Abstract
Background
The clinical utility of cellular therapies is being investigated in a broad range of therapeutic areas. This phase 1 study represents the first exploration of PDA001, a preparation of cells cultured from human placental tissue, in subjects with Crohn’s disease.
Methods
Twelve subjects with active, moderate-to-severe Crohn’s disease unresponsive to previous therapy were given 2 intravenous infusions of PDA001 1 week apart, monitored weekly for 5 weeks, and assessed at 6 months, 1 year, and 2 years after infusion. Six subjects received 2 infusions of 2 × 108 cells (low dose), and 6 subjects received 2 infusions of 8 × 108 cells (high dose).
Results
Mean baseline Crohn’s Disease Activity Index in the low-dose and high-dose groups was 305 and 364, respectively, and mean C-reactive protein was 8 mg/L and 49 mg/L, respectively. All subjects in the low-dose group achieved a clinical response (a Crohn’s Disease Activity Index decrease of ≥70 points versus baseline), and 3 achieved remission (a Crohn’s Disease Activity Index decrease of ≥100 to <150 points). Two subjects in the high-dose group achieved response, and none met remission criteria. Most adverse events were mild to moderate in severity and included headache, nausea, fever, and infusion site reactions.
Conclusions
PDA001 infusions appear safe and well-tolerated in subjects with treatment-resistant Crohn’s disease. A response was seen in all subjects in the low-dose group. The high-dose group, with a higher baseline disease activity, had only 2 responders, suggesting a more treatment-resistant population. A phase 2 study in this patient population is ongoing.
Keywords: Crohn’s disease, cellular therapies, biologic therapies, immunomodulators
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract that affects more than 1 million people in the United States. Its pathogenesis is complex and remains unclear.1 Clinical features of CD include diarrhea, pain, narrowing of the gut lumen leading to strictures and bowel obstruction, abscess formation, and fistulization to skin and internal organs. Extraintestinal inflammatory manifestations in the joints, eyes, skin, mouth, and liver can also occur.2
Currently available conventional therapies are still the mainstay of therapy for CD, including nonspecific anti-inflammatory (salicylates or steroids) and immunomodulatory agents (6-MP or azathioprine).3,4 Increased use of biologic therapies that target specific pathogenic immunologic pathways, such as the anti–tumor necrosis factor monoclonal antibodies, has offered an important advance in therapy for some patients with CD4,5; however, despite these advances in therapeutic options, many patients remain unresponsive or show a diminished response to therapy over time.6 Multiple other approaches, including anti-adhesion molecules, T-cell inhibitors, and anti-inflammatory cytokines, are being investigated.4
Cellular therapy, a relatively new approach to the treatment of CD, may provide both immunosuppressive and regenerative potential to correct immunologic abnormalities and repair damaged intestinal tissue.7 Both hematopoietic and, more recently, mesenchymal cells have been explored for the use as therapy for CD.8,9 Human placenta-derived adherent cells are also being assessed as a novel therapeutic agent of CD. PDA001 is a preparation of mesenchymal-like adherent cells derived from postpartum placentas. Flow cytometric analysis of PDA001 shows expression of CD200, CD105, and CD73 but not CD34, CD19, CD3, or CD45. The cells can suppress immune function in several assays in both mouse and man.10,11
The current phase 1 study was conducted to investigate (1) the safety of PDA001 for more than 2 years in subjects with moderate-to-severe CD refractory to oral corticosteroids and immune modulators, and (2) its clinical effect, including their effect on the quality of life of the subjects.
MATERIALS AND METHODS
Study Design
The study was an open-label, dose-escalation, phase 1 study conducted at 4 sites in the United States between March 2009 and August 2010. Subjects eligible for inclusion were aged 18 to 75 years with demonstrated CD and an inadequate response to oral corticosteroids or immunomodulators with no serious concomitant medical conditions. The screening score of Crohn’s Disease Activity Index (CDAI) was required to be between 220 and 400 within the 21 days of dosing.
There were 6 subjects per group across the study sites. Group A (low dose) (n = 6) subjects were enrolled and dosed first. Following day 36 evaluation of group A, the Data Monitoring Committee recommended enrollment of group B (high dose) (n = 6). A pretreatment phase of up to 90 days, but generally lasting approximately 4 weeks, allowed completion of eligibility determinations and for concomitant medications to be held stable. This was followed by an 8-day treatment phase (infusion of PDA001 on day 0 and day 7) plus a 2-year follow-up phase. Efficacy and safety assessments were performed at or immediately before the baseline and then at weekly visits through day 36, then at 6, 12, 18, and 24 months after the initial infusion. The baseline was considered to be the last measurements immediately before the infusion of PDA001; the baseline CDAI score was taken the week before dosing.
The first 6 subjects received 2 infusions of 2 × 108 cells (group A, low dose), and the next 6 received 2 infusions of 8 × 108 cells (group B, high dose). Infusion occurred over 1 to 2 hours by means of a 20-gauge to 22-gauge catheter connected to a volumetric infusion pump, and subjects were monitored for at least 2 hours after each infusion for heart rate, respiration, blood pressure, body temperature, and blood oxygen saturation. Premedications, such as antihistamines and corticosteroids, were recommended but not required before infusion. Diphenhydramine was administered to 75% of patients, and hydrocortisone to 75% of patients. Concomitant medications were allowed to be continued but needed to be at a stable dose as follows: 5-aminosalicylic acid and steroids (28 days), small molecule immunomodulators (90 days), and biological response modulators (42 days). To the extent possible, they were maintained at a constant dosage throughout the study.
Primary and Secondary End points
Outcome measurements included, for clinical efficacy, the CDAI,12 and for quality of life, the Inflammatory Bowel Disease Questionnaire (IBDQ).13 Disease activity was also monitored by evaluating serum C-reactive protein (CRP). A response was defined as a decrease in CDAI score of ≥70 points from baseline without a concomitant increase in CD medications, and a remission was defined as a decrease in CDAI score of ≥100 to <150 points. Subjects who achieved a response and subsequently flared were eligible for one retreatment course at their previously assigned dose (2 infusions). Endoscopy was not required as an efficacy end point.
Subjects underwent computed tomography of the chest and abdomen at baseline and at 12 and 24 months to rule out the possibility of ectopic tissue formation. To monitor the potential immunogenicity, HLA class I and class II panel reactive antibodies were assayed by Luminex methodology at screening, baseline, and each subsequent study visit. When a positive was detected, the specificity of the HLA antibody was determined. Additional safety testing including serum chemistry, blood counts, and Clostridium difficile toxin (screening period only) measurements were obtained at each visit.
Statistical Analysis
All efficacy variables chosen for this study were analyzed descriptively, and no formal statistical tests were planned. Descriptive summaries for each end point included safety (for the safety population and by group and efficacy end points) and efficacy (for the modified intent-to-treat population and by group). Both the safety and modified intent-to-treat populations included all 12 subjects. Continuous, quantitative, variable summaries included the number of subjects (N), mean, standard deviation (SD), median, minimum, and maximum. Categorical, qualitative, variable summaries included the frequency and percentage of subjects in each category. Baseline value was the last assessment result before the initial administration of PDA001; all results thereafter were considered postbaseline.
Ethical Considerations
The study protocol was approved by the local institutional review boards. Subjects provided written informed consent, and the study was conducted in accordance with the ethical principles of Good Clinical Practice as required by the major regulatory authorities and in accordance with the Declaration of Helsinki. Steps were taken to assure data quality and Good Clinical Practice compliance: selection of qualified investigators and study sites, prestudy review of protocol procedures with the study sites, and periodic monitoring visits.
RESULTS
Subject Disposition and Baseline Characteristics
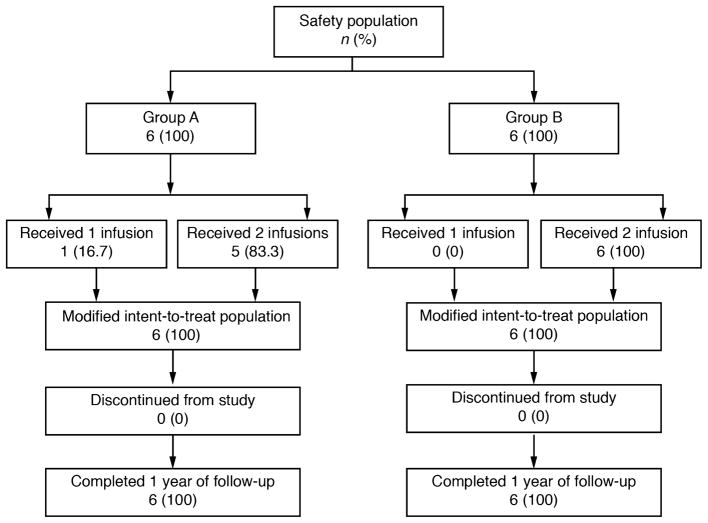
Subject disposition is shown in Figure 1. Of the 12 subjects, 11 received both infusions, and all 12 received at least 1 infusion and completed the study assessments, including at least 1 year of follow-up. Demographics and baseline characteristics of the subjects are shown in Table 1. The mean baseline CDAI was notably higher in group B (high dose) compared with group A (low dose) (364 versus 305, respectively), and the mean baseline CRP in group B was approximately 4 times that in group A (49 mg/L versus 8 mg/L, respectively), indicating greater baseline disease severity in these subjects. Of the 6 subjects in group A, 6 came from 1 study center because it opened to enrollment before the other centers. In group B, 5 of the 6 subjects came from 2 other centers, which could account for the discrepant severities of the disease between group A and group B. In addition, all subjects in group A were female, whereas half were male in group B, although no other imbalances in medical history, previous surgical history (including intestinal resections in both groups), the presence of fistulae, or previous treatments were noted.
FIGURE 1.
Subject disposition flow diagram.
TABLE 1.
Demographics and Baseline Characteristics
| Treatment Group
|
|||
|---|---|---|---|
| Group A (Low Dose) (n = 6) | Group B (High Dose) (n = 6) | Total (N = 12) | |
| Age, yr | |||
| Mean (SD) | 42.7 (9.81) | 34.0 (9.14) | 38.3 (10.11) |
| Median (minimum, maximum) | 46.0 (24, 52) | 33.5 (22, 50) | 38.0 (22, 52) |
| Gender, n (%) | |||
| Male | 0 (0) | 3 (50.0) | 3 (25.0) |
| Female | 6 (100) | 3 (50.0) | 9 (75.0) |
| Race, n (%) | |||
| Black or African | 0 (0) | 1 (16.7) | 1 (8.3) |
| American White | 6 (100) | 5 (83.3) | 11 (91.7) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 1 (16.7) | 0 (0) | 1 (8.3) |
| Not Hispanic or Latino | 5 (83.3) | 6 (100) | 11 (91.7) |
| Baseline CDAI score | |||
| Mean (minimum, maximum) | 304.8 (234, 380) | 363.5 (253, 485) | 334.2 (234,485) |
| Baseline CRP | |||
| Mean (SD) | 8 (4.9) | 49 (42.1) | 28 (35.4) |
| Baseline IBDQ Score | |||
| Mean (minimum, maximum) | 130.0 (106, 157) | 118.7 (91, 166) | 124.3 (91, 166) |
Previous and Concomitant Medications
All 12 subjects were taking at least 1 previous medication and took at least 1 concomitant medication, with similar usage between the 2 treatment groups. Common concomitant medications included paracetamol (66.7%), prednisone (58.3%), hydrocodone (50.0%), folic acid (41.7%), and mesalamine (41.7%). In group A, 1 subject was taking adalimumab and 1 infliximab. In group B, 3 subjects took adalimumab and 1 infliximab. Although information on failed medications before the 30-day period before screening was not formally collected, within that period, additional subjects had been administered monoclonal antibodies (adalimumab, vedolizumab, and certolizumab), and 1 subject had been administered 6-mercaptopurine.
Clinical Response
Crohn’s Disease Activity Index
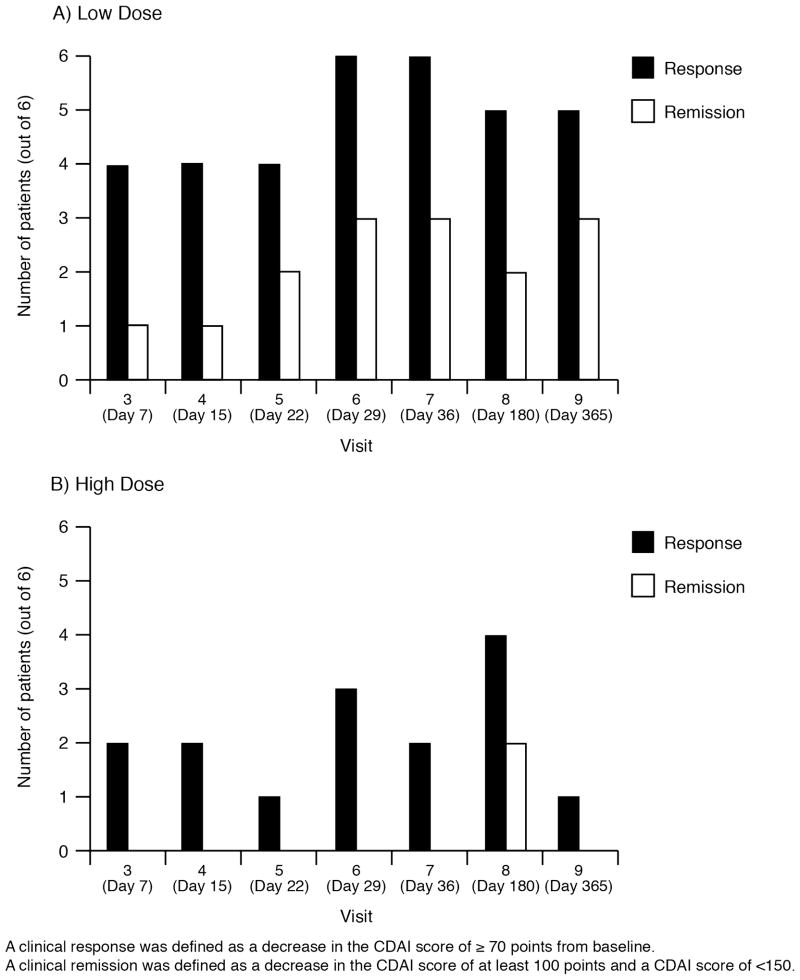
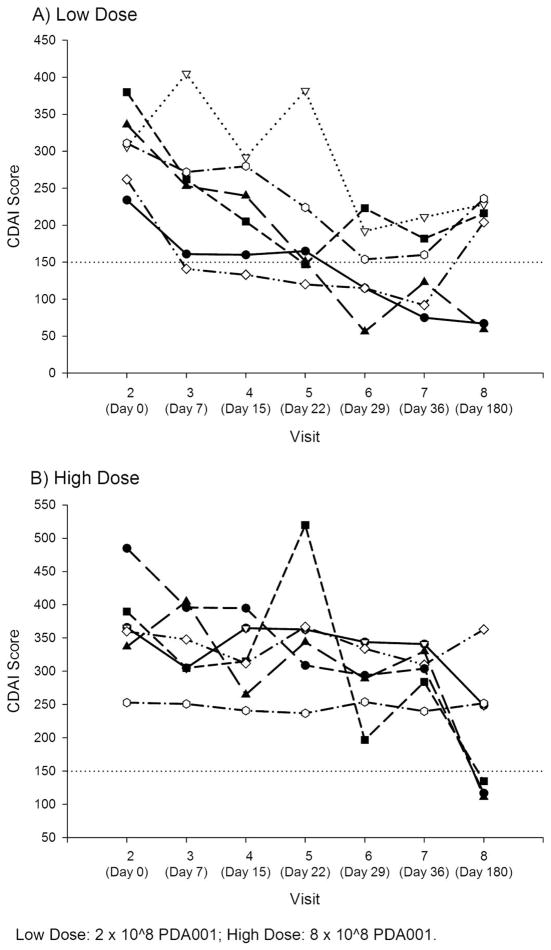
A summary of clinical response and remission is shown in Figure 2, and individual CDAI scores over time are shown in Figure 3. Responses were seen in 4 subjects in group A at day 7 of treatment, although maximum response was seen at day 29 (visit 6) and day 36 (visit 7). All 6 subjects in group A demonstrated a clinical response (a decrease of ≥70 points at 4 weeks compared with baseline). Three subjects achieved remission (decrease of ≥100 to <150 points). After 6 months of follow-up, 5 of 6 subjects in group A had maintained this response, although in some cases, additional CD medications, such as infliximab, were added. In group B, 3 subjects demonstrated a clinical response at 4 weeks, and none achieved remission. In this group, 2 of 6 subjects achieved a response, which lasted for at least 2 consecutive visits. Individual CDAI data from all subjects are shown in Figure 3. If a more stringent criterion of a reduction of 100 points in the CDAI score had been used to determine response at 4 weeks; all 6 group A subjects and 2 group B subjects would have been considered responders.
FIGURE 2.
CDAI response and remission summary of low-dose (A) high-dose (B) groups.
FIGURE 3.
Individual CDAI score over time in low-dose (A) high-dose (B) groups.
C-Reactive Protein
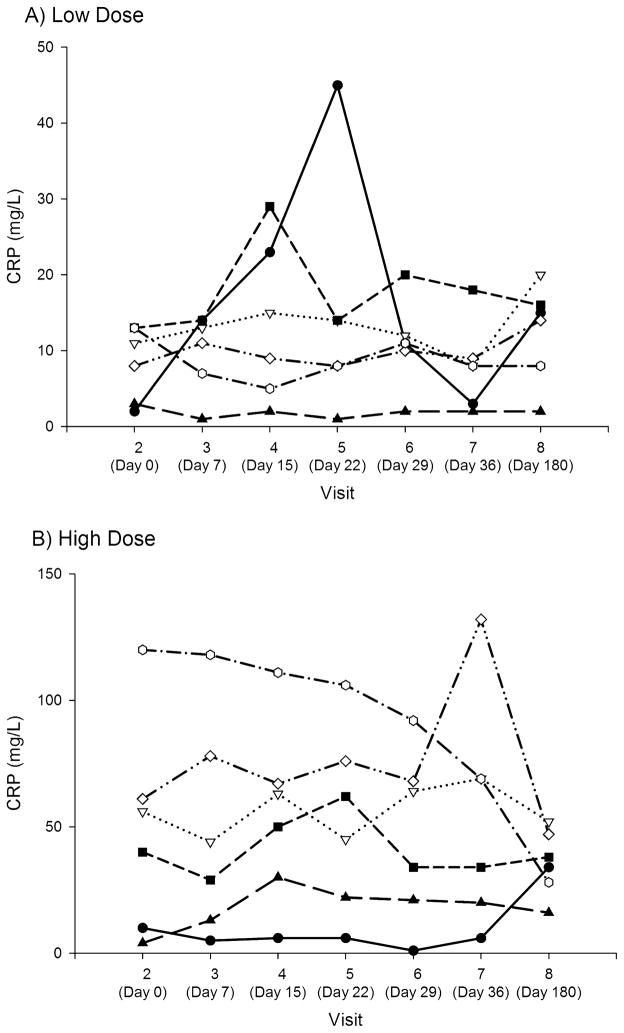
No subject in either group demonstrated a decrease in CRP (see Figure 4). In group B (high dose), CRP remained notably higher throughout. The normal range was 0 to 8 mg/L.
FIGURE 4.
Individual CRP over time in low-dose (A) high-dose (B) groups.
Inflammatory Bowel Disease Questionnaire
Scores on the IBDQ improved in group A (low dose) from a mean of 130.0 at baseline to a mean of 158.3 at week 4 with a median change of 30. In group B (high dose), IBDQ score improved from a mean of 118.7 at baseline to a mean of 128.2 at week 4 with a median change of 3.5. As a change of 27 is considered clinically significant in the IBDQ,14 most patients in group A achieved clinically significant improvement. Subscores (total and change from baseline) for bowel, systemic, emotional, and social IBDQ assessments showed a similar pattern.
Adverse Events
PDA001 was generally well-tolerated. The overall incidence of treatment-emergent adverse events (AEs) was similar at both dose levels, and the most frequently reported treatment-emergent AEs were headache (58.3%), anemia (25%) and hematuria (25%) (Table 2). Hematuria occurred more frequently in group A (low dose), and it was not associated with changes in renal function. Anemia occurred more frequently in group B (high dose). It is notable that the median baseline hemoglobin was 13.5 g/dL and 9.7 g/dL in the 2 treatment groups, respectively, suggesting that many subjects in the high-dose group were anemic at baseline. The anemia responded to therapy with iron. Of the 12 subjects, 10 (5 in each treatment group) experienced a treatment-emergent AE suspected to be related to study medication. Suspected events in group A (low dose) included nausea, vomiting, infusion-related reaction, pyrexia, and headache. Suspected events in group B (high dose) included infusion-related reaction, infusion site induration, infusion site pain, infusion site phlebitis, pyrexia, limb discomfort, headache, hyperesthesia, cough, and photosensitivity reaction. One subject experienced a grade 1 infusion reaction consisting of rash, nasal congestion, and cough, responding to hydrocortisone, which precluded the second infusion. No subject experienced a decrease in blood oxygen saturation during the infusions.
TABLE 2.
Summary of Treatment-Emergent AEs
| Parameter | Group A (Low Dose) (n = 6) | Group B (High Dose) (n = 6) | Total (N = 12) |
|---|---|---|---|
| No. of subjects with at least 1 TEAE, n (%) | 6 (100) | 6 (100) | 12 (100) |
| No. of subjects with at least 1 SAE, n (%) | 2 (33.3) | 3 (50.0) | 5 (41.7) |
| No. of subjects with at least 1 AE leading to permanent study medication withdrawal, n (%) | 0 (0) | 0 (0) | 0 (0) |
| No. of subjects with TEAE by severity, n (%) | |||
| Mild/Grade 1 | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Moderate/Grade 2 | 3 (50.0) | 2 (33.3) | 5 (41.7) |
| Severe/Grade 3 | 2 (33.3) | 3 (50.0) | 5 (41.7) |
| Life-threatening/Grade 4 | 0 (0) | 0 (0) | 0 (0) |
| Death/Grade 5 | 0 (0) | 0 (0) | 0 (0) |
| No. of subjects with TEAE by relationship to study medication (%) | |||
| Not suspected | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Suspected | 5 (83.3) | 5 (83.3) | 10 (83.3) |
| System Organ Class Preferred Term | |||
| Any TEAE (%) | 6 (100) | 6 (100) | 12 (100) |
| Blood or lymphatic system disorders, n (%) | 0 (0) | 3 (50.0) | 3 (25.0) |
| Anemia, n (%) | 0 (0) | 3 (50.0) | 3 (25.0) |
| Nervous system disorders, n (%) | 4 (66.7) | 4 (66.7) | 8 (66.7) |
| Headache (%) | 4 (66.7) | 3 (50.0) | 7 (58.3) |
| Renal and urinary disorders, n (%) | 3 (50.0) | 0 (0) | 3 (25.0) |
| Hematuria (%) | 3 (50.0) | 0 (0) | 3 (25.0) |
TEAE, treatment-emergent AE.
No subject withdrew from the study as a result of an AE, and no deaths were reported during the study. Five subjects experienced serious AEs (SAEs), none of which was considered treatment related. One subject was diagnosed with a localized lobular breast carcinoma 4 months after the treatment. The most frequently reported SAEs were gastrointestinal disorders and infections (Table 3). No subject showed evidence of ectopic tissue formation on the computed tomography of the chest and abdomen performed at 1 year or 2 years after the infusion time points. No serious AEs related to PDA001 were reported during the long-term follow-up period. Two subjects, 1 in group A and 1 in group B, had positive panel reactive antibodies to class I HLA antigens after infusion. One of the 2 subjects with a transient, de novo antibody was in group A and the other was in group B. In both subjects, specificity to HLA A2, which is present on the infused cells, was demonstrated, and both subjects demonstrated a clinical response. In addition, 1 of the 2 subjects was retreated on flare approximately 17 months after the first infusion and subsequently achieved a second response. This second response was also associated with de novo Class I A2 specific anti-HLA antibody production. No subject developed a de novo class II panel reactive antibody response.
TABLE 3.
Serious AEs by System Organ Class
| System Organ Class Preferred Term | Group A (Low Dose) (n = 6), n (%) | Group B (High Dose) (n = 6), n (%) | Total (N = 12), n (%) |
|---|---|---|---|
| No. of subjects with at least 1 SAE | 2 (33.3) | 3 (50.0) | 5 (41.7) |
| Blood and lymphatic system disorders | 0 (0) | 1 (16.7) | 1 (8.3) |
| Anemia | 0 (0) | 1 (16.7) | 1 (8.3) |
| Gastrointestinal disorders | 1 (16.7) | 2 (33.3) | 3 (25.0) |
| Anal fistula | 0 (0) | 1 (16.7) | 1 (8.3) |
| Anal stenosis | 0 (0) | 1 (16.7) | 1 (8.3) |
| CD | 0 (0) | 1 (16.7) | 1 (8.3) |
| Intestinal obstruction | 1 (16.7) | 0 (0) | 1 (8.3) |
| Nausea | 1 (16.7) | 0 (0) | 1 (8.3) |
| Infections and infestations | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Anal abscess | 0 (0) | 1 (16.7) | 1 (8.3) |
| Urinary tract infections | 1 (16.7) | 0 (0) | 1 (8.3) |
| Neoplasms benign, malignant, and unspecified | 1 (16.7) | 0 (0) | 1 (8.3) |
| Breast cancer | 1 (16.7) | 0 (0) | 1 (8.3) |
| Nervous system disorders | 0 (0) | 1 (16.7) | 1 (8.3) |
| Dizziness | 0 (0) | 1 (16.7) | 1 (8.3) |
| Headache | 0 (0) | 1 (16.7) | 1 (8.3) |
DISCUSSION
PDA001 infusions appear safe and well-tolerated in subjects with treatment-resistant CD in this phase 1 study. Although further clinical studies are required for a clearer safety profile, possible AEs associated with PDA001 include headache, fever, and infusion-related reactions. A slightly higher rate of SAEs in group B (high dose) could be attributable to the higher disease activity in this group.
Although no fixed time points for the evaluation of efficacy were prespecified in the Statistical Analysis Plan, it is most useful to highlight the 4-week time point, which is frequently used in studies evaluating CD therapies.15 CDAI response and remission rates at 4 weeks were encouraging; the change in IBDQ scores from baseline in group A (low dose) were in a range considered clinically relevant (20–23.5).16,17
The low-dose group was notable for a response in all subjects, with 2 subjects achieving remission for as long as 6 months after the infusions. The high-dose group demonstrated higher baseline disease activity (CRP and CDAI) and had only 2 responders, suggesting that this may have been a more treatment-resistant population. Because most subjects in the low-dose group came from 1 study center and most subjects in the high-dose group came from 2 other centers, it is possible that this baseline disease severity difference reflects differences in referral patterns to the different study centers.
Several case studies have reported CD regression after transplantation of autologous hematopoietic stem cells,8,9 and a phase 3 study is ongoing to investigate their effects after high-dose immune ablation in nonresponding subjects with severe CD.18 Autologous adipose-derived mesenchymal cell transplantation has also provided clinical benefit in limited reports.19–21
The results of the current study are comparable with the limited data available on the effects of bone marrow–derived mesenchymal stem cells in a similar population of subjects with CD. For example, in a phase 2 study of 10 subjects with similarly resistant CD, a preparation of cells obtained from the bone marrow of healthy adult donors led to CDAI score reduction.22
Although most subjects experienced AEs, these tended to be mild or moderate, and no safety issues that would preclude continuing to phase 2 development were identified. A limited number of subjects developed antibodies to HLA determinants of the infused cells, but these did not appear to be associated with AEs or lack of efficacy. Compared with cellular therapies derived from other sources, PDA001 has potentially significant benefits such as derivation from a safe and plentiful source of nonembryonic cells and scalability comparable with traditional pharmaceutical therapies.
These initial results are promising, and a phase 2 study of PDA001 in subjects with treatment-resistant CD is ongoing.23 As a result of treatment-group differences in baseline disease severity, the dose–response relationship remains unclear. Larger studies with more balanced treatment groups, as well as a placebo treatment group, are warranted and should provide a clearer understanding of the efficacy and safety of PDA001.
Acknowledgments
This study was funded by Celgene Cellular Therapeutics, Warren, NJ. Writing support (funded by CCT) was provided by Watermeadow Medical USA. The authors have had full access to the study data and take full responsibility for the contents of this manuscript.
References
- 1.Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9–16. doi: 10.1007/s00535-009-0138-3. [DOI] [PubMed] [Google Scholar]
- 2.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 3.Bamias G, Cominelli F. Novel strategies to attenuate immune activation in Crohn’s disease. Curr Opin Pharmacol. 2006;6:401–407. doi: 10.1016/j.coph.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Podolsky D. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 6.Kucharzik T, Maaser C, Lugering A, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 7.Singh UP, Singh NP, Singh B, et al. Stem cells as potential therapeutic targets for inflammatory bowel disease. Front Biosci (Schol Ed) 2010;2:993–1008. doi: 10.2741/s115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzoni G, Roda G, Belluzzi A, et al. Inflammatory bowel disease: moving toward a stem cell-based therapy. World J Gastroenterol. 2008;14:4616–4626. doi: 10.3748/wjg.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Bosch O, Ricart E, Panés J. Review article: stem cell therapies for inflammatory bowel disease—efficacy and safety. Aliment Pharmacol Ther. 2010;32:939–952. doi: 10.1111/j.1365-2036.2010.04439.x. [DOI] [PubMed] [Google Scholar]
- 10.Paludan C, Harbacheuski R, Murray R, et al. Placenta derived stem cells (PDAC) suppress the allo-MLR and the EBV regression assay. Blood. 2006;108:1737. [Google Scholar]
- 11.Li X, Ling W, Pennisi A, et al. Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells. 2011;29:263–273. doi: 10.1002/stem.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 13.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–810. [PubMed] [Google Scholar]
- 14.Hlavaty T, Persoons P, Vermeire S, et al. Values of inflammatory bowel disease questionnaire in Crohn’s disease. Inflamm Bowel Dis. 2006;12:199–204. doi: 10.1097/01.MIB.0000217768.75519.32. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer S, Sandborn W, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Higgins P, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54:782–788. doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irvine E, Greenberg G, Feagan B, et al. Quality of life rapidly improves with budesonide therapy for active Crohn’s disease. Canadian Inflammatory Bowel Disease Study Group. Inflamm Bowel Dis. 2000;6:181–187. doi: 10.1097/00054725-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 18.European Group for Blood and Marrow Transplantation. [Accessed February 3, 2012];ASTIC autologous stem cell transplantation for Crohn’s disease [ClinTrials.gov Web site] 2010 Oct 14; Available at: http://www.clinicaltrials.gov/ct2/show/NCT00297193?term=ASTIC+crohn%27s&rank=1.
- 19.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Olmo D, García-Arranz M, García LG, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451–454. doi: 10.1007/s00384-003-0490-3. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 22.Onken JE, Gallup D, Hanson J, et al. Successful outpatient treatment of refractory Crohn’s disease using adult mesenchymal stem cells. Presented at: ACG 2006 Final Program Book; October 20–25, 2006; Las Vegas, Nevada. 2006. p. 121. [Google Scholar]
- 23.Celgene Corporation. [Accessed February 3, 2012];A multi-center study to evaluate the safety and efficacy of intravenous infusion of human placenta-derived cells (PDA001) for the treatment of adults with moderate-to-severe Crohn’s disease [ClinTrials.gov Web site] 2011 Oct 11; Available at: http://www.clinicaltrials.gov/ct2/show/NCT01155362?term=celgene+crohn%27s&rank=1.