Abstract
Background
The bacterium Chlamydia trachomatis is one of the leading causes of sexually transmitted diseases worldwide. Since no simple and effective tool exists to diagnose C. trachomatis infections, we evaluated a novel point-of-care (POC) test, aQcare Chlamydia TRF kit, which uses europium-chelated nanoparticles and a time-resolved fluorescence reader.
Methods
The test performance was evaluated by comparing the results obtained using the novel POC testing kit with those obtained using a nucleic acid amplification test (NAAT), using 114 NAAT-positive and 327 NAAT-negative samples.
Results
The cut-off value of the novel test was 20.8 with a detection limit of 0.27 ng/mL. No interference or cross-reactivity was observed. Diagnostic accuracy showed an overall sensitivity of 93.0% (106/114), specificity of 96.3% (315/327), positive predictive value (PPV) of 89.8% (106/118), and negative predictive value (NPV) of 97.5% (315/323). The sensitivity of the novel test was much higher than that of currently available POC tests. Furthermore, the relative ease and short turnaround time (30 min) of this assay enables C. trachomatis-infected individuals to be treated without a diagnostic delay.
Conclusions
This simple and novel test is a potential tool to screen a larger population, especially those in areas with limited resources.
Keywords: Chlamydia trachomatis, Europium, Point-of-care systems
INTRODUCTION
Chlamydia trachomatis is an obligate, intracellular, gram-negative bacterium that infects the columnar epithelium of the cervix and is responsible for a broad spectrum of sexually transmitted diseases (STDs) in humans. There has been a nine-fold increase in the number of reported cases of C. trachomatis infection in Korea from 2001 (354 reported cases) to 2007 (3,196 reported cases) [1]. Although treatment with antibiotics can cure an uncomplicated C. trachomatis infection, 80-94% of infected women are reported to be asymptomatic [2, 3, 4, 5], which makes diagnosis and treatment difficult.
Serology, cell culture, direct immunofluorescence assays (DFA), ELISA, and nucleic acid amplification tests (NAATs) are all available laboratory-based methods to detect C. trachomatis infections [2, 6, 7]. Earlier studies have assessed the culture method to be the gold standard for diagnosis owing to its high sensitivity (85%) and specificity (100%) [2, 6]. However, other diagnostic methods needed to be developed, because the culture method is difficult to standardize, expensive, time-consuming, and labor intensive [2]. Although many NAATs, having a high sensitivity for detecting C. trachomatis infections even in asymptomatic patients, have become available recently, these still require trained personnel to run the tests and expensive testing platform settings. A delay between receiving the test outcome and initiating the treatment of the patient results in a low rate of patient return and increases disease transmission, which compromises the utility of the test for the control of such communicable infections. Therefore, there is a great need for a rapid and simple assay that would allow diagnosis and treatment in a single visit; this would have widespread use even in areas with few resources. To that end, some point-of-care (POC) commercial kits for the diagnosis of C. trachomatis infections have become available recently. These tests can be easily performed and the results are obtained in less than 30 min, thereby significantly shortening the delay between diagnosis and treatment to minimize the spread of infection and the risk of further complications. Unfortunately, an evaluation of the performance of the currently available rapid POC tests revealed an alarmingly poor sensitivity of less than 20% [8, 9, 10, 11].
In this study, we evaluated the performance of a newly developed lateral flow immunoassay (LFIA)-based POC testing kit, aQcare Chlamydia TRF kit (Medisensor, Inc., Daegu, Korea) for detecting C. trachomatis. LFIA is a well-established technology, and LFIA-based tests are used in commercially available pregnancy and drug tests. Instead of conventional colloidal gold or latex, the kit evaluated here uses europium (Eu) (III) chelated nanoparticles as the labeling substance, which is a promising novel reporter for stronger and more reliable signals [12, 13, 14, 15]. This assay was developed to meet the demand for a rapid, simple, and sensitive tool for detecting C. trachomatis infections, which could ultimately aid in controlling the transmission of these diseases. Furthermore, this test may aid in the diagnosis and prompt treatment of urogenital C. trachomatis infection even in areas with limited resources where NAAT cannot be used.
METHODS
1. Study population and sample collection
Samples were collected from men and women aged 20-80 yr, who visited a hospital for the evaluation of STD symptoms from January 2012 to June 2013. Samples were stably stored until further examination according to the recommendations of the manufacturers of the relevant tests. Urine samples, urethral swabs, and endocervical swabs were collected. All samples were collected before initiation of any antibiotic regimen. In total, 441 (positive and negative; 93 urine samples, 8 urethral swabs, and 340 endocervical swabs) anonymized samples were selected for this study; 340 samples were obtained from women and 101 samples were obtained from men. This study was approved by the institutional review board of Kyungpook National University Hospital, Korea (IRB No.2013-05-033).
2. Testing protocols
The aQcare Chlamydia TRF assay and NAAT were performed by 2 persons each in the clinical microbiology laboratory at the Department of Laboratory Medicine according to the manufacturers' instructions after receiving adequate training by the suppliers of the test. The laboratory evaluated the performance of the newly developed assay in accordance with the corresponding CLSI guidelines [16, 17, 18].
3. Testing samples with the newly developed assay
aQcare Chlamydia TRF kit is a very rapid and user-friendly LFIA-based POC test kit. The kit has a sample detection region outside a plastic cassette, with a control line and a test line marked on the rectangular result-viewing area. The sample pad, conjugate pad, white nitrocellulose membrane, and absorption pad are attached to the internal test strip in that order. Each strip has a control line coated with goat anti-mouse immunoglobulin G, which is the internal control, and a test line coated with streptavidin. The C. trachomatis antigen in the sample forms a complex with the genus specific, biotin-labeled mouse monoclonal anti-Chlamydia antibody in the reagent, which then binds to the streptavidin-coated test line. The reagent and extraction tubes are included in the test kit package. Each kit contains a colorless reagent that is prepared such that precise pipetting is not necessary, and the extraction tube is easily converted to a dropper with a cap.
The portable, small signal-acquisition device, having dimensions of 348×240×221 mm, is equipped with an ultraviolet (UV) light source and a digital camera for detection, and a front display for the observation of quantitative measurement levels. The detector has a fixed absorption wavelength of 333 nm and an emission wavelength of 613 nm, which are the standard wavelengths for the detection of europium (Eu) (III) chelated nanoparticles. Since europium (Eu) (III) chelates have a large Stokes shift, a time-resolved fluorescence (TRF) reading system for specific signal detection was implemented to obtain results that are more accurate.
For swab samples, 7 drops (approximately 240 µL) of the reagent were added. The swab was inserted into the tube and rotated 10 times while pushing the swab into the base of the tube to extract any C. trachomatis antigens from sample. The testing kit was placed on a flat surface and 2 drops (about 60 µL) of the sample solution were added to the detection area of the testing kit prior to incubation for 15 min. The results were analyzed by using the TRF reader device. For urine samples, 200 µL of urine was added to the tube by using disposable droppers, and the other steps were identically performed.
4. Testing samples with the nucleic acid amplification test
For NAATs, the AccuPower CT & NG real-time PCR kit (Bioneer, Inc., Daejeon, Korea) was used to qualitatively detect C. trachomatis. This kit was approved by the Korea Food & Drug Administration (KFDA) and is used as the gold standard for diagnosing C. trachomatis infection. The recommended protocol as described in the package inserts was followed. All samples were tested in duplicate without prior knowledge of the results of other tests.
5. Performance evaluation
To determine the cut-off value for this new assay, the 327 NAAT-negative samples were used. Analysis was performed over 5 days with 2 runs per day. After analyzing these samples with the novel test, the average value and SD from the TRF reading device were used to calculate the cut-off value to discriminate between positive and negative C. trachomatis infections. This value was used throughout the performance evaluation to determine positive and negative infection.
To determine the detection limit, serial dilutions using 4 ng/mL of C. trachomatis standard antigen (Chlamydia grade 2 antigen: Microbix Biosystems Inc., Toronto, Canada) were performed. Serially diluted samples of known concentration were measured 20 times, and the lowest concentration that was consistently detected (over 95%) was considered the detection limit of the test. The detection limits for each serotype of the C. trachomatis strain were also evaluated with the same testing protocol using standards for the D (ATCC.VR-885), E (ATCC.VR-228B), F (ATCC.VR-346), G (ATCC.VR-878), and H (ATCC.VR-879) serotypes of C. trachomatis.
Potential nonspecific interference was determined in multiple testing conditions by mixing various biomaterials into the patient samples. Whole blood, 4 mg/dL tetracycline, 30 mg/dL erythromycin, 20 mg/dL acetaminophen, 50 mg/dL salicylic acid, 40 mg/dL ibuprofen, 20 mg/dL bilirubin, and 1 mg/mL albumin were added to observe possible interference in the negative samples, low-positive swab samples, and low-positive urine samples. The biomaterials were added at concentrations deemed high enough to cause interference.
Additionally, 15 clinically considered pathogens, including C. trachomatis, were tested to evaluate cross-reactivity, which could result in false positive results.
Diagnostic accuracy was evaluated by using 114 NAAT-positive and 327 NAAT-negative samples. Each sample was tested in duplicate, for 10 consecutive days using the novel test kit, without prior knowledge of the NAAT test results. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) with 95% confidence intervals (CI) were calculated by comparing the values obtained from the novel test to those obtained from the NAAT.
6. Analysis
Performance values were determined based on the recommended CLSI guidelines and analyzed using SPSS ver.17.0 statistical package (SPSS, Inc., Chicago, IL, USA). Sensitivity, specificity, PPV, and NPV of aQcare Chlamydia TRF compared to NAAT were calculated by using standard methods.
RESULTS
The cut-off value of the TRF reader was 20.8 (data not shown). We used this value to detect C. trachomatis infection during this study, where values ≥20.8 were considered positive and values <20.8 were considered negative. Quantitative values were obtained from the TRF reader, but they were used to determine the results qualitatively.
Using aQcare Chlamydia TRF, a C. trachomatis standard antigen level as low as 0.27 ng/mL showed positive results with 95% consistency over 20 repeated trials. Below a concentration of 0.27 ng/mL, the consistency of the positive result was lower than 95%; therefore, we determined the detection limit of the aQcare Chlamydia TRF to be 0.27 ng/mL. However, detection limits of different C. trachomatis serotypes were variable. Serotype D and E were consistently positive at 1×103 inclusion forming unit (IFU)/mL and 1×104 IFU/mL, respectively, while serotypes F, G, and H were consistently positive at 1×102 IFU/mL.
Eight substances, including whole blood, drugs, and other biomaterials, that could potentially interfere with the test were added to the negative and low-positive samples at a high concentration to investigate possible false positives. However, the readings did not change in the presence of any of these substances; negative samples remained negative and low-positive swab and urine samples remained low-positive (data not shown).
Fifteen human pathogens that can cause various urogenital infections (C. trachomatis as a positive control) were used to evaluate potential cross-reactivity. Each pathogen was added to the C. trachomatis-negative samples at a high concentration of 1×107 colony forming unit (CFU)/mL. Only the addition of C. trachomatis resulted in a positive test; none of the other pathogens tested positive (Table 1).
Table 1.
Cross-reactivity evaluation using aQcare Chlamydia TRF kit
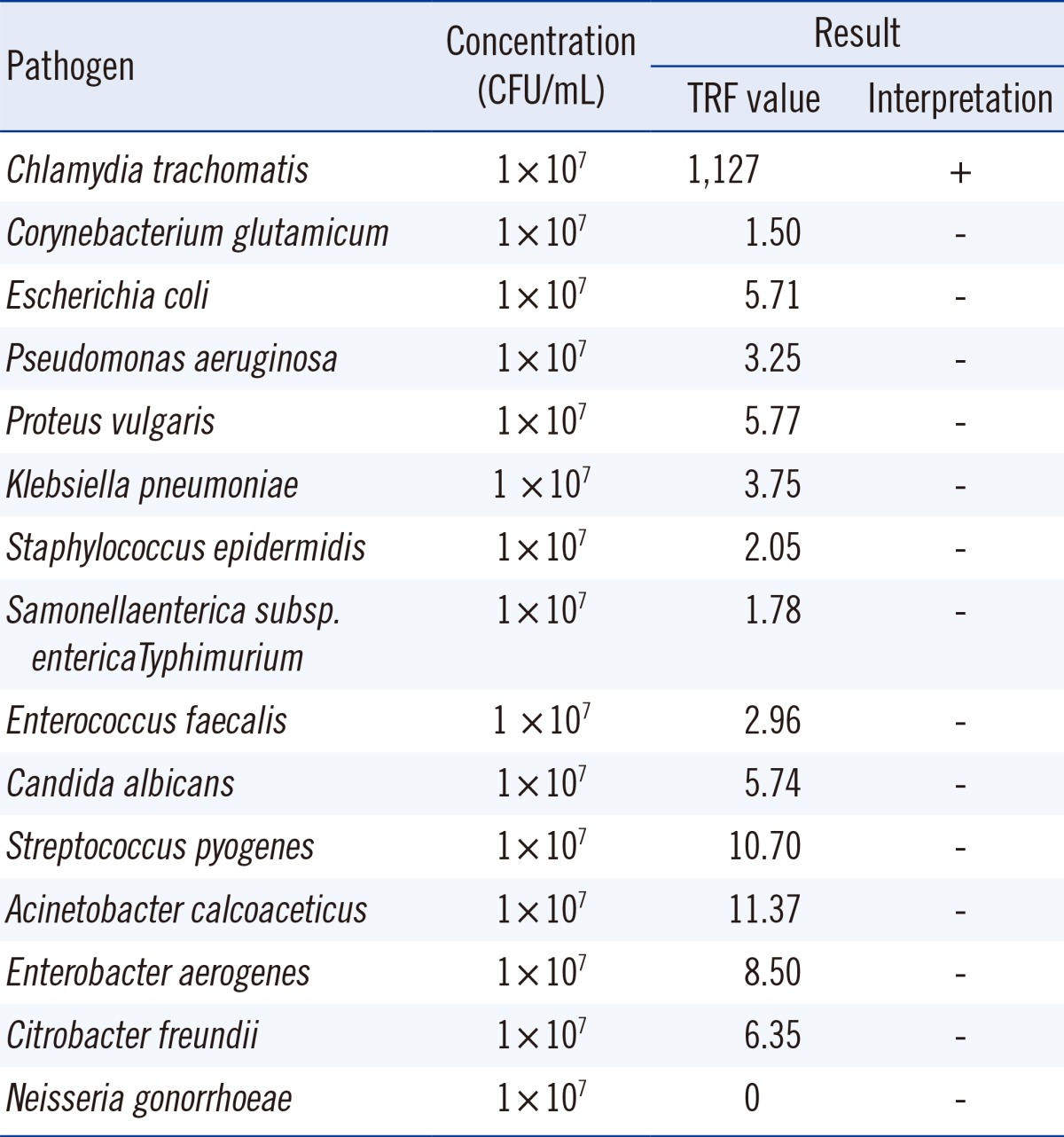
Abbreviations: CFU, colony-forming units; TRF, time-resolved fluorescence.
The diagnostic accuracy of aQcare Chlamydia TRF was evaluated by using NAAT as the gold standard; aQcare Chlamydia TRF showed a high sensitivity of 93.0% (106/114; 95% CI, 0.880-0.963) and overall specificity of 96.3% (315/327; 95% CI, 0.946-0.975) (Table 2). Sensitivity of aQcare Chlamydia TRF using the swab samples was 93.8% (91/97; 95% CI, 0.886-0.970) with a specificity of 96.8% (243/251; 95% CI, 0.948-0.981), while the sensitivity and specificity using the urine samples were slightly lower at 88.2% (15/17; 95% CI, 0.674-0.977) and 94.7% (72/76; 95% CI, 0.901-0.969), respectively (Table 2). Overall, PPV was 89.8% (106/118; 95% CI, 0.850-0.930), and NPV was 97.5% (315/323; 95% CI, 0.958-0.987) (Table 2).
Table 2.
Diagnostic accuracy evaluation using aQcare Chlamydia TRF kit
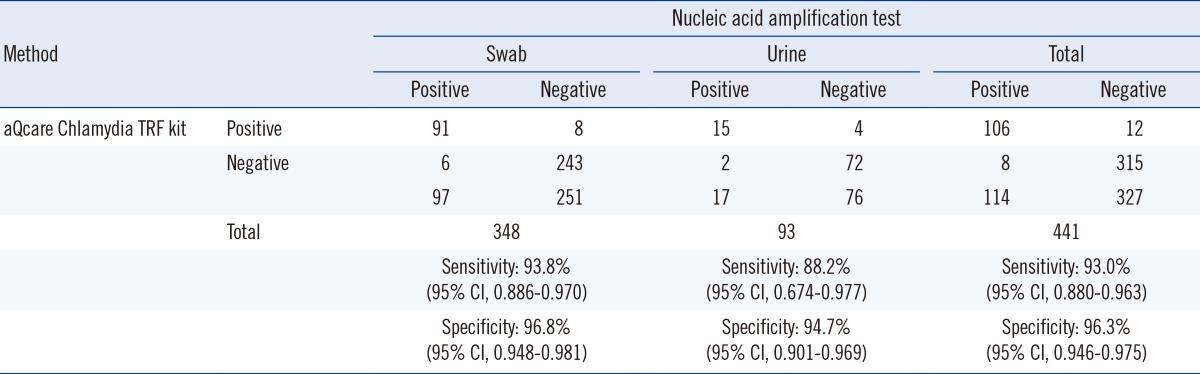
Abbreviation: CI, confidence interval.
DISCUSSION
The goal of this study was to evaluate a newly developed POC testing kit (aQcare Chlamydia TRF kit) for the detection of C. trachomatis; this kit is expected to be a potential tool for the rapid and sensitive detection of C. trachomatis infections.
Although LFIA has been widely used as a qualitative screening tool in various diseases, its poor signal intensity is a major obstacle for increasing its sensitivity. Colloidal gold nanoparticles and latex have generally been used in LFIA kits, and many other studies have been conducted to overcome the low sensitivity, albeit with only limited success [15]. aQcare Chlamydia TRF uses europium (Eu) (III) chelated nanoparticles to detect C. trachomatis antigen with better sensitivity and specificity. Europium (Eu) (III) is a luminescent lanthanide element that is characterized by a large Stokes shift (excitation 333 nm; emission 613 nm) when compared with other fluorescent labels. It allows easy discrimination owing to its own unique emission signals, which eliminates the background fluorescence associated with the use of many existing fluorophores [13, 15]. The longer half-life of europium (Eu) (III) chelated nanoparticles makes it ideal for use in a TRF detection system, which recognizes specific resultant signals after a certain time interval. Europium (Eu) (III) chelates are covalently bonded to polystyrene in multiple layers to produce a strong signal with high sensitivity. The calculated number of europium (Eu) (III) chelates per nanoparticle in the kit was as high as 2×106, allowing for high sensitivity, which is the critical limitation of LFIA-based POC tests. While a technically identical kit for detecting C. trachomatis is not currently on the market, the utility of europium (Eu) (III) chelated nanoparticles has been evaluated in various fields [12, 15, 19, 20]. Trials using it to detect human chorionic gonadotropin (hCG) or cardiac troponin I (cTnI), both requiring fast and reliable results, revealed its potential [12, 21]. The detection limit of europium (Eu) (III) has been reported to be 7- to over 100-times more sensitive than LFIAs labeled with colloidal gold nanoparticles [14, 19, 20]. Similarly, the detection limit of the aQcare Chlamydia TRF was 0.27 ng/mL of standard antigen, which improves sensitivity 8-fold when compared with the detection limit of commercial POC kits that use colloidal gold nanoparticles.
Many studies have revealed that the advantages of most commercial kits over NAAT were limited, owing to their low sensitivity, despite their simplicity and rapidness (Table 3). Among them, the Chlamydia Rapid Test (Diagnostics for the Real World, Cambridge, UK) showed exceptionally high sensitivity, nearing 90% in some studies; however, one study reported its sensitivity to be below 50% [22] (Table 3). The aQcare Chlamydia TRF had a consistent overall sensitivity of 92.9%, which is considerably better than that of currently available commercial POC tests. Its sensitivity using swab samples and urine samples was also consistently better; however, this may need to be reexamined with more samples in the future. Since we used only endocervical swab samples, it may not be reasonable to compare these data to the results of studies using other vaginal swabs. However, it has been demonstrated that the bacterial load found in vaginal swabs is similar to that found in endocervical swabs [23]. Therefore, the swab source is unlikely to influence the general trend observed in our results. Recently, a report of a new POC test developed at the University of Massachusetts Medical School showed a somewhat similar sensitivity of approximately 92.9% [24], demonstrating a promising new POC test that was also cost-effective (Table 3). The cost-effectiveness of aQcare Chlamydia TRF is yet to be determined, and this will be important to evaluate its overall utility in clinical settings. Moreover, there might be a selection bias with the samples, which were selected on the basis of positive/negative results of NAATs. Hence, the test needs to be assessed by using consecutive samples for more accurate performance evaluation. Additionally, although cross-reactivity was not observed in our experiments, the test had a slightly lower overall specificity of 96.3%. False positive cases can cause critical adverse effects especially in low prevalence areas; therefore, further evaluation of causative factors is necessary. Furthermore, to interpret the results quantitatively, assessment of the quantitative performance including imprecision, linearity, and the analytical reportable range of this device is required. Examining the correlation between quantitative values and bacterial load would add more clinically beneficial information.
Table 3.
Reported performance of other point-of-care testing kits
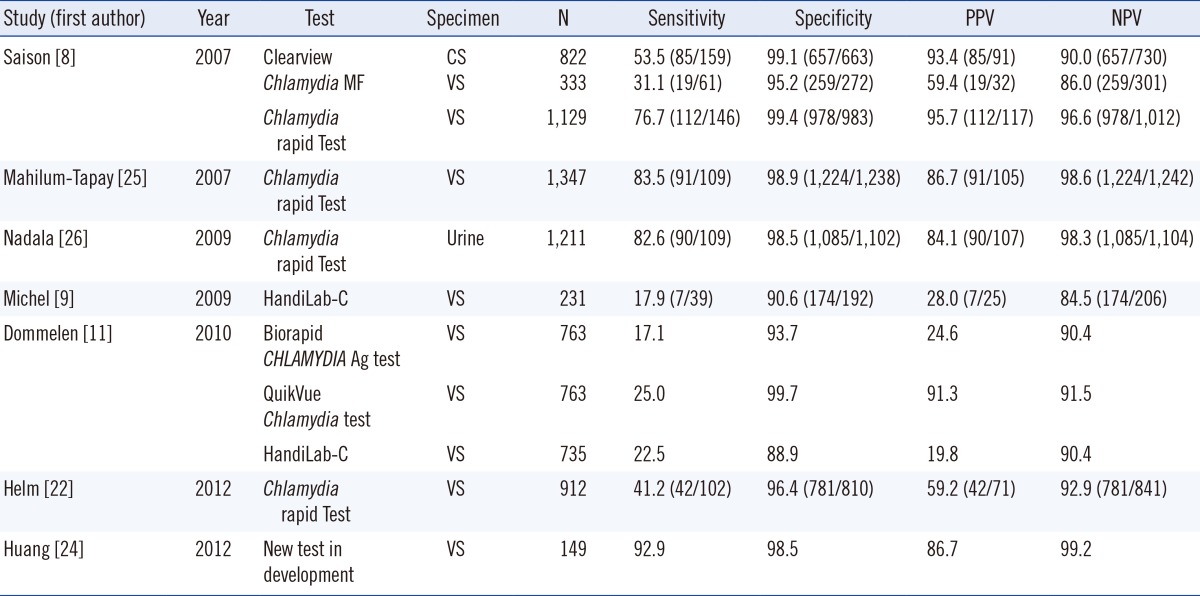
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; CS, endocervical swab; VS, vaginal swab.
Despite these limitations, the advantages of the aQcare Chlamydia TRF kit are promising. Since reliable results are available within 30 min, infected individuals can be identified rapidly and can be prescribed the proper regimen of antibiotics, without having to return to the clinic. Furthermore, we observed a greatly improved sensitivity compared with currently available POC tests, which is expected to provide significant benefits and ultimately aid in achieving the long-term goal of public infection control. Advantages of a POC test are thus maintained in the aQcare Chlamydia TRF kit without the disadvantages of bulky instruments or complex testing techniques. Thus, this novel test can be used to screen wider populations, including those in areas with limited resources where NAAT is unavailable.
Acknowledgments
This research was supported by Bilateral International Collaborative R&D Program of MOTIE/KIAT (GT-2009-ME-SI-0065), Republic of Korea.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Lee IS. Historical Changes and the Present Situation of Sexually Transmitted Diseases. J Korean Med Assoc. 2008;51:868–874. [Google Scholar]
- 2.Watson EJ, Templeton A, Russell I, Paavonen J, Mardh PA, Stary A, et al. The accuracy and efficacy of screening tests for Chlamydia trachomatis: a systematic review. J Med Microbiol. 2002;51:1021–1031. doi: 10.1099/0022-1317-51-12-1021. [DOI] [PubMed] [Google Scholar]
- 3.Torrone EA, Geisler WM, Gift TL, Weinstock HS. Chlamydia trachomatis infection among women 26 to 39 yr of age in the United States, 1999 to 2010. Sex Transm Dis. 2013;40:335–337. doi: 10.1097/OLQ.0b013e31827cd60d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, Serwadda D, et al. What proportion of episodes of gonorrhoea and Chlamydia becomes symptomatic? Int J STD AIDS. 2002;13:91–101. doi: 10.1258/0956462021924712. [DOI] [PubMed] [Google Scholar]
- 5.Patel AL, Sachdev D, Nagpal P, Chaudhry U, Sonkar SC, Mendiratta SL, et al. Prevalence of Chlamydia infection among women visiting a gynaecology outpatient department: evaluation of an in-house PCR assay for detection of Chlamydia trachomatis. Ann Clin Microbiol Antimicrob. 2010;9:24. doi: 10.1186/1476-0711-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajikhani B, Motellaebi T, Norouzi J, Bahador A, Bagheri R, Asgari S, et al. Classical and molecular methods for evaluation of Chlamydia trachomatis infection in women with tubal factor infertility. J Reprod Infertil. 2013;14:29–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Sachdeva P, Patel AL, Sachdev D, Ali M, Mittal A, Saluja D. Comparison of an in-house PCR assay, direct fluorescence assay and the Roche AMPLICOR Chlamydia trachomatis kit for detection of C. trachomatis. J Med Microbiol. 2009;58:867–873. doi: 10.1099/jmm.0.008698-0. [DOI] [PubMed] [Google Scholar]
- 8.Saison F, Mahilum-Tapay L, Michel CE, Buttress ND, Nadala EC, Jr, Magbanua JP, et al. Prevalence of Chlamydia trachomatis infection among low- and high-risk Filipino women and performance of Chlamydia rapid tests in resource-limited settings. J Clin Microbiol. 2007;45:4011–4017. doi: 10.1128/JCM.01343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel CE, Saison FG, Joshi H, Mahilum-Tapay LM, Lee HH. Pitfalls of internet-accessible diagnostic tests: inadequate performance of a CE-marked Chlamydia test for home use. Sex Transm Infect. 2009;85:187–189. doi: 10.1136/sti.2008.035055. [DOI] [PubMed] [Google Scholar]
- 10.Moi H. Handilab C Chlamydia for home testing is not what it claims. Tidsskr Nor Laegeforen. 2007;127:2083–2085. [PubMed] [Google Scholar]
- 11.van Dommelen L, van Tiel FH, Ouburg S, Brouwers EE, Terporten PH, Savelkoul PH, et al. Alarmingly poor performance in Chlamydia trachomatis point-of-care testing. Sex Transm Infect. 2010;86:355–359. doi: 10.1136/sti.2010.042598. [DOI] [PubMed] [Google Scholar]
- 12.von Lode P, Rosenberg J, Pettersson K, Takalo H. A europium chelate for quantitative point-of-care immunoassays using direct surface measurement. Anal Chem. 2003;75:3193–3201. doi: 10.1021/ac0340051. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Nchimi Nono K, Syrjanpää M, Charbonnière LJ, Hovinen J, Härmä H. Stable and highly fluorescent europium (III) chelates for time-resolved immunoassays. Inorg Chem. 2013;52:8461–8466. doi: 10.1021/ic400384f. [DOI] [PubMed] [Google Scholar]
- 14.Xia X, Xu Y, Ke R, Zhang H, Zou M, Yang W, et al. A highly sensitive europium nanoparticle-based lateral flow immunoassay for detection of chloramphenicol residue. Anal Bioanal Chem. 2013;405:7541–7544. doi: 10.1007/s00216-013-7210-9. [DOI] [PubMed] [Google Scholar]
- 15.Xia X, Xu Y, Zhao X, Li Q. Lateral flow immunoassay using europium chelate– loaded silica nanoparticles as labels. Clin Chem. 2009;55:179–182. doi: 10.1373/clinchem.2008.114561. [DOI] [PubMed] [Google Scholar]
- 16.CLSI. User protocol for evaluation of qualitative test performance; approved guideline-second edition. CLSI document EP12-2A. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 17.CLSI. Protocols for determination of limits of detection and limits of quantitation; approved guideline-second edition. CLSI document EP17. Wayne, PA: Clinical and Laboratory Standards Institute; 2004. [Google Scholar]
- 18.CLSI. Interference testing in clinical chemistry; approved guideline-second edition. CLSI document EP07-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2005. [Google Scholar]
- 19.Juntunen E, Myyryläinen T, Salminen T, Soukka T, Pettersson K. Performance of fluorescent europium (III) nanoparticles and colloidal gold reporters in lateral flow bioaffinity assay. Anal Biochem. 2012;428:31–38. doi: 10.1016/j.ab.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Xia X, Xu Y, Ke W, Yang W, Li Q. Application of europium (III) chelates-bonded silica nanoparticle in time-resolved immunofluorometric detection assay for human thyroid stimulating hormone. Anal Chim Acta. 2012;722:95–99. doi: 10.1016/j.aca.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 21.Järvenpää ML, Kuningas K, Niemi I, Hedberg P, Ristiniemi N, Pettersson K, et al. Rapid and sensitive cardiac troponin I immunoassay based on fluorescent europium (III)-chelate-dyed nanoparticles. Clin Chim Acta. 2012;414:70–75. doi: 10.1016/j.cca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 22.van der Helm JJ, Sabajo LO, Grunberg AW, Morré SA, Speksnijder AG, de Vries HJ. Point-of-care test for detection of urogenital Chlamydia in women shows low sensitivity. A performance evaluation study in two clinics in Suriname. PLoS One. 2012;7:e32122. doi: 10.1371/journal.pone.0032122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knox J, Tabrizi SN, Miller P, Petoumenos K, Law M, Chen S, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29:647–654. doi: 10.1097/00007435-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Gaydos CA, Barnes MR, Jett-Goheen M, Blake DR. Comparative effectiveness of a rapid point-of-care test for detection of Chlamydia trachomatis among women in a clinical setting. Sex Transm Infect. 2013;89:108–114. doi: 10.1136/sextrans-2011-050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahilum-Tapay L, Laitila V, Wawrzyniak JJ, Lee HH, Alexander S, Ison C, et al. New point of care Chlamydia Rapid Test--bridging the gap between diagnosis and treatment: performance evaluation study. BMJ. 2007;335:1190–1194. doi: 10.1136/bmj.39402.463854.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadala EC, Goh BT, Magbanua JP, Barber P, Swain A, Alexander S, et al. Performance evaluation of a new rapid urine test for Chlamydia in men: prospective cohort study. BMJ. 2009;339:b2655. doi: 10.1136/bmj.b2655. [DOI] [PMC free article] [PubMed] [Google Scholar]


