Abstract
Background
Acinetobacter species are the leading cause of bloodstream infection (BSI), but their correct identification is challenging. We evaluated the matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS)-based VITEK MS (bioMérieux, France), and two automated systems, VITEK 2 (bioMérieux) and MicroScan (Siemens, USA) for identification of Acinetobacter BSI isolates.
Methods
A total of 187 BSI isolates recovered at a university hospital in Korea between 2010 and 2012 were analyzed. The identification results obtained using VITEK MS and two automated systems were compared with those of rpoB sequencing.
Results
Of 187 isolates analyzed, 176 were identified to the species level by rpoB sequencing: the Acinetobacter baumannii group (ABG; 101 A. baumannii, 43 A. nosocomialis, 10 A. pittii isolates) was most commonly identified (82.4%), followed by Acinetobacter genomic species 13BJ/14TU (5.3%), A. ursingii (2.1%), A. soli (2.1%), A. bereziniae (1.1%), and A. junii (1.1%). Correct identification rates to the species group (ABG) level or the species level was comparable among the three systems (VITEK MS, 90.3%; VITEK 2, 89.2%; MicroScan, 86.9%). However, VITEK MS generated fewer misidentifications (0.6%) than VITEK 2 (10.8%) and MicroScan (13.1%) (P<0.001). In addition, VITEK MS demonstrated higher specificity (100%) for discrimination between ABG and non-ABG isolates than the other systems (both, 31.8%) (P<0.001).
Conclusions
The VITEK MS system is superior to the VITEK 2 and MicroScan systems for identification of Acinetobacter BSI isolates, with fewer misidentifications and better discrimination between the ABG and non-ABG isolates.
Keywords: VITEK MS, VITEK 2, MicroScan, Acinetobacter, Identification
INTRODUCTION
Acinetobacter species are one of the leading causative agents of healthcare-associated infections worldwide, including bloodstream infection (BSI), pneumonia, urinary tract infection, and meningitis [1, 2, 3]. To date, more than 32 species of the Acinetobacter genus have been identified by gene sequencing [4, 5, 6, 7, 8]. Members of the Acinetobacter baumannii group (ABG), which consists of A. baumannii, A. pittii, and A. nosocomialis, share important clinical and epidemiological characteristics that cannot be distinguished by most of the currently available phenotypic identification (ID) systems [2]. Although the ABG remains the most common Acinetobacter species recovered from clinical specimens, the non-ABG species are also often clinically relevant [3, 4, 9, 10, 11]. Because Acinetobacter species may differ in their pathogenicity, epidemiology, antimicrobial susceptibility, and clinical outcomes [5, 9, 11, 12, 13], information on the relative frequency of various Acinetobacter species causing BSI may be useful for establishing protocols for infection control and treatment of Acinetobacter BSI. However, to date, data regarding distributions of BSI isolates of Acinetobacter species in hospitals are limited, because the correct ID of Acinetobacter isolates is difficult to determine when using the currently available, common phenotypic methods [2, 3, 4, 9, 10, 14].
Recent studies have shown that matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) offers an opportunity for rapid, cost-effective, convenient, and high-throughput bacterial ID in routine diagnostic procedures conducted in clinical microbiology laboratories [15, 16]. However, information regarding the suitability of the MALDI-TOF MS-based VITEK MS system (VITEK MS; bioMérieux, Marcy l'Etoile, France) for the ID of clinical isolates of Acinetobacter species is scarce as compared to that of other commonly used automated ID systems. In this study, we performed molecular ID to investigate the rank order of occurrence of the various species of Acinetobacter isolated from blood cultures obtained from a university hospital in Korea between 2010 and 2012. We performed a comparative evaluation of MALDI-TOF MS-based VITEK MS system versus the VITEK 2 (VITEK2 XL; bioMérieux) and MicroScan (MicroScan WalkAway-96 Plus; Siemens, Deerfield, IL, USA) automated systems for the correct ID of Acinetobacter BSI isolates, including evaluation of their discriminative abilities for the ABG versus other (non-ABG) Acinetobacter species.
METHODS
1. Acinetobacter isolation and molecular ID
A total of 187 isolates molecularly identified as belonging to the genus Acinetobacter were analyzed in this study. All isolates were obtained from the blood cultures of 187 patients at the Chonnam National University Hospital (a 1,000-bed tertiary-care hospital in Gwangju, Korea) between January 2010 and December 2012. Duplicate isolates of Acinetobacter species from the same patient were excluded. For molecular ID, DNA was extracted from the isolates as described previously [17], and a 450-bp sequence (zone 2) of the rpoB gene region of each isolate was sequenced [18]. The primers Ac1055F (5'-GTGATAARATGGCBGGTCGT-3') and Ac1598R (5'-CGBGCRTGCATYTTGTCRT-3') were used to amplify the rpoB region. All loci were sequenced in both the forward and reverse directions with the same primers as those used for amplification. The amplification products were purified and sequenced by using an ABI 3730XL sequencer (Applied Biosystems, Foster City, CA, USA). Sequence data were assembled and compared with previously reported sequences by using the basic local alignment search tool (BLAST) of the national center for biotechnology information (NCBI) database (http://www.ncbi.nlm.nih.gov/blast).
2. ID using the VITEK MS, VITEK 2, and MicroScan systems
ID with VITEK MS (in vitro-diagnostic [IVD] mode) was performed according to the manufacturer's instructions by directly smearing an overnight-cultured bacterial specimen onto disposable target slides with a 1.0-µL matrix solution (VITEK MS-CHCA). Escherichia coli ATCC 8739 was used as the calibration strain. The advanced spectrum classifier software of the VITEK MS system proposes 3 confidence levels: (a) single choice, with one significant choice (confidence value ≥ 60); (b) low discrimination, with more than one significant choice (maximum 4 choices); and (c) unidentified organism, with no significant choice (no class with probability and score higher than the defined thresholds) or a number of significant choices greater than the defined threshold of low discrimination.
ID using the VITEK 2 and MicroScan systems was performed by using GN ID Card (bioMérieux) and Gram Negative Breakpoint Combo Panel Type 42 (Siemens), respectively, according to the manufacturers' instructions. The ID results from these systems are proposed automatically by the respective accompanying software. The tests were repeated only if the initial results indicated "low discrimination" or "no ID", and the repeat result was used for data analysis.
3. Data analysis
The ID capability of the 3 systems was evaluated by using the Acinetobacter isolates that were identified to the species level by rpoB sequencing. The ID results from the 3 systems were classified into 3 categories by comparison with the results of the reference rpoB sequencing: (a) "correct ID" to the species (identical to sequence-based ID) or species group level; (b) "mis-ID", when the ID result of the system differed from that of the sequence-based reference method; and (c) "no ID", when the system could not identify the isolate. Because the ID result of the isolates belonging to the ABG (isolates of A. baumannii, A. pittii, or A. nosocomialis) is assigned by these 3 systems at the species group level only, the ABG isolates that were identified as "A. baumannii complex" by using VITEK MS IVD and VITEK 2, or those identified as "A. baumannii/haemolyticus" by using the MicroScan system were categorized under the "correct ID" category. Statistical analysis to compare the ID performance of the 3 systems was performed with the chi-square test, Fisher's exact test, and McNemar test using the IBM SPSS Statistics (version 21, IBM, Armonk, NY, USA) and GraphPad Prism (version 5, GraphPad Software, San Diego, CA,USA) software packages.
RESULTS
1. Acinetobacter isolation and molecular ID
Table 1 presents the species distribution of the 187 Acinetobacter BSI isolates determined by partial rpoB sequencing. Of the 187 isolates collected during the 3-yr period, 154 (82.4%) isolates belonged to the ABG, which included 101 (54.0% of the total 187 Acinetobacter isolates) A. baumannii, 43 (23.0%) A. nosocomialis, and 10 (5.3%) A. pittii isolates. The remaining 33 (17.6%) isolates belonged to the non-ABG: 10 (5.3%) isolates of Acinetobacter genomic species 13BJ/14TU, 4 (2.1%) of A. ursingii, 4 (2.1%) of A. soli, 2 (1.1%) of A. bereziniae, 2 (1.1%) of A. junii, and 11 (5.9%) of other miscellaneous Acinetobacter species, which were identified as the genus Acinetobacter but not identified to the species level. Overall, a total of 176 isolates were identified to the species level by partial rpoB sequencing.
Table 1.
Species distribution of 187 Acinetobacter bloodstream isolates recovered during the 3-yr period
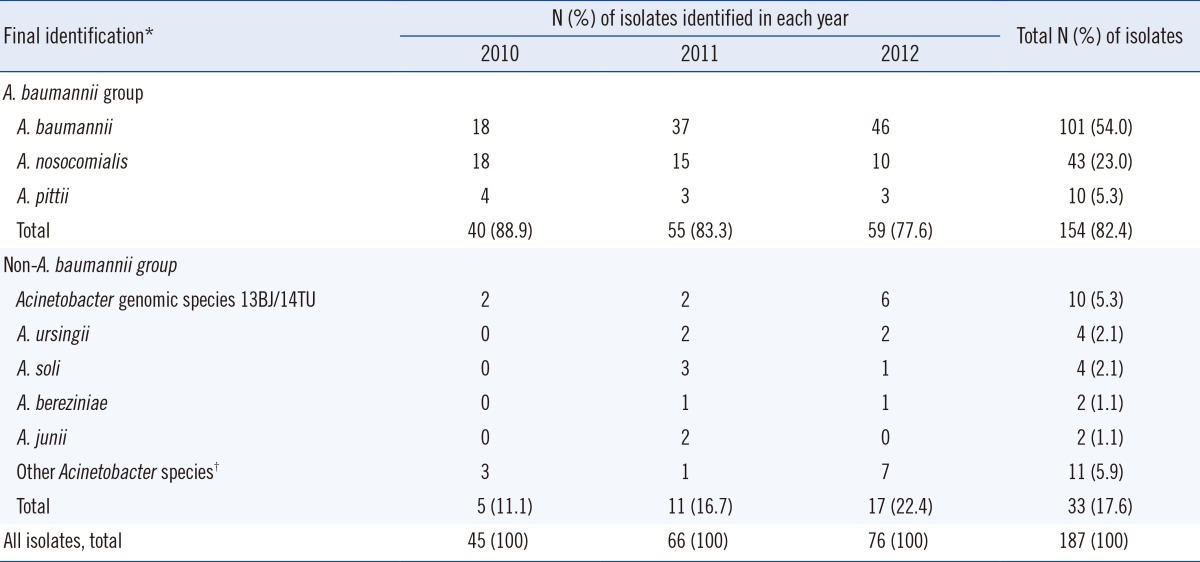
*The final identification results were obtained by rpoB sequencing; †Isolates were identified as belonging to genus Acinetobacter, but not identified up to the species level by rpoB sequencing.
2. ID using the VITEK MS, VITEK 2, and MicroScan systems
Details of the ID results from rpoB sequencing and the VITEK MS, VITEK 2, and MicroScan systems are provided in Table 2. Of the 154 ABG isolates, 153 (99.4%) were correctly identified by all 3 systems. The VITEK MS system correctly identified all 4 A. ursingii isolates and 2 A. junii isolates, and VITEK 2 correctly identified 2 of 4 A. ursingii isolates and 2 A. junii isolates, which were present in their respective databases; by contrast, the MicroScan system misidentified each of the 6 isolates absent from its database. Among the 16 isolates of Acinetobacter genomic species 13BJ/14TU, A. soli, and A. bereziniae, which were absent in the databases of the 3 systems, VITEK MS showed 1 (6.3%) mis-ID and 15 (93.8%) no ID results, whereas both VITEK 2 and MicroScan showed 16 (100%) mis-ID and 0 (0%) no ID results (P<0.001).
Table 2.
Detailed identification results by rpoB sequencing and the MALDI-TOF MS-based VITEK MS, VITEK 2, and MicroScan systems for 176 Acinetobacter blood isolates
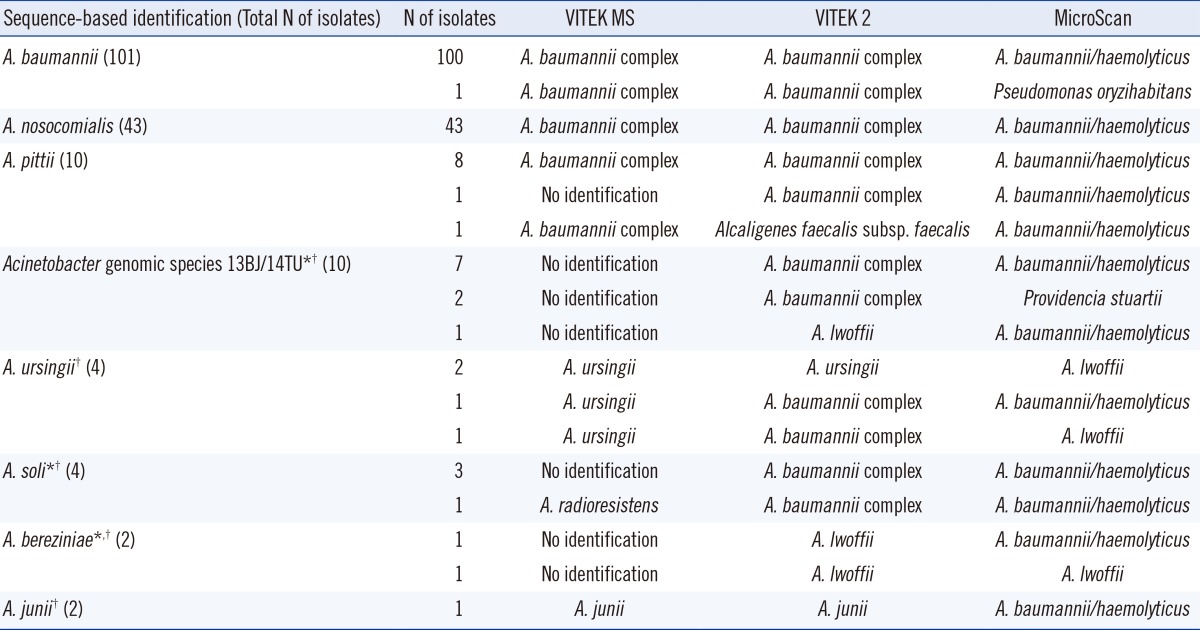
*Species not included in the databases of VITEK MS and VITEK 2 systems; †Species not included in the database of MicroScan system.
Abbreviation: MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry.
3. ID results for the ABG and non-ABG isolates
The ID results from the 3 tested systems for the 154 ABG and 22 non-ABG isolates are summarized in Table 3. All 3 systems correctly identified 99.4% (153/154) of the ABG isolates. However, the test results for the 22 non-ABG isolates, including 10 Acinetobacter genomic species 13BJ/14TU, 4 A. ursingii, 4 A. soli, 2 A. bereziniae, and 2 A. junii isolates, showed correct ID rates of 27.3%, 18.2%, and 0% for the VITEK MS, VITEK 2, and MicroScan systems, respectively (VITEK MS and VITEK 2 versus MicroScan, P<0.05). The mis-ID rate for the 22 non-ABG isolates was 4.5% (1/22) using VITEK MS, which was significantly lower than that obtained using VITEK 2 (81.8%) and MicroScan (100%) (P<0.001). Of the 22 non-ABG isolates, no (0.0%) and 15 (68.2%) isolates were misidentified as A. baumannii complex when using VITEK MS and VITEK 2, respectively, and 15 (68.2%) isolates were misidentified as A. baumannii/haemolyticus with MicroScan. Therefore, the specificity of VITEK MS for discrimination between the ABG and non-ABG isolates was much higher (100% [22/22]) than that of VITEK 2 and MicroScan (both, 31.8% [7/22]; P<0.001), while the sensitivity of the 3 systems was identical at 99.4% (153/154). The false-positive rate of VITEK MS for discrimination between the ABG and non-ABG isolates was 0% (0/22), while that of both VITEK 2 and MicroScan was 68.2% (15/22) (P<0.001).
Table 3.
Identification results of 176 Acinetobacter bloodstream isolates by the MALDI-TOF MS-based VITEK MS, VITEK 2, and MicroScan systems as compared to sequence-based identification
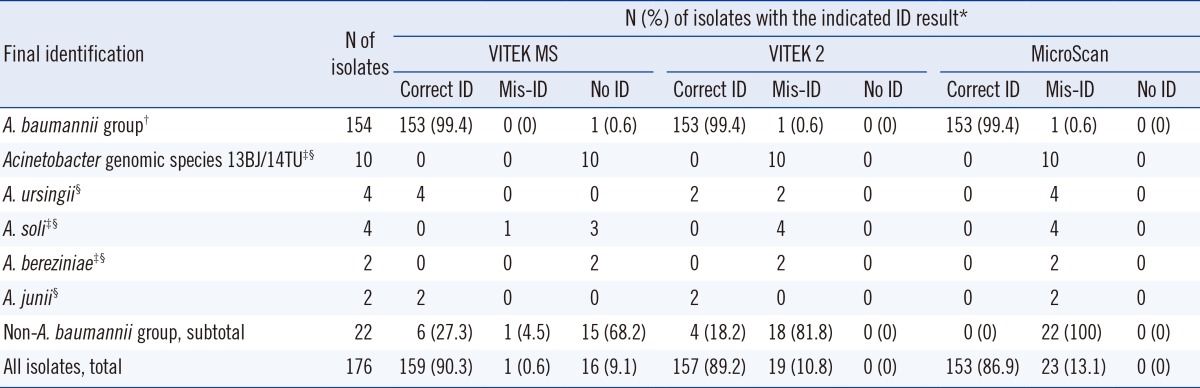
*Correct ID (identification) included "correct ID" up to the (i) species level; (ii) species group level for the A. baumannii group (e.g., "A. baumannii complex" by using VITEK MS and VITEK 2 or "A. baumannii/haemolyticus" by using MicroScan); †A. baumannii, A. pittii, and A. nosocomialis were included in the A. baumannii group; ‡Species not included in the databases of VITEK MS and VITEK 2 systems; §Species not included in the database of the MicroScan system.
Abbreviations: MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry; correct ID, identification up to the species or species group level; mis-ID, identification result of the system differed from that of the sequence-based reference method; no ID, the system could not identify the isolate.
Overall, for all 176 isolates tested, the correct ID rates obtained with the VITEK MS, VITEK 2, and MicroScan systems were 90.3%, 89.2%, and 86.9%, respectively, which indicate similar rates of correct ID among the 3 systems. However, VITEK MS showed a lower mis-ID rate (0.6%) than VITEK 2 (10.8%) and MicroScan (13.1%) (P<0.001) and a higher no ID rate (9.1%) than VITEK 2 and MicroScan (both, 0%) (P<0.001).
DISCUSSION
To the best of our knowledge, this study reports the first comparative evaluation of MALDI-TOF MS-based VITEK MS with both the VITEK 2 and MicroScan systems for the ID of Acinetobacter species from BSI isolates. The current findings demonstrate that the mis-ID rate of VITEK MS was significantly lower (0.6%) than that obtained with the VITEK 2 (10.8%) and MicroScan (13.1%) systems for the ID of Acinetobacter BSI isolates. Moreover, VITEK MS showed the best discrimination between the ABG and non-ABG isolates in this study. Thus, we believe that VITEK MS is a useful system for the rapid and accurate ID of BSI isolates of Acinetobacter species in routine diagnostic procedures conducted in clinical microbiology laboratories.
For molecular ID, we analyzed a partial sequence of the rpoB gene region, which has a higher discriminatory power for routine ID of different Acinetobacter species compared to the 16S rRNA gene [18, 19]. Sequence-based analysis of 187 BSI isolates recovered during the 3-yr period (2010-2012) revealed that the ABG isolates represented 82.4% of all Acinetobacter BSI cases in our hospital. This finding, together with data from other countries, confirmed that the ABG continues to be the leading cause of Acinetobacter BSI worldwide [4, 9, 10]. Although A. baumannii is the most frequent etiological agent of Acinetobacter BSI in our hospital, the prevalence of A. baumannii (54.0%) detected in this study was slightly lower than that reported for the USA (63.4%) [9], but higher than that reported for Norway (8.8%) and Japan (17.9%) in previous studies [4, 10]. Karah et al. [4] reported that the most prevalent species was A. nosocomialis (46.9%), followed by A. pittii (19.5%) and A. baumannii (8.8%) among the Acinetobacter BSI isolates in Norway. On the other hand, Kishii et al. [10] reported that the most prevalent species was A. pittii (34.1%), followed by A. baumannii (17.9%) and A. nosocomialis (15.4%) in Japan. Our recent report revealed that Acinetobacter genomic species 13BJ/14TU is innately resistant to colistin, but susceptible to most of the other clinically relevant antimicrobial agents, and that BSI patients infected with this species show excellent clinical outcomes with cleared bacteremia [11]. In the current study, among the 33 (17.6%) non-ABG isolates, 10 (5.3%) isolates of Acinetobacter genomic species 13BJ/14TU were identified as causative agents of BSI. Overall, our results, together with data from other countries reported previously, demonstrate that the rank order of the occurrence of various Acinetobacter species that cause BSI may differ among hospitals and countries and with respect to time [4, 9, 10].
To date, few evaluations of the VITEK MS system using molecularly identified Acinetobacter clinical isolates have been performed, with the exception of one multi-center study for the ID of non-Enterobacteriaceae gram-negative bacilli isolated from various samples [16]. In this previous study, when the VITEK MS ID results of 108 Acinetobacter isolates were compared with those determined by gene sequencing, the rates of correct ID to the species level, mis-ID, and no ID were 83.3% (90/108), 0.9% (1/108), and 13.0% (14/108), respectively. Similarly, we found that the rates of correct ID, mis-ID, and no ID using VITEK MS for 176 Acinetobacter BSI isolates were 90.3%, 0.6%, and 9.1%, respectively. In addition, the current study demonstrated that the VITEK MS system was better at correctly identifying the ABG isolates (99.4%) than the non-ABG isolates (27.3%). In our study, all isolates of A. ursingii and A. junii, which are included in the VITEK MS database, were correctly identified. Because the isolates described herein represent almost all of the Acinetobacter BSI isolates encountered in a single hospital over a 3-yr period, our finding that VITEK MS showed a lower correct ID rate for the non-ABG isolates than for the ABG isolates will provide valuable information for the diagnosis and treatment of BSI patients infected with Acinetobacter species.
To date, no comparative evaluation of the VITEK MS, VITEK 2, and MicroScan systems for the ID of BSI isolates of Acinetobacter species has been reported. The present study revealed that the correct ID rate of VITEK MS (90.3%) was comparable to that of the VITEK 2 (89.2%) and MicroScan (86.9%) systems; however, VITEK MS showed a lower rate of mis-ID (0.6%) than VITEK 2 (10.8%) and MicroScan (13.1%) for these isolates. Instead, VITEK MS yielded more "no ID" results (9.1%) than VITEK 2 and MicroScan (both, 0%). These results suggest that the VITEK MS system can be advantageous in the routine diagnostic procedures conducted in clinical microbiology laboratories, because an isolate not identified by the primary testing method can be routinely retested or tested by using other methods [15, 20]. In addition, the low rate of mis-ID obtained using the VITEK MS system offers an important clinical advantage along with offering more rapid and reliable ID of other bacterial isolates [16].
Although the need for species ID of Acinetobacter isolates in routine clinical microbiology laboratories has been questioned, recent studies have highlighted the importance of discriminating the ABG from non-ABG BSI isolates since non-ABG BSI has a more benign clinical course than ABG BSI, and the two groups differ in their antimicrobial susceptibilities [2, 9, 11, 21, 22, 23]. Of the 3 systems tested, VITEK MS showed the best discrimination ability between the ABG and non-ABG isolates, with 100% specificity (0% false-positive rate), while the VITEK 2 and MicroScan systems both showed 31.8% specificity (68.2% false-positive rate) for discriminating between these groups. This finding highlights the effectiveness of VITEK MS for discriminating the ABG from non-ABG isolates in Acinetobacter BSI cases. Thus, use of VITEK MS will facilitate prediction of the clinical course and outcome of BSI patients as well as the selection of an appropriate antimicrobial regimen.
The current and previous studies have demonstrated that the limitation of the current VITEK MS system for ID of Acinetobacter species [15, 16]. It cannot differentiate among the ABG species such as A. baumannii, A. pittii, and A. nosocomialis. However, when a cluster analysis was performed on the Acinetobacter isolates tested in this study by using VITEK MS RUO (research-use-only, Saramis database, bioMérieux) mode, the A. baumannii, A. pittii, and A. nosocomialis isolates were relatively well-clustered (data not shown). In addition, a recent report showed that another MALDI-TOF MS-based system that was unable to reliably differentiate between these closely related species has now been improved by using an alternative protocol [24]. Thus, the VITEK MS system shows potential for offering reliable species-level ID of ABG isolates in routine diagnostic procedures conducted in clinical microbiology laboratories.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Chuang YC, Sheng WH, Li SY, Lin YC, Wang JT, Chen YC, et al. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin Infect Dis. 2011;52:352–360. doi: 10.1093/cid/ciq154. [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turton JF, Shah J, Ozongwu C, Pike R. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J Clin Microbiol. 2010;48:1445–1449. doi: 10.1128/JCM.02467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karah N, Haldorsen B, Hegstad K, Simonsen GS, Sundsfjord A, Samuelsen Ø. Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. J Antimicrob Chemother. 2011;66:738–744. doi: 10.1093/jac/dkq521. [DOI] [PubMed] [Google Scholar]
- 5.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, Baik KS, Kim MS, Park SC, Kim SS, Rhee MS, et al. Acinetobacter soli sp. nov., isolated from forest soil. J Microbiol. 2008;46:396–401. doi: 10.1007/s12275-008-0118-y. [DOI] [PubMed] [Google Scholar]
- 7.Nemec A, Musílek M, Maixnerová M, De Baere T, van der Reijden TJ, Vaneechoutte M, et al. Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. Int J Syst Evol Microbiol. 2009;59:118–124. doi: 10.1099/ijs.0.001230-0. [DOI] [PubMed] [Google Scholar]
- 8.Vaneechoutte M, De Baere T, Nemec A, Musílek M, van der Reijden TJ, Dijkshoorn L. Reclassification of Acinetobacter grimontii Carr et al. 2003 as a later synonym of Acinetobacter junii Bouvet and Grimont 1986. Int J Syst Evol Microbiol. 2008;58:937–940. doi: 10.1099/ijs.0.65129-0. [DOI] [PubMed] [Google Scholar]
- 9.Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, Edmond MB, et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect. 2012;64:282–290. doi: 10.1016/j.jinf.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Kishii K, Kikuchi K, Matsuda N, Yoshida A, Okuzumi K, Uetera Y, et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for species identification of Acinetobacter strains isolated from blood cultures. Clin Microbiol Infect. 2014;20:424–430. doi: 10.1111/1469-0691.12376. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Shin JH, Park KH, Kim JH, Shin MG, Suh SP, et al. Identification, genotypic relation, and clinical features of colistin-resistant isolates of Acinetobacter genomic species 13BJ/14TU from bloodstreams of patients in a university hospital. J Clin Microbiol. 2014;52:931–939. doi: 10.1128/JCM.02868-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YC, Huang YT, Tan CK, Kuo YW, Liao CH, Lee PI, et al. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J Antimicrob Chemother. 2011;66:1839–1846. doi: 10.1093/jac/dkr200. [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011;52:879–891. doi: 10.3349/ymj.2011.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernards AT, van der Toorn J, van Boven CP, Dijkshoorn L. Evaluation of the ability of a commercial system to identify Acinetobacter genomic species. Eur J Clin Microbiol Infect Dis. 1996;15:303–308. doi: 10.1007/BF01695662. [DOI] [PubMed] [Google Scholar]
- 15.Dubois D, Grare M, Prere MF, Segonds C, Marty N, Oswald E. Performances of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid identification of bacteria in routine clinical microbiology. J Clin Microbiol. 2012;50:2568–2576. doi: 10.1128/JCM.00343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manji R, Bythrow M, Branda JA, Burnham CA, Ferraro MJ, Garner OB, et al. Multi-center evaluation of the VITEK® MS system for mass spectrometric identification of non-Enterobacteriaceae Gram-negative bacilli. Eur J Clin Microbiol Infect Dis. 2014;33:337–346. doi: 10.1007/s10096-013-1961-2. [DOI] [PubMed] [Google Scholar]
- 17.Khamis A, Raoult D, La Scola B. rpoB gene sequencing for identification of Corynebacterium species. J Clin Microbiol. 2004;42:3925–3931. doi: 10.1128/JCM.42.9.3925-3931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Scola B, Gundi VA, Khamis A, Raoult D. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol. 2006;44:827–832. doi: 10.1128/JCM.44.3.827-832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gundi VA, Dijkshoorn L, Burignat S, Raoult D, La Scola B. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology. 2009;155:2333–2341. doi: 10.1099/mic.0.026054-0. [DOI] [PubMed] [Google Scholar]
- 20.Won EJ, Shin JH, Lee K, Kim MN, Lee HS, Park YJ, et al. Accuracy of species-level identification of yeast isolates from blood cultures from 10 university hospitals in South Korea by use of the matrix-assisted laser desorption ionization-time of flight mass spectrometry-based Vitek MS system. J Clin Microbiol. 2013;51:3063–3065. doi: 10.1128/JCM.00945-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol. 1991;29:277–282. doi: 10.1128/jcm.29.2.277-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seifert H, Strate A, Schulze A, Pulverer G. Bacteremia due to Acinetobacter species other than Acinetobacter baumannii. Infection. 1994;22:379–385. doi: 10.1007/BF01715492. [DOI] [PubMed] [Google Scholar]
- 23.Wareham DW, Bean DC, Khanna P, Hennessy EM, Krahe D, Ely A, et al. Bloodstream infection due to Acinetobacter spp: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis. 2008;27:607–612. doi: 10.1007/s10096-008-0473-y. [DOI] [PubMed] [Google Scholar]
- 24.Šedo O, Nemec A, Křížová L, Kačalová M, Zdráhal Z. Improvement of MALDI-TOF MS profiling for the differentiation of species within the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. Syst Appl Microbiol. 2013;36:572–578. doi: 10.1016/j.syapm.2013.08.001. [DOI] [PubMed] [Google Scholar]


