200μl) were cultured either with DC (1~4 x 104) and various amounts of OVA peptide323-339 antigen or with variable concentrations of immobilized anti-CD3 (2C11) and 2.5μg/ml of soluble anti-CD28 (37N5) in each well of 96-well plates at 37°C with 5% CO2. In some experiments, rat anti-mouse 4-1BB (3H3, originally from Dr. Robert Mittler, Emory University), rat anti-mouse 4-1BBL (19H3, also from Dr. Robert Mittler), or 4-1BB-Fc (15) were added to the culture in soluble or immobilized forms (20μg/ml). Rat IgG (Sigma) and human IgG1 Fc (Millipore) were used as controls, respectively, and added soluble or immobilized in the exact manner as the 4-1BB/4-1BBL reagents. For endocytosis inhibition assays, Chlorpromazine or Genistein (Sigma) was added to the culture for the final six hours. For Treg conversion experiments, 4-1BB-expressing CD11c+ MHC Class IIhi DC from mesenteric lymph nodes (MLN) were pre-enriched by removing T/B lymphocytes, NK cells, and γδT cells, using biotinylated anti-CD3ε, anti-B220, anti-DX5α, and anti-γδTCR (eBioscience) along with SAV RapidSpheres (StemCell Technologies) according to the manufacturer's instruction, and then further sorted by FACS using FITC-conjugated anti-CD11c, Pacific-Blue conjugated anti-I-A/I-E (BioLegend), biotinylated anti-4-1BB, with APC-conjugated streptavidin (eBioscience). Recombinant human TGF-β1 (R&D Systems) was used for Treg conversion assays. In some cases, purified naïve CD4 T cells were labeled with 0.5μM CFSE (Invitrogen).
T hybridoma cells were generated from activated 4-1BB−/− OT-II T cells fused with the BW5147 thymoma, and selected based on production of IL-2. Myc-tagged full-length or cytoplasmic region deleted 4-1BB vectors were packaged in HEK293T cells, and supernatants were used to introduce the constructs into the T hybridoma cells by centrifugation at 3K rpm for 2 hrs at RT. 4-1BB-expressing hybridoma cells were selected with 0.75μM puromycin treatment, and purified by FACS based on Myc tag expression. In experiments, 1 x 105 cells were co-cultured with mock-transfected T hybridoma cells (1 x 105) in 200μl volumes for 24 hours. To stimulate 4-1BBL via cell-to-cell interaction, recombinant 4-1BB was introduced into 3T3 fibroblast cells or thymoma cells by retroviral transduction as described above, and used as 4-1BB-expressing accessary cells (AC) along with 4-1BB-negative mock cells as controls.
Adoptive transfer, antigen administration, and ex vivo analysis
Purified naïve WT or 4-1BBL−/− OT-II T cells (2 x 106) were intravenously transferred into congenic Ly5.1 B6 mice. In some experiments, WT and 4-1BB−/− B6 mice were used as recipients of WT Ly5.1+ congenic OT-II T cells. Twenty-four hours post-transfer, 5 μg of OVA peptide323-339 was injected in PBS into each mouse through the tail vein. At day 3, 6, or 9 post peptide administration, spleens and lymph nodes were analyzed by flow cytometry for accumulation of OT-II T cells (Vα2+Vβ5+Ly5.2+) with an effector phenotype (CD44hiCD62Llo) or Treg phenotype (Foxp3+). Splenocytes, harvested at day 3, were also restimulated with PMA (5ng/ml) and ionomycin (500ng/ml), and culture supernatants assayed for production of IL-2 and IFN-γ by ELISA. In an alternate protocol, soluble OVA peptide was injected 24hrs after adoptive transfer (25μg), and then again 4 days later (10μg). Transferred OT-II T cells from spleens and lymph nodes were then analyzed 3 days after the second challenge (day 7) by flow cytometry.
Flow Cytometry
For 4-1BB and 4-1BBL detection, biotinylated anti-4-1BB (17B5) and anti-4-1BBL (TKS-1) antibodies were used, respectively, along with biotinylated Rat IgG2a as an isotype control, plus streptavidin-conjugated APC (all from eBioscience). For analyzing activated T cells, anti-CD44-FITC, anti-CD62L-APC, anti-Vα2-Pacific Blue, anti-Vβ5-PE and anti-CD4-APC-Cy7 were used, along with anti-CD45.1 or anti-CD45.2-PerCP-Cy5.5. For detection of Foxp3+ Treg cells, anti-Foxp3-PerCP-Cy5.5 was used along with other T cell markers including anti-CD25-PE-Cy7 (all from eBioscience).
ELISA
Unlabeled anti-mouse IL-2 (0.5μg/ml) or anti-mouse IFN-γ (0.5μg/ml), biotinylated anti-mouse IL-2 or IFN-γ and streptavidin-conjugated HRP (BD Biosciences) were used in conventional ELISA assays to assess IL-2 or IFN-γ secretion using TMB Substrate Reagents (BioLegend). The O.D. was read at 450nm on a SpectraMax 250 (Molecular Devices). The amount of IL-2 or IFN-γ in each sample was determined based on the standard curve generated with serially diluted recombinant murine IL-2 or IFN-γ (Peprotech).
Immunofluorescence Microscopy
Activated T Cells (2 x 105) were loaded onto each poly-L-lysine-coated coverslip (BD Biosciences), fixed with 4% paraformaldehyde/PBS for 15 min., permeabilized with 0.3% saponin/PBS for 5 min., treated with 5% BSA/PBS for 30 min., and stained with anti-4-1BBL goat pAb (R&D Systems) and biotinylated anti-4-1BB (3E1) mAb (biotinylated using 3E1 Ab, a kind gift from Dr. Robert Mittler, Emory Univ.) for 1 hr, followed by donkey anti-goat IgG (H+L)-AlexaFluor 488 and streptavidin-AlexaFluor 647 (Invitrogen) for another hour. Cells were washed with PBS three times between the steps. Cellular nuclei were revealed with DAPI staining. Immunofluorescent images were obtained under Zeiss Axiovert 200M microscope integrated with Intelligent Imaging Innovations slidebook 4.2 and analyzed using Image J software.
Statistics
All statistical analyses were performed using a two-tailed Student t test.
Results
4-1BBL is induced on activated T cells but downregulated when 4-1BB is strongly expressed
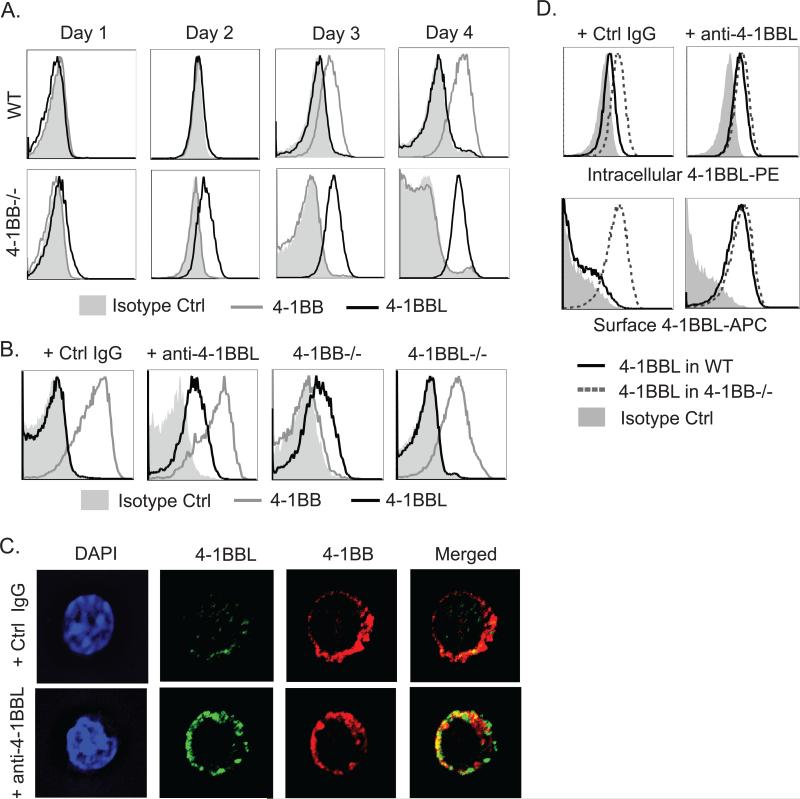
Certain TNF family ligands have been characterized as primarily expressed on T cells, notably molecules such as CD40L and LIGHT. However, it is not clear whether ligands for many of the TNF family receptors that are costimulatory for T cells, such as 4-1BB, OX40, GITR, and CD27, are also ubiquitously present, and what might regulate their availability. Understanding this is also complicated by the fact that many TNF family ligands might be cleaved from the cell membrane or regulated by endocytosis. 4-1BBL is normally hard to detect on T lymphocytes, but we found that 4-1BB-deficient T cells displayed high levels of 4-1BBL on their surface upon activation (Fig. 1A). This type of regulation is consistent with previous studies that revealed higher levels of 4-1BBL on hematopoietic stem cells and dendritic cell precursor when obtained from 4-1BB-deficient animals (4). Suggesting this was not related to abnormal activity of 4-1BB-deficient T cells, we also found that an anti-4-1BBL antibody that can block the interaction of 4-1BB with 4-1BBL also revealed strong expression of surface 4-1BBL by flow and in confocal experiments when added in soluble form into WT T cell cultures (Fig. 1B & C). Anti-4-1BBL may have simply captured 4-1BBL on the cell surface, however, antibodies directed to 4-1BB also revealed surface 4-1BBL (data not shown), suggesting an interaction between the two molecules reduces the amount of 4-1BBL present on the T cell surface. We also found that intracellular levels of 4-1BBL were higher in 4-1BB−/− than WT T cells, and in WT T cells cultured in the presence of anti-4-1BBL (Fig. 1D). 4-1BBL became detectable on the cell surface after 15 minutes at low levels and was highly expressed between 24-48 hr upon addition of soluble anti-4-1BBL, correlating with the normal induction kinetics of the receptor (data not shown and Fig. 1). This suggests that 4-1BBL is produced and potentially available to play a functional role after T cells are activated, but when 4-1BB is made at high levels, it can limit the expression of its own ligand.
Figure 1. Expression of 4-1BB and 4-1BBL on activated T cells.
WT, 4-1BB−/−, and 4-1BBL−/− naive OT-II CD4 T cells were either activated with WT DCs and 1μM OVA peptide for varying lengths of time (A) or anti-CD3 and anti-CD28 in the presence of soluble anti-4-1BBL (19H3) or Ctrl IgG for 48 hrs (B-D). (A and B) 4-1BB and 4-1BBL surface expression in gated CD44hi Vα2+Vβ5+ T cells by flow cytometry. (C) 4-1BB and 4-1BBL expression in T cells from (B) analyzed by confocal microscopy. Green, 4-1BBL; red, 4-1BB. (D) Intracellular staining for 4-1BBL (top) after surface 4-1BBL was stained with saturating amounts of the same detection Ab conjugated to a different dye. Data are representative of three to five independent experiments.
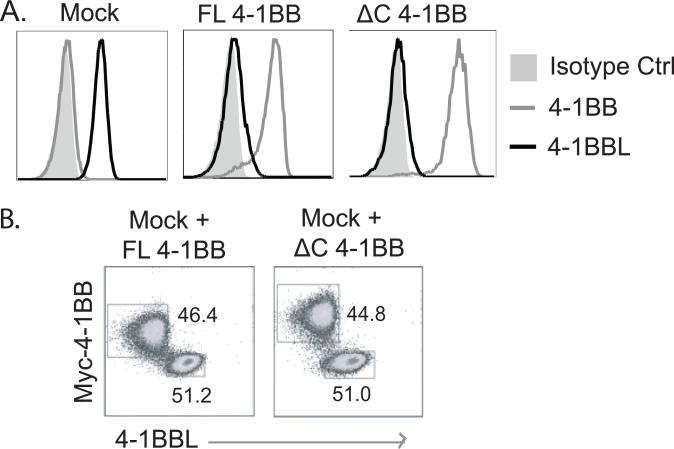
To further study this, a T cell hybridoma was generated from 4-1BB-deficient primary activated T cells and then retrovirally transduced with 4-1BB. Parent T hybridoma cells endogenously expressed surface 4-1BBL due to the lack of 4-1BB, and transduction with mock vector (Mock) did not alter this endogenous expression (Fig. 2A). In contrast, transduction with full length (FL) 4-1BB resulted in strongly diminished the levels of surface 4-1BBL. Introduction of a cytoplasmic domain-deleted mutant form of 4-1BB (ΔC 4-1BB) also reduced surface 4-1BBL to a similar degree. This showed that the cytoplasmic tail of 4-1BB and signaling through 4-1BB is dispensable for this activity, and again suggested that the reduction of expression of 4-1BBL requires an interaction with 4-1BB. To then determine whether 4-1BB could suppress surface 4-1BBL expressed on a neighboring cell (in trans), mock-transduced cells expressing endogenous 4-1BBL were co-cultured at 1:1 ratio for 24 hours with cells transduced with FL or ΔC 4-1BB. 4-1BB expressed in trans did not alter the level of 4-1BBL expressed on the mock cells (Fig. 2B), indicating that T cell intrinsic expression of 4-1BB limits 4-1BBL levels in the same cell through a cis-interaction, either intracellularly or on the membrane.
Figure 2. T cell intrinsic suppression of 4-1BBL expression by 4-1BB.
(A) 4-1BB-deficient CD4 T hybridoma cells mock transfected or transfected with Myc-tagged full length (FL) 4-1BB or C-term-deleted mutant (ΔC) 4-1BB were stained for membrane 4-1BB and 4-1BBL. (B) FL 4-1BB- or ΔC 4-1BB-transfected T hybridoma cells (1 x 105) were co-cultured at a 1:1 ratio with mock-transfected T hybridoma cells (expressing endogenous 4-1BBL) for 24 hours, and stained for membrane 4-1BB and 4-1BBL. All data are representative of two independent experiments.
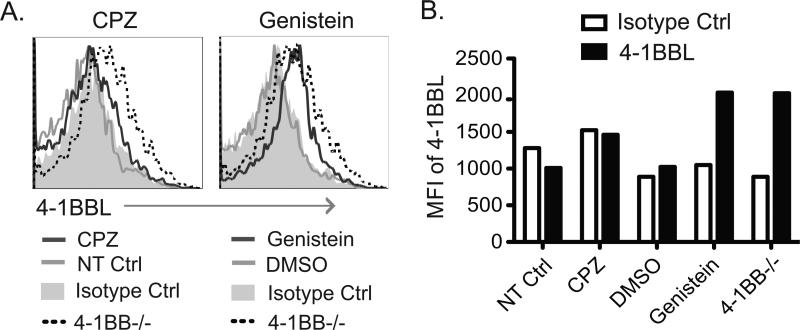
We then performed assays to block endocytosis to determine if this is one of the mechanisms by which 4-1BBL expression is controlled on the T cell surface (Fig. 3). A 6 hr treatment of WT T cells with Genistein, an inhibitor of caveolin-mediated endocytosis, resulted in expression of surface 4-1BBL at similar levels to those detectable on 4-1BB-deficient T cells. In contrast, an inhibitor of clathrin-mediated endocytosis, Chlorpromazin (CPZ), had little effect on the expression of 4-1BBL. Quantitative PCR analysis from activated WT and 4-1BB−/− T cells showed no difference in expression of mRNA for 4-1BBL (data not shown), suggesting that the dominant mechanism for 4-1BB-facilitated down-regulation of 4-1BBL is post-transcriptional. As intracellular levels of 4-1BBL were also decreased in WT T cells compared to 4-1BB-deficient T cells, or in WT T cells treated with anti-4-1BBL blocking antibody (Fig. 1D), these data then suggest that 4-1BBL protein is degraded after endocytosis triggered by 4-1BB.
Figure 3. 4-1BBL surface expression is regulated by endocytosis.
WT naive CD4 T cells were activated with anti-CD3 and anti-CD28. After 42 hrs, endocytosis blockers, Chlorpromazine (CPZ: 10μg/ml) or Genistein (500μM), were added to the cultures and incubated for an additional 6 hrs. Controls were non-treated (NT) or DMSO. Cells were stained for membrane 4-1BBL. 4-1BB−/− T cells were also cultured as an additional positive control for 4-1BBL expression. (A) Flow plots. (B) MFI of 4-1BBL among the different groups. Data are representative of two independent experiments.
4-1BBL signaling limits T cell activation in vitro
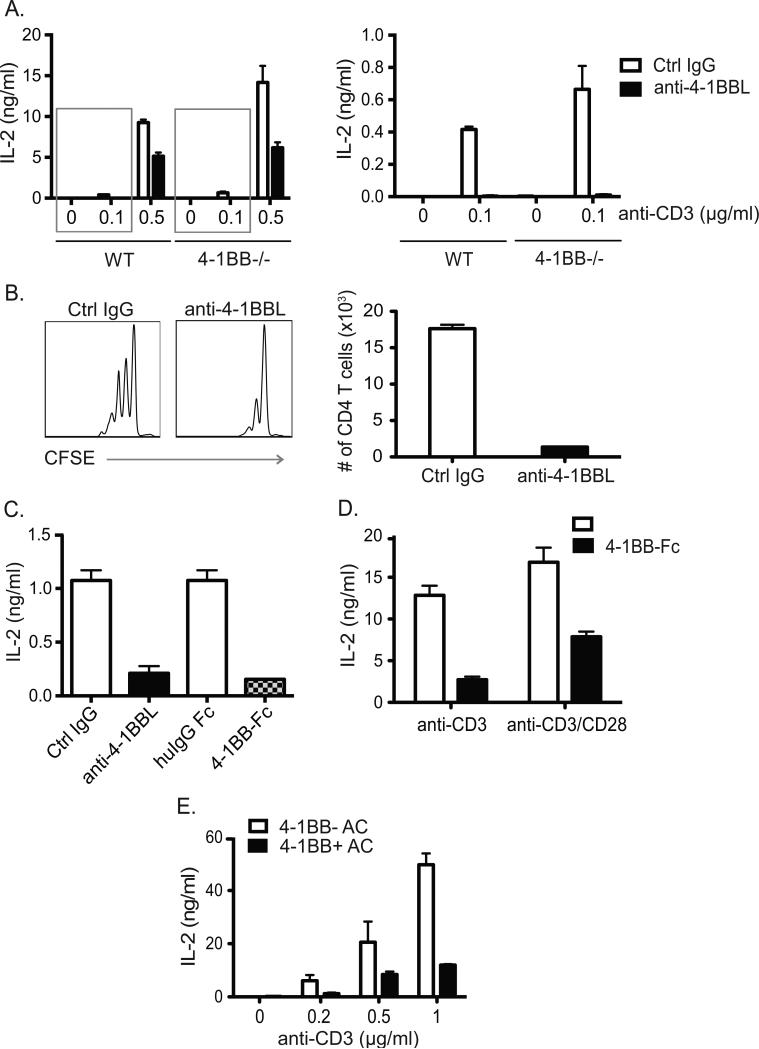
As there was a reciprocal relationship between expression of 4-1BBL and its receptor, this implied that 4-1BBL might be functionally active in activated T cells in certain situations where 4-1BB might not be optimally induced. Thus, we first tested whether signaling through 4-1BBL, brought about by cross-linking with immobilized anti-4-1BBL or an Fc fusion of 4-1BB, would have any effect on the response of T cells to TCR stimulation with or without co-stimulation through CD28. Significantly, T cell activation to varying doses of anti-CD3 with anti-CD28 was suppressed in the presence of immobilized anti-4-1BBL, as determined by assessing the levels of secreted IL-2 (Fig. 4A). Notably, this activity of binding 4-1BBL was strongest under conditions of suboptimal stimulation with anti-CD3. Varying the dose of anti-CD28 did not alter the suppressive effect (not shown). CFSE dilution assays also showed a significant decrease in the number of cell divisions as well as the total number of dividing cells in the presence of immobilized anti-4-1BBL, with this effect limited to low doses of anti-CD3, correlating with the action on IL-2 production (Fig. 4B and data not shown). To verify that suppression of T cell activation was a direct result of signaling through 4-1BBL and not because of disrupting 4-1BB/4-1BBL interactions, 4-1BB−/− T cells that express higher levels of endogenous 4-1BBL were also tested. Immobilized anti-4-1BBL suppressed IL-2 production to a similar extent in 4-1BB−/− T cells compared to WT T cells (Fig. 4A). An equivalent effect was also seen with immobilized 4-1BB-Fc (Fig. 4C) showing suppression was not antibody specific but could result when 4-1BBL bound its natural partner. Similarly, 4-1BBL-expressing T hybridoma cells derived from activated 4-1BB−/− OT-II cells also produced lower levels of IL-2 when stimulated with anti-CD3 or anti-CD3/anti-CD28 in the presence of immobilized 4-1BBFc (Fig. 4D). The suppressive activity was further confirmed by using irradiated accessory cells that expressed 4-1BB to ligate 4-1BBL on these T cells. Reduced IL-2 production was observed compared to T cells cultured with accessory cells that lacked 4-1BB (Fig. 4E). These data collectively suggest that 4-1BBL plays a suppressive role in T cell activation under conditions of suboptimal stimulation. This negative activity is controlled when T cells are stimulated strongly to express high levels of 4-1BB, which in cis configuration facilitates the removal of 4-1BBL from the T cell surface by endocytosis.
Figure 4. T cell activation is suppressed by 4-1BBL signaling.
(A) WT and 4-1BB−/− naïve CD4 T cells were stimulated with various concentrations of anti-CD3 and 2.5μg/ml of anti-CD28 in the presence of plate-bound anti-4-1BBL (20 μg/ml) or Ctrl IgG. IL-2 was assessed at 48 hr by ELISA. Right graph is data magnified from left graph (gray boxes). (B) CFSE-labeled naïve CD4 T cells were stimulated with 0.1μg/ml of anti-CD3 and 2.5μg/ml of anti-CD28 in the presence of plate-bound anti-4-1BBL or control IgG for 48 hours. CFSE dilution was assessed (left) and CD4 T cell recovery calculated (right). (C) Naïve 4-1BB−/− CD4 T cells were stimulated with low dose plate-bound anti-CD3 and anti-CD28 as in (A) in the presence of plate-bound anti-4-1BBL or 4-1BB-Fc (20μg/ml), or control Rat IgG or human IgG1 Fc. IL-2 was assessed at 48 hr by ELISA. (D) 4-1BB−/− T hybridoma cells were activated with anti-CD3 (0.1μg/ml) with or without anti-CD28 (2.5μg/ml), in the presence of plate-bound 4-1BB-Fc or control human IgG1 Fc (20μg/ml). IL-2 was assessed at 6 hr by ELISA. (E) 4-1BB−/− T hybridoma cells were activated with various concentrations of anti-CD3 in the presence of irradiated accessory cells (AC) that did or did not express 4-1BB. IL-2 was assessed at 6 hr by ELISA. Data are representative of five independent experiments, and are means ± sem from replicate cultures.
4-1BBL signaling limits effector T cell development in vivo under non-inflammatory conditions
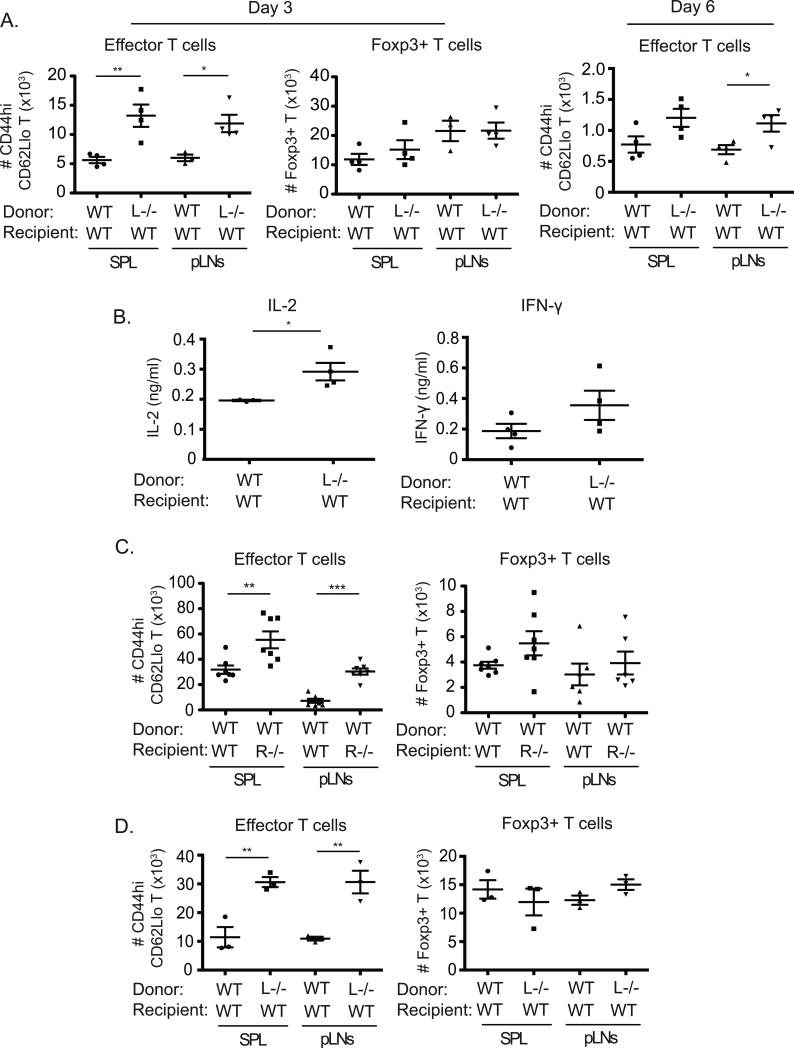
To investigate any physiological relevance of these results, we assessed conditions in vivo where peptide was recognized under non-inflammatory/tolerogenic conditions that favor development of Foxp3+ Treg cells, and that might mimic the scenario we found in vitro where 4-1BBL was actively suppressive in T cells (16). The response of naïve TCR transgenic T cells that could or could not express 4-1BBL was tracked when adoptively transferred into WT hosts. With systemic injection of a low dose of OVA peptide antigen in PBS, we found that the absence of 4-1BBL−/− on the responding naive T cells resulted in accumulation of approximately 3-fold more effector T cells (CD44hi, CD62lo) in spleens or lymph nodes when assessed after 3 days (Fig. 5A, left). In contrast, a similar number of Foxp3+ OT-II Treg cells developed regardless of the presence or absence of 4-1BBL on the responding T cells (Fig. 5A, middle). The enhanced numbers of effector T cells generated in the absence of 4-1BBL was maintained at day 6, although the absolute numbers were reduced compared to day 3 regardless of being WT or 4-1BBL−/− (Fig. 5A, left). After 9 days, we could not detect effector T cells regardless of being WT or 4-1BBL−/− (not shown). Consistent with this being a tolerogenic response, Foxp3+ Treg cells were maintained over this time period and similar in number in both groups (not shown). This data suggested that 4-1BBL principally acted to limit the generation of effector T cells as Treg cells were forming to aid in the development of tolerance. In line with this, higher levels of IL-2 and IFN-γ were detected in splenic cultures from mice receiving 4-1BBL−/− T cells (Fig. 5B). To ascertain whether the suppressive activity of 4-1BBL on T cells came from its interaction with 4-1BB expressed in the hosts, presumably on antigen-presenting cells, 4-1BB−/− mice were used as recipients of WT OT-II T cells. 2-3-fold higher numbers of OVA-specific T cells of the effector phenotype were generated in 4-1BB−/− recipients paralleling the observation with 4-1BBL-deficient T cells (Fig. 5C). In contrast, there was no significant difference in the numbers of Foxp3+ Treg cells generated in both groups.
Figure 5. 4-1BBL limits T cell activation in vivo under non-inflammatory conditions.
(A) Sorted naïve WT or 4-1BBL−/− (L−/−) Ly5.2+ OT-II T cells (2 x 106) were adoptively transferred into WT Ly5.1+ congenic recipient mice. One day later, mice were immunized i.v. with 5μg of OVA peptide (323-339) in PBS. After 3 days (left and middle) or 6 days (right), the number of effector (CD44hi CD62Llo) or Foxp3+ OT-II (Vα2+Vβ5+Ly5.2+) T cells was calculated in spleens (SPL) and peripheral lymph nodes (pLNs). (B) Splenocytes from (A), taken at day 3, were stimulated with PMA (5ng/ml) and ionomycin (500ng/ml) for 5 hr, and IL-2 and IFN-γ production were measured by ELISA. (C) Sorted naïve Ly5.1+ congenic WT OT-II T cells were transferred into WT or 4-1BB−/− (R−/−) Ly5.2+ recipients. Mice were immunized and analyzed at day 3 as in (A). (D) Recipients of 4-1BBL−/− T cells were challenged with 25μg OVA peptide in PBS similar to (A) and then re-challenged with 10μg OVA peptide 4 days later. Accumulation of effector and Foxp3+ OT-II T cells was analyzed after a further 3 days. All data show numbers of T cells or amounts of each cytokine in individual recipient mice, with means ± sem for each group, and are representative of at least two independent experiments in each case.
To test the effect of 4-1BBL in another system, we challenged mice twice with soluble OVA peptide in PBS, with the second injection given after 4 days, and then assessed the number of effector T cells generated after a further 3 days (7 days total). In this scenario, higher numbers of effector T cells were maintained over this time frame compared to a single peptide injection, but importantly the difference between WT and 4-1BBL−/− T cells was maintained at approximately a 1:3 ratio (Fig. 5D). Again, Foxp3+ Treg cells were generated equally regardless of the absence of 4-1BBL. Furthermore, we observed no significant difference in the response of 4-1BBL-deficient T cells compared to WT T cells when the adjuvant alum was given along with OVA peptide using a similar immunization protocol that does not generate significant numbers of Treg cells (data not shown). Thus, 4-1BBL expressed on T cells suppresses the initial accumulation and differentiation of effector populations under non-inflammatory conditions where Treg cells are also generated, but it has no apparent role in the T cell response under inflammatory conditions.
4-1BB-4-1BBL interactions between regulatory DC and T cells limits T cell activation
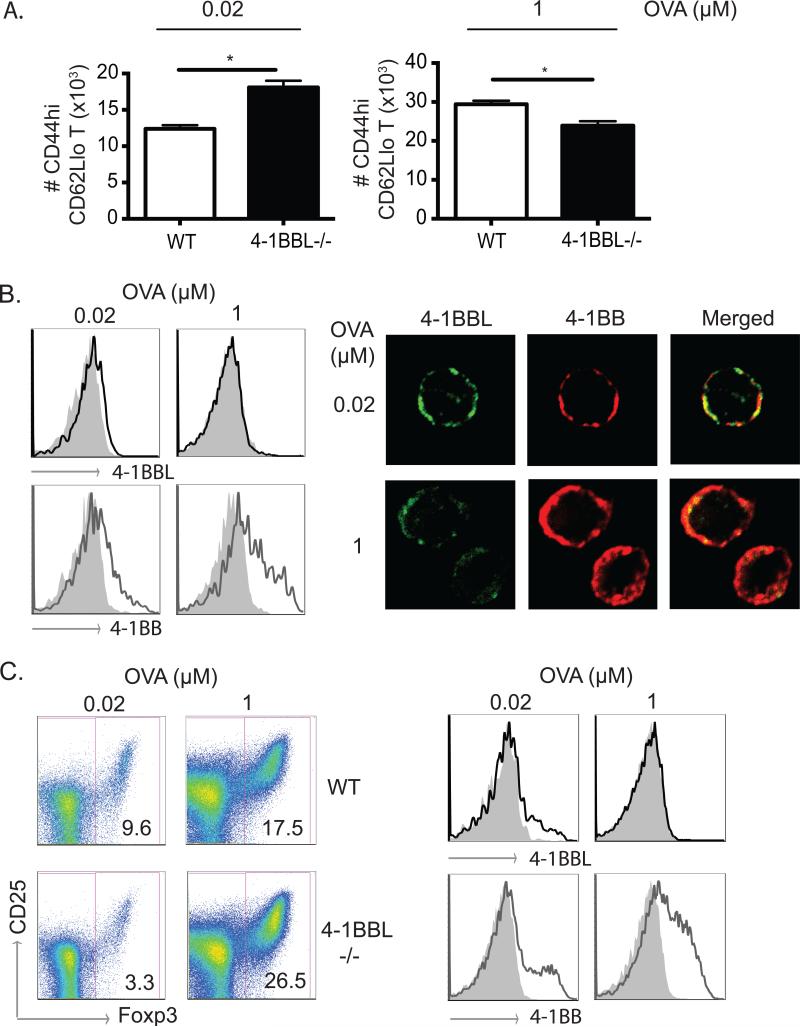
Previously we reported that a proportion of mesenteric lymph node (MLN) dendritic cells implicated in promoting the generation of Foxp3+ Treg cells constitutively expressed 4-1BB. This is the subset that also expresses CD103 and makes high levels of the regulatory enzyme RALDH that controls retinoic acid production. We furthermore found that 4-1BB participated in the development of this subset of regulatory DC from precursors by determining the level of expression of RALDH (5). To assess whether 4-1BB on these DC may also promote suppressive activity by binding T cell-expressed 4-1BBL, WT or 4-1BBL−/− naïve OT-II T cells were co-cultured with sorted 4-1BB-expressing MLN DCs (CD11c+ MHC Class IIhi 4-1BB+). 4-1BBL−/−effector T cells accumulated to a greater extent with a low dose of antigen (Fig. 6A), in line with limiting antigen or inflammation revealing the suppressive effect of 4-1BBL. With a high dose of antigen, 4-1BB was strongly induced on WT T cells whereas its expression was weaker with a low dose of antigen (Fig. 6B). Correspondingly, 4-1BBL was readily detectable with a low dose of antigen, expressed together with 4-1BB, but it was much more weakly detectable with a high dose of antigen when 4-1BB was present at higher levels (Fig. 6B). This likely accounts for why it was functionally relevant under the former conditions.
Figure 6. 4-1BBL signaling in T cells limits T cell activation and favors Treg cell generation with low dose antigen.
(A) Naïve WT or 4-1BBL−/− OT-II T cells were cultured with sorted 4-1BB+ MLN DCs (MHC Class IIhi, CD11c+) at 20:1 ratios with 0.02 or 1μM of OVA peptide for 72 hours. Recovery of effector phenotype OT-II T cells (CD44hiCD62Llo) was determined. Data are means ± sem from triplicate cultures. (B) T cells from cultures in (A) were analyzed for expression of 4-1BBL and 4-1BB by flow cytometry (left) and confocal microscopy (right). (C) T cells were cultured as in (A) but in the presence of 5ng/ml TGF-β1. Percentages of Foxp3+ OT-II T cells were determined with co-staining for CD25 after 5 days (left), and expression of 4-1BBL and 4-1BB on total OT-II T cells assessed after 3 days (right). All data are representative of three independent experiments.
Despite the fact that 4-1BBL signaling did not alter Foxp3+ Treg cell differentiation in the conditions of immunization we used in vivo (Fig. 5), it remained possible that 4-1BBL signaling might contribute to the proportion of Treg cells that are generated in certain microenvironments such as the GALT where TGF-β is strongly expressed and 4-1BB-expressing DCs are found. To test this in vitro, sorted 4-1BB+ MLN DC were cultured with 4-1BBL−/− T cells in the presence of TGF-β. A significantly reduced percentage of Foxp3+ Treg cells were generated when 4-1BBL could not be expressed (3.3% vs 9.6%). This was limited to the cultures with a low dose of antigen whereas with high dose antigen no defect in Treg cell generation was apparent (Fig. 6C). This again correlated with little detectable 4-1BBL and strong 4-1BB expression under the latter conditions, whereas we again observed with low dose antigen that 4-1BBL was more strongly expressed and the majority of the 4-1BBL-expressing cells also co-expressed 4-1BB (Fig. 6C, and not shown). In the cultures with limiting antigen, there were also many T cells that neither expressed 4-1BB or 4-1BBL, most likely because they were not stimulated well through the TCR. Thus, 4-1BBL can exert suppressive activity in T cells to limit effector cell development under conditions of weak antigen presentation or no inflammation, and in certain conditions 4-1BBL may also participate in allowing greater development of Treg cells by ligating 4-1BB on APC.
Discussion
A growing number of auto-inflammatory and allergic disorders have been identified in humans, most of which are attributed to hyper-responsiveness of T lymphocytes against potentially harmless foreign- or self-antigens. T cells have multiple ways to limit their responsiveness to non-pathogenic stimuli. The current study demonstrates that ligation of 4-1BBL on T cells can function in this regard and suppress T cell activation and early expansion of effector T cells. 4-1BBL was induced upon antigen-dependent activation of T cells under tolerizing conditions in vitro as well as in vivo and was active in this inhibitory capacity when inflammation and antigen presentation were sub-optimal.
Although much of the literature on the interaction of 4-1BB and 4-1BBL has focused on the stimulatory capacity of 4-1BB on various cell types including T cells, there is a growing body of research showing that 4-1BBL itself can signal. However, whether this provides a stimulatory or inhibitory stimulus appears to vary and may be both cell-type specific and context dependent. 4-1BBL signaling has been illustrated in myeloid cells as well as in lymphocytes. Cross-linking of 4-1BBL with immobilized 4-1BB-Fc, anti-4-1BBL mAb, or 4-1BB-expressing cells, can lead to monocyte and dendritic cell activation, proliferation, maturation, production of pro-inflammatory cytokines such as IL-6, IL-8, TNF, or IL-12, and/or cell survival (11, 17-22). However, there is accumulating evidence of negative regulatory roles for 4-1BBL signaling in activation and/or differentiation of varying types of cells, including bone marrow myeloid precursors, osteoclasts, as well as T cells (3, 4, 9, 10, 12, 23, 24). In particular, Schwarz et al. first suggested the potential regulatory activity of 4-1BBL signaling by showing suppressed proliferation of human PBMC T cells when co-cultured with fixed 4-1BB-expressing cells (12). The inhibitory activity of 4-1BBL signaling was not restricted to human PBMCs, but was also replicated in mouse splenocytes stimulated with anti-CD3 where the proliferation of 4-1BB-deficient T cells was reduced again if these cells were incubated with 4-1BB-expressing cells (3, 25).
We now add to these studies and suggest the suppressive activity of 4-1BBL on T cells is regulated by its own receptor. 4-1BB has long been known as an inducible molecule on T cells that transmits costimulatory signals to augment division, survival, and cytokine production under inflammatory conditions. We found that when 4-1BB was strongly induced, this resulted in the downregulation of 4-1BBL via a cis-interaction that may largely occur on the T cell surface but might also be functional intracellularly. This was most dramatically illustrated with T cells that could not express 4-1BB where 4-1BBL was readily and easily detected at high levels on the cell surface. The plausible mechanisms limiting expression of 4-1BBL included shedding or cleavage from the membrane, and internalization. We do not favor the former because although we could detect soluble 4-1BBL in the supernatant of activated T cells, we found as much, and in some cases more, soluble 4-1BBL in cultures of 4-1BB−/− T cells (data not shown). This is opposite to what would be predicted if a cis-interaction with 4-1BB enhanced shedding or cleavage. In contrast, our data with confocal analyses and using endocytosis inhibitors support the idea that 4-1BB binding to 4-1BBL results in its internalization and subsequent degradation. This appears to be a mechanism by which the strong induction of 4-1BB maximizes its potential to be a costimulatory molecule for the T cell when 4-1BBL is upregulated on APC under inflammatory conditions, reducing any potential negative effect of its ligand if a T cell encounters an APC that expresses 4-1BB.
The physiological significance of the immunoregulatory role of 4-1BBL engagement on T cells will be primarily evident in non-inflammatory states or tolerizing conditions based on our data in vitro and in vivo. The mechanism we describe depends on a source of 4-1BB in trans, and this is most likely provided by APC that can express 4-1BB. These are largely predicted to be dendritic cells. Given the inhibitory activity of 4-1BBL, it would make sense that 4-1BB was provided on a tolerogenic or regulatory DC. The most obvious example of this cell is the CD103+ DC found in the mesenteric lymph nodes, which we previously described expressed 4-1BB directly ex-vivo (5). In line with this, we showed that expression of 4-1BB on a subpopulation of MLN DC coincided with those cells that had the greatest ability to make retinoic acid. Indeed, ligation of 4-1BB sustained the activity of the enzyme RALDH which promotes RA production in these MLN DC. 4-1BB signaling additionally promoted RALDH activity in splenic DC when they upregulated 4-1BB after stimulation with TLR2-ligand or GMCSF. This in turn led to a greater ability of the DC to promote the generation of Foxp3+ Treg cells (5). Therefore, by blocking T cell activation through 4-1BBL, as well as upregulating RA production through 4-1BB signaling to the DC, there can be synergistic suppression of the T cell response. As we show in vitro, this may in some cases also aid the generation of Foxp3+ Treg cells. Such bidirectional signaling activity is likely to occur in mucosal tissues such as the gastrointestinal tract where TGF-β is available along with constant or periodic low-level stimulation of T cells by innocuous antigens from food or commensals. This hypothesis is further supported by our unpublished data, which show reduced numbers of Foxp3+ Treg cells in the Peyer's Patches and small intestinal lamina propria of 4-1BB−/− and 4-1BBL−/− mice.
In summary, we show a rate-limiting activity of 4-1BBL when expressed by recently activated T cells that suppresses T cell activation and effector cell development under tolerizing conditions or conditions where antigen presentation occurs at a low level with little inflammation. The data highlight the complex interplay between ligands and receptors in the TNF/TNFR superfamily. How intracellular signals from 4-1BBL exert a suppressive effect in T cells is not known, but understanding this will be important for future studies.
Acknowledgments
This work was supported by NIH grants AI042944 and AI089624 to M.C. This is manuscript 1729 from the La Jolla Institute for Allergy and Immunology.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croft M, Duan W, Choi H, Eun SY, Madireddi S, Mehta A. TNF superfamily in inflammatory disease: translating basic insights. Trends Immunol. 2012;33:144–152. doi: 10.1016/j.it.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE, Vinay DS. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, Croft M. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat Immunol. 2008;9:917–926. doi: 10.1038/ni.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol. 2012;189:2697–2701. doi: 10.4049/jimmunol.1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Jenkins NA, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 8.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011;89:21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- 9.Saito K, Ohara N, Hotokezaka H, Fukumoto S, Yuasa K, Naito M, Fujiwara T, Nakayama K. Infection-induced up-regulation of the costimulatory molecule 4-1BB in osteoblastic cells and its inhibitory effect on M-CSF/RANKL-induced in vitro osteoclastogenesis. J Biol Chem. 2004;279:13555–13563. doi: 10.1074/jbc.M303791200. [DOI] [PubMed] [Google Scholar]
- 10.Shin HH, Lee JE, Lee EA, Kwon BS, Choi HS. Enhanced osteoclastogenesis in 4-1BB-deficient mice caused by reduced interleukin-10. J Bone Miner Res. 2006;21:1907–1912. doi: 10.1359/jbmr.060813. [DOI] [PubMed] [Google Scholar]
- 11.Kang YJ, Kim SO, Shimada S, Otsuka M, Seit-Nebi A, Kwon BS, Watts TH, Han J. Cell surface 4-1BBL mediates sequential signaling pathways ‘downstream’ of TLR and is required for sustained TNF production in macrophages. Nat Immunol. 2007;8:601–609. doi: 10.1038/ni1471. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz H, Blanco FJ, von Kempis J, Valbracht J, Lotz M. ILA, a member of the human nerve growth factor/tumor necrosis factor receptor family, regulates T-lymphocyte proliferation and survival. Blood. 1996;87:2839–2845. [PubMed] [Google Scholar]
- 13.Michel J, Pauly S, Langstein J, Krammer PH, Schwarz H. CD137-induced apoptosis is independent of CD95. Immunology. 1999;98:42–46. doi: 10.1046/j.1365-2567.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim W, Kim J, Jung D, Kim H, Choi HJ, Cho HR, Kwon B. Induction of lethal graft-versus-host disease by anti-CD137 monoclonal antibody in mice prone to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:306–314. doi: 10.1016/j.bbmt.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Madireddi S, Eun SY, Lee SW, Nemcovicova I, Mehta AK, Zajonc DM, Nishi N, Niki T, Hirashima M, Croft M. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J Exp Med. 2014;211:1433–1448. doi: 10.1084/jem.20132687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang J, Huddleston SJ, Fraser JM, Khoruts A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J Leukoc Biol. 2008;83:1230–1239. doi: 10.1189/jlb.1207851. [DOI] [PubMed] [Google Scholar]
- 17.Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol. 1998;160:2488–2494. [PubMed] [Google Scholar]
- 18.Langstein J, Michel J, Schwarz H. CD137 induces proliferation and endomitosis in monocytes. Blood. 1999;94:3161–3168. [PubMed] [Google Scholar]
- 19.Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H. CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. J Leukoc Biol. 2002;72:35–42. [PubMed] [Google Scholar]
- 20.Kim YJ, Li G, Broxmeyer HE. 4-1BB ligand stimulation enhances myeloid dendritic cell maturation from human umbilical cord blood CD34+ progenitor cells. J Hematother Stem Cell Res. 2002;11:895–903. doi: 10.1089/152581602321080556. [DOI] [PubMed] [Google Scholar]
- 21.Lippert U, Zachmann K, Ferrari DM, Schwarz H, Brunner E, Mahbub-Ul Latif AH, Neumann C, Soruri A. CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response. Eur J Immunol. 2008;38:1024–1032. doi: 10.1002/eji.200737800. [DOI] [PubMed] [Google Scholar]
- 22.Kim DK, Lee SC, Lee HW. CD137 ligand-mediated reverse signals increase cell viability and cytokine expression in murine myeloid cells: involvement of mTOR/p70S6 kinase and Akt. Eur J Immunol. 2009;39:2617–2628. doi: 10.1002/eji.200939292. [DOI] [PubMed] [Google Scholar]
- 23.Shin HH, Lee EA, Kim SJ, Kwon BS, Choi HS. A signal through 4-1BB ligand inhibits receptor for activation of nuclear factor-kappaB ligand (RANKL)-induced osteoclastogenesis by increasing interferon (IFN)-beta production. FEBS Lett. 2006;580:1601–1606. doi: 10.1016/j.febslet.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 24.Senthilkumar R, Lee HW. CD137L- and RANKL-mediated reverse signals inhibit osteoclastogenesis and T lymphocyte proliferation. Immunobiology. 2009;214:153–161. doi: 10.1016/j.imbio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Lee SW, Vella AT, Kwon BS, Croft M. Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol. 2005;174:6803–6808. doi: 10.4049/jimmunol.174.11.6803. [DOI] [PubMed] [Google Scholar]