ABSTRACT
Animal African trypanosomosis (AAT), caused by Trypanosoma congolense, is widespread throughout sub-Saharan Africa. There are significant concerns related to the current drugs available for the treatment of AAT due to their limited effectiveness across species and their adverse effects. Moreover, drug resistant trypanosomes have recently been reported in the field. High throughput screening (HTS) of large chemical compound library collections is a promising approach for identifying novel drug candidates. While HTS for Trypanozoon trypanosomes, T. brucei sspp. and T. evansi is well established, no assays have been developed for T. congolense. In the present study, the authors developed an ATP-based luciferase viability assay for T. congolense in a 96-well plate format. The calculated 50% inhibitory concentration (IC50) values for pentamidine and diminazene were 10–100 times higher in T. congolense than in T. brucei. This result suggests that the transporters for the 2 tested compounds differ between T. congolense and T. brucei. This assay could further be applied to screen novel chemical compounds for the treatment of AAT caused by T. congolense.
Keywords: Animal African trypanosomosis, drug screening, luciferase assay, Trypanosoma congolense
Animal African trypanosomosis (AAT) in domesticated and wild animals, known as Nagana, is mainly caused by Trypanosoma congolense, T. vivax and T. brucei brucei [9]. The most consistent clinical features of Nagana in livestock are intermittent fever and anemia, although general leukopenia, splenomegaly and hepatomegaly, and weight loss are also present in some cases. T. congolense and T. vivax only proliferate in blood circulation, whereas T. b. brucei has also been found to infect the central nervous system [2, 14, 16, 21]. The FAO estimates that AAT causes a combined economic loss of agricultural gross domestic product of US$ 4.75 billion per year (http://www.fao.org/ag/againfo/programmes/en/paat/disease.html). Currently, 3 drugs are commercially available for the treatment of AAT, namely: diminazene, isometamidium and homidium. The latter 2 drugs can also be used for chemoprophylaxis [9]. In the case of human African trypanosomosis (HAT) treatment, pentamidine and suramine are the first choice of drugs for the treatment of T. b. rhodesiense and T. b. gambiense, respectively [2]. However, drug resistant trypanosomes and drug refractory trypanosomosis have been reported from the field for both HAT and AAT [1, 6, 7, 19]. Novel trypanocidal compounds are therefore needed for the treatment of drug resistant trypanosomosis.
High throughput screening (HTS) assay of large compound libraries has been undertaken to discover novel therapeutic candidates for several parasites [18]. This has been performed for a number of parasitic diseases, such as American trypanosomosis (Chagas’ disease), leishmaniosis and malaria [8, 13, 22, 25]. The HTS approach has been proven to have the potential to identify new drugs with novel modes of action. HTS approaches with specific targets have been well established and reported for Trypanozoon trypanosomes, T. b. brucei, T. b. gambiense, T. b. rhodesiense and T. evansi [10, 20, 23, 24, 26]. A viability assay, utilizing Alamar BlueTM dye, has been developed for all T. brucei sspp. [20, 23]. However, the Alamar BlueTM dye assay has some problems in that it is time-consuming to perform and its sensitivity is low. Recently, a luciferase viability assay for measuring the ATP concentration of cells was adapted to HTS to evaluate viable T. b. brucei and T. b. gambiense cell numbers [24, 26]. In comparison, the luciferase assay method offers greater sensitivity and is less time-consuming than the Alamar BlueTM assay system [23, 24]. This assay could therefore be used in HTS for important animal parasites, such as T. congolense. However, no HTS assay for T. congolense has previously been developed or reported.
In the present study, we initially established an ATP-based luciferase viability assay for T. congolense using the 2 available drugs against trypanosomosis: diminazene and pentamidine. We applied this assay in evaluating ATP congruent to the T. congolense cell number in culture to develop an HTS system for this trypanosome.
MATERIALS AND METHODS
Parasites andin vitro culture: Trypanosoma: Trypanosoma congolense IL3000, a savannah type strain isolated near the Kenya/Tanzania border in 1966, was used in this study. The blood stream form (BSF) of T. congolense IL3000 was propagated at 33°C in air using Hirum’s modified Iscove’s medium (HMI)-9 composed of Iscove’s modified Dulbecco’s medium (Sigma-Aldrich, Tokyo, Japan) supplemented with 20% heat inactivated-fetal bovine serum (HI-FBS), 60 mM HEPES (Sigma-Aldrich), 1 mM pyruvic acid sodium salt (Sigma-Aldrich), 0.1 mM bathocuproine (Sigma-Aldrich), 1 mM hypoxanthine and 16 µM thymidine (HT supplement: Invitrogen, Tokyo, Japan), 10 µg/l insulin, 5.5 µg/l transferrin and 6.7 ng/l sodium selenite (ITS-X: Invitrogen), 0.0001% 2-β-mercaptoethanol (Sigma-Aldrich), 0.4 g/l BSA (Sigma-Aldrich) and 2 mM L-cysteine (Sigma-Aldrich), as previously reported [12]. The BSF cultures were maintained by replacing the entire culture supernatant with fresh medium every other day.
Optimization of cell density and calculation of doubling time: A growth curve was plotted to estimate the maximum number of cells and calculate the doubling time. One hundred µl of T. congolense (at a density of 1 × 105, 5 × 104 and 2.5 × 104 cells/ml) was incubated in a 96-well plate at 33°C. Trypanosomes were counted every 24 hr post-inoculation until day 6 using a counting chamber after appropriate dilution by phosphate buffered saline with glucose (PSG). Doubling time (Td) was calculated by counting the cells in the log phase of growth and using the equation: Td=(t2 − t1) × log (2)/log (q2/q1). Two measurements were made: the initial count (q1) at time (t1) and the resultant density following 24 hr incubation (q2, t2) [23, 24].
Optimization of dimethylsufoxide concentration: Fifty µl of different concentrations of dimethylsufoxide (DMSO; Wako Pure Chemical Industries, Ltd., Osaka, Japan) diluted in HMI-9 medium were added into a 96-well plate containing 50 µl of T. congolense at a density of 2 × 105 cells/ml. The plate was incubated for a further 72 hr, and the inhibition rate was calculated. The optimized luciferase assay protocol was then performed (see next section).
Finally optimized HTS assay conditions: After optimization, the final conditions for the assay were established for conducting the tests. Fifty µl of various concentrations of reference compounds in HMI-9 with 0.5% DMSO were added to a Nunc® MicroWell 96-well optical bottom plate (Thermo Fisher Scientific K.K., Yokohama, Japan) containing 50 µl of T. congolense at a density of 2 × 105 cells/ml. The final concentration of the parasite cell used in the assay was 1 × 105 cells/ml, and the DMSO was at a final concentration of 0.25% in 100 µl of HMI-9 per well. The cells were incubated for 72 hr, and subsequently, 50 µl of CellTiter-GloTM Luminescent Cell Viability Assay reagent (Promega Japan, Tokyo, Japan) was added to evaluate intracellular ATP concentration. The plate was shaken for 500 shakes/min by MS3 basic plate shaker (IKA® JAPAN K.K., Osaka, Japan) in 2 min to facilitate cell lysis and release intracellular ATP, and then incubated for another 10 min at room temperature. The plate was read using a GloMax®-Multi+ Detection System plate reader (Promega Japan).
Statistical analysis of samples: The quality of this assay was evaluated by Z’-factor [28] with the following formula: Z’-factor =1−{(3×SD A + 3×SD B)/(mean A − mean B)}; where A=the mean signal of each assay (cell growth detected in wells containing T. congolense in HMI-9 without compounds) and B=the signal of 500 ng/ml of reference compounds to the cells to cause 100% culture death. The coefficient of the determination value (R2) was calculated using GraphPad PRISM 5 (GraphPad Software Inc., CA, U.S.A.).
Determination of reference compounds 50% inhibitory concentration (IC50): Diminazene aceturate (Sigma-Aldrich) and pentamidine (Sigma-Aldrich) were used for estimating drug sensitivity in this assay. Both compounds were stored as 10 mg/ml stock solutions in DMSO. Two-fold drug dilutions were made in triplicate from 500–1.95 ng/ml in HMI-9 with 0.5% DMSO. The IC50 value of each reference compound was calculated by plotting the% inhibition (0% inhibition=the luminescence of trypanosome culture well without any chemicals) against log in GraphPad PRISM 5 software (GraphPad Software Inc.).
RESULTS
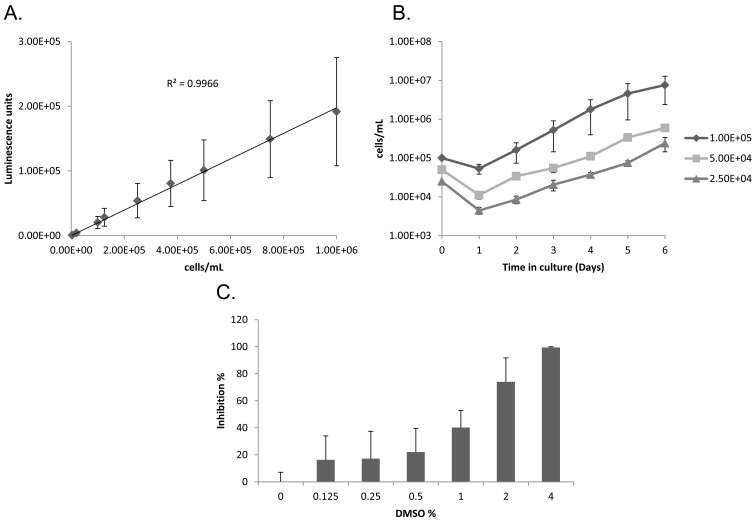
Establishment of HTS assay: For establishment of ATP-based luciferase viability assay for HTS in T. congolense, the maximum cell concentration linearly correlated with luminescence was used to optimize the test. The luminescence signal was well correlated, in a linear fashion, with trypanosome cell concentrations of up to 1 × 106cells/ml in 100 µl per well (R2≥0.99) (Fig. 1A). However, this linear correlation was not seen when a higher concentration was used (Data not shown). The condition of the cell culture that leads to a final concentration of 1 × 106 cells/ml in day 3 was optimized using different concentrations as shown in Fig. 1B. In our in vitro study, a start of 1 × 105cells/mlculture reached nearly 1 × 106 cells/ml after 72 hr incubation (Fig. 1B). This condition was therefore utilized in the present study. Finally, the DMSO concentration needed for the assay was optimized by testing the effect of 0–4% DMSO on trypanosome growth. The results showed that 4% DMSO completely inhibited cell proliferation, whereas concentrations of ≤0.25% inhibited cell proliferation by <20% (Fig. 1C). In addition, using the information on the T. congolense culture day 1 to day 5 log phase proliferation, the doubling time was calculated to be 15 ± 2 hr (Fig. 1B). HMI-9 containing 0.25% DMSO was therefore utilized for the chemical compound dilution.
Fig. 1.
Optimization of 96-well based screening assay conditions. (A) Correlation of cell number and luminescence. Cell concentration of T. congolense was evaluated using the ATP-based luciferase viability assay in 100 µl assay volume per well. Fifty µl of luciferase reagent was added to 50 µl of the cell culture for evaluation. All plots were calculated from 5 independent experiments and shown as the mean luminescence signal ± standard deviation. (B) Optimization of cell culture condition. T. congolense in 1 × 105, 5 × 104 and 2.5 × 104 cells/ml as the initial cell densities was cultured in a 96-well plate for 6 days. Trypanosome number was counted microscopically every day using a cell-counting chamber and plotted onto the graph. Each plot shows the mean number of trypanosomes ± standard deviation. (C) Estimation of trypanocidal effect of dimethylsufoxide (DMSO). Trypanocidal effect of DMSO was estimated by the ATP-based luciferase viability assay in the 96-well plate. Inhibition rate (%) was calculated from 4 independent experiments. Each column shows the mean inhibition rate (%) ± standard deviation.
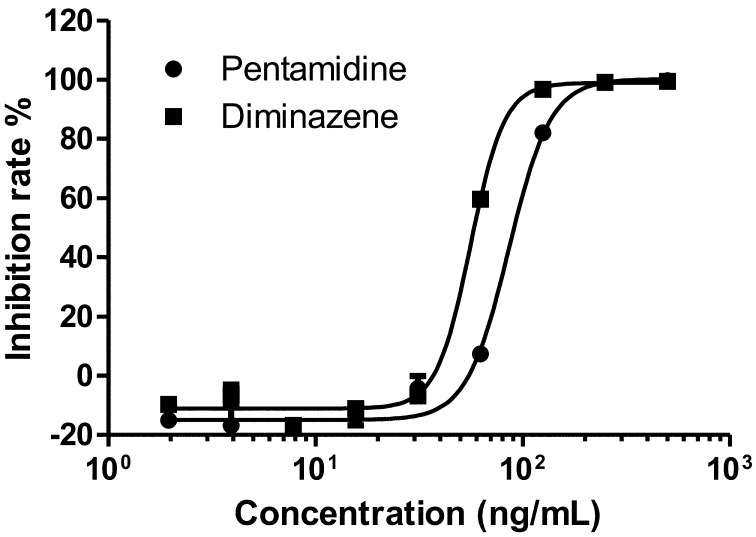
Evaluation of the HTS assay: To evaluate the established assay system, the authors initially evaluated pentamidine and diminazene aceturate, calculating its IC50 values (Fig. 2). The IC50 values of pentamidine and diminazene were calculated as 100.45 ± 26.08 ng/ml (169.48 ± 44.00 nM) and 55.98 ± 13.15 ng/ml (108.65 ± 25.25 nM), respectively (Table 1). In addition, the Z’-factor values of pentamidine and diminazene were calculated as 0.86 ± 0.06 and 0.94 ± 0.03, respectively (Table 1). These 2 compounds were also tested against T. b. brucei GUTat3.1 strain and T. evansi Tansui strain using this assay system (Supplemental Fig. 1). The IC50 values of pentamidine and diminazene in T. b. brucei were 0.64 ± 0.30 ng/ml (1.09 ± 0.50 nM) and 6.75 ± 0.40 ng/ml(13.09 ± 2.94 nM), respectively (Supplemental Table 1). In addition, those of T. evansi were 0.68 ± 0.40 ng/ml (1.15 ± 0.67 nM) and 8.15 ± 2.84 ng/ml (13.09 ± 2.94 nM), respectively (Supplemental Table 1). The results showed that the IC50 values of these compounds for T. b. brucei and T. evansi were 6–100 times lower than those of T. congolense.
Fig. 2.
The IC50 values of pentamidine and diminazene for T. congolense. The IC50 values of diminazene and pentamidine were evaluated for T. congolense using the ATP-based luciferase viability assay. The percentage of inhibition was relative to that of the control well (where no chemical compounds were added). The sigmoidal dose response curve graphs were plotted by GraphPad PRISM5 software.
Table 1. Evaluation of the ATP-based luciferase viability assay for T. congolense using reference compounds.
| Pentamidine | Diminazene | |
|---|---|---|
| IC50 (ng/ml) | 100.45 ± 26.08 | 55.98 ± 13.15 |
| IC50 (nM) | 169.48 ± 44.00 | 108.65 ± 25.51 |
| Z’-factor | 0.86 ± 0.06 | 0.94 ± 0.03 |
| R2 | 0.98 ± 0.02 | 0.99 ± 0.01 |
All values were calculated from 6 independent experiments and shown as the mean value ± standard deviation. IC50: 50% inhibitory concentration, R2: coefficient of determination.
DISCUSSION
In this study, the authors established and evaluated the ATP-based luciferase viability assay in 96-well plate for T. congolense. Because of the intracellular ATP levels detected in viable cells, the luminescence levels and the numbers of trypanosomes were directory proportional (Fig. 1A). To optimize the cell culture with a final concentration of 1 × 106 cells/ml on day 3, T. congolense was cultivated with different starting concentrations in 96-well plate (Fig. 1B). The concentration of T. congolense on day 1 was shown lower than that on day 0 (Fig. 1B). T. congolense BSF proliferates in a cell adhesion dependent manner at the vascular endothelium in vivo or at the bottom of the culture flask in vitro [11, 12]. Therefore, BSF that failed to adhere will eventually die on the first day of culture, resulting in a lower number of trypanosomes on day 1 than on day 0. To evaluate this assay, the Z’-factor was calculated. The Z’-factor values of pentamidine and diminazene for T. congolense were calculated as 0.86 ± 0.06 and 0.94 ± 0.03, respectively (Table 1). In addition, the Z’-factors for T. b. brucei and T. evansi were also >0.5 (Supplemental Table 1). These results suggested that the assay system could be applied to the HTS system for trypanosome, since it showed a remarkable Z’-factor value of 1> Z’-factor ≥0.5 [28].
Previous reports showed that the IC50 values of pentamidine and diminazene for T. b. brucei and T. evansi were 2–100 times lower than our results with T. congolense [10, 20, 23, 24, 26]. These results suggest that pentamidine and diminazene transporter of T. congolense could differ to that of the T. brucei. It was previously reported that T. brucei adenosine transporter 1 (TbAT1), identified as a P2 type aminopurine transporter of T. brucei, is responsible for the diminazene and pentamidine uptake [17]. The orthologue of this gene exists in Trypanozoon trypanosomes, T. brucei sspp., T. evansi and T. equiperdum [3, 4, 15, 27]. TbAT1 orthologous gene has recently been identified in T. congolense and was named as T. congolense adenosine transporter 1 (TcoAT1) [5]. However, sequencing analysis suggested that TcoAT1 was not a true orthologous gene of P2-type purine transporter, TbAT1, but rather that it seemed to be an orthologous gene of P1-type purine transporter, TbNT10 [17]. In addition, it was reported that the IC50 value of pentamidine and diminazene became >200 µM when TcoAT1 was overexpressed in diminazene resistant T. b. brucei (B48 strain) [17]. The IC50 values of T. congolense in our study were more than 1,000 times lower than in the aforementioned report, suggesting that in T. congolense, pentamidine and diminazene uptake likely occurs via an unknown aminopurine transporter.
HTS systems are very well established for HAT and leishmaniosis, where they are utilized for screening chemical compound libraries to facilitate the development of therapeutic medicine. In the case of T. congolense, which causes a neglected tropical parasitic animal disease, this report is useful for the development of a more sensitive assay, which is necessary for establishing HTS. This will therefore offer a great advantage and be an efficient tool for screening and determining novel animal trypanocidal drugs in future studies.
Supplementary Material
Acknowledgments
This study was financially supported by the Japan Society for the Promotion of Science (JSPS), Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Japan International Cooperation Agency (JICA) and the JST/JICA, SATREPS.
REFERENCES
- 1.Baker N., de Koning H. P., Mäser P., Horn D.2013. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 29: 110–118. doi: 10.1016/j.pt.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett M. P., Burchmore R. J., Stich A., Lazzari J. O., Frasch A. C., Cazzulo J. J., Krishna S.2003. The trypanosomiases. Lancet 362: 1469–1480. doi: 10.1016/S0140-6736(03)14694-6 [DOI] [PubMed] [Google Scholar]
- 3.Barrett M. P., Zhang Z. Q., Denise H., Giroud C., Baltz T.1995. A diamidine-resistant Trypanosoma equiperdum clone contains a P2 purine transporter with reduced substrate affinity. Mol. Biochem. Parasitol. 73: 223–229. doi: 10.1016/0166-6851(95)00120-P [DOI] [PubMed] [Google Scholar]
- 4.de Koning H. P., Anderson L. F., Stewart M., Burchmore R. J., Wallace L. J., Barrett M. P.2004. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in african trypanosomes. Antimicrob. Agents Chemother. 48: 1515–1519. doi: 10.1128/AAC.48.5.1515-1519.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delespaux V., Chitanga S., Geysen D., Goethals A., van den Bossche P., Geerts S.2006. SSCP analysis of the P2 purine transporter TcoAT1 gene of Trypanosoma congolense leads to a simple PCR-RFLP test allowing the rapid identification of diminazene resistant stocks. Acta Trop. 100: 96–102. doi: 10.1016/j.actatropica.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 6.Delespaux V., Dinka H., Masumu J., Van den Bossche P., Geerts S.2008. Five-fold increase in Trypanosoma congolense isolates resistant to diminazene aceturate over a seven-year period in Eastern Zambia. Drug Resist. Updat. 11: 205–209. doi: 10.1016/j.drup.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 7.Delespaux V., Geysen D., Van den Bossche P., Geerts S.2008. Molecular tools for the rapid detection of drug resistance in animal trypanosomes. Trends Parasitol. 24: 236–242. doi: 10.1016/j.pt.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Engel J. C., Ang K. K., Chen S., Arkin M. R., McKerrow J. H., Doyle P. S.2010. Image-based high-throughput drug screening targeting the intracellular stage of Trypanosoma cruzi, the agent of Chagas’ disease. Antimicrob. Agents Chemother. 54: 3326–3334. doi: 10.1128/AAC.01777-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geerts S., Holmes P. H., Eisler M. C., Diall O.2001. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 17: 25–28. doi: 10.1016/S1471-4922(00)01827-4 [DOI] [PubMed] [Google Scholar]
- 10.Gillingwater K., Buscher P., Brun R.2007. Establishment of a panel of reference Trypanosoma evansi and Trypanosoma equiperdum strains for drug screening. Vet. Parasitol. 148: 114–121. doi: 10.1016/j.vetpar.2007.05.020 [DOI] [PubMed] [Google Scholar]
- 11.Hemphill A., Ross C. A.1995. Flagellum-mediated adhesion of Trypanosoma congolense to bovine aorta endothelial cells. Parasitol. Res. 81: 412–420. doi: 10.1007/BF00931503 [DOI] [PubMed] [Google Scholar]
- 12.Hirumi H., Hirumi K.1991. In vitro cultivation of Trypanosoma congolense bloodstream forms in the absence of feeder cell layers. Parasitology 102: 225–236. doi: 10.1017/S0031182000062533 [DOI] [PubMed] [Google Scholar]
- 13.Lang T., Goyard S., Lebastard M., Milon G.2005. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell. Microbiol. 7: 383–392. doi: 10.1111/j.1462-5822.2004.00468.x [DOI] [PubMed] [Google Scholar]
- 14.MacLean L., Myburgh E., Rodgers J., Price H. P.2013. Imaging African trypanosomes. Parasite Immunol. 35: 283–294. doi: 10.1111/pim.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matovu E., Stewart M. L., Geiser F., Brun R., Mäser P., Wallace L. J., Burchmore R. J., Enyaru J. C., Barrett M. P., Kaminsky R., Seebeck T., de Koning H. P.2003. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2: 1003–1008. doi: 10.1128/EC.2.5.1003-1008.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulenga C., Mhlanga J. D., Kristensson K., Robertson B.2001. Trypanosoma brucei brucei crosses the blood-brain barrier while tight junction proteins are preserved in a rat chronic disease model. Neuropathol. Appl. Neurobiol. 27: 77–85. doi: 10.1046/j.0305-1846.2001.00306.x [DOI] [PubMed] [Google Scholar]
- 17.Munday J. C., Rojas, López K. E., Eze A. A., Delespaux V., Van Den Abbeele J., Rowan T., Barrett M. P., Morrison L. J. , Koning H. P.2013. Functional expression of TcoAT1 reveals it to be a P1-type nucleoside transporter with no capacity for diminazene uptake. Int. J. Parasitol. Drugs Drug Resist. 3: 69–76. doi: 10.1016/j.ijpddr.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pécoul B.2004. New drugs for neglected diseases: from pipeline to patients. PLoS Med. 1: e6. doi: 10.1371/journal.pmed.0010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinder M., Authie E.1984. The appearance of isometamidium resistant Trypanosoma congolense in West Africa. Acta Trop. 41: 247–252. [PubMed] [Google Scholar]
- 20.Räz B., Iten M., Grether-Bühler Y., Kaminsky R., Brun R.1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 68: 139–147. doi: 10.1016/S0001-706X(97)00079-X [DOI] [PubMed] [Google Scholar]
- 21.Rodgers J., McCabe C., Gettinby G., Bradley B., Condon B., Kennedy P. G.2011. Magnetic resonance imaging to assess blood-brain barrier damage in murine trypanosomiasis. Am. J. Trop. Med. Hyg. 84: 344–350. doi: 10.4269/ajtmh.2011.10-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smilkstein M., Sriwilaijaroen N., Kelly J. X., Wilairat P., Riscoe M.2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48: 1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sykes M. L., Avery V. M.2009. Development of an Alamar Blue viability assay in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Am. J. Trop. Med. Hyg. 81: 665–674. doi: 10.4269/ajtmh.2009.09-0015 [DOI] [PubMed] [Google Scholar]
- 24.Sykes M. L., Avery V. M.2009. A luciferase based viability assay for ATP detection in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Parasit. Vectors 2: 54. doi: 10.1186/1756-3305-2-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka T. Q., Williamson K. C.2011. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol. Biochem. Parasitol. 177: 160–163. doi: 10.1016/j.molbiopara.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Reet N., Pyana P., Rogé S., Claes F., Büscher P.2013. Luminescent multiplex viability assay for Trypanosoma brucei gambiense. Parasit. Vectors 6: 207. doi: 10.1186/1756-3305-6-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witola W. H., Inoue N., Ohashi K., Onuma M.2004. RNA-interference silencing of the adenosine transporter-1 gene in Trypanosoma evansi confers resistance to diminazene aceturate. Exp. Parasitol. 107: 47–57. doi: 10.1016/j.exppara.2004.03.013 [DOI] [PubMed] [Google Scholar]
- 28.Zhang J. H., Chung T. D., Oldenburg K. R.1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4: 67–73. doi: 10.1177/108705719900400206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.