ABSTRACT
The entry mechanisms of Akabane virus (AKAV), Bunyaviridae family, have not yet been determined. In this study, chemical inhibitors were used to analyze endocytic mechanisms during AKAV infection of mammalian cell lines. The analyses using drug treatments followed by quantitative measurement of viral RNA and N protein revealed that AKAV enters non-bovine-derived cell lines (Vero, HmLu-1 and BHK cells) in a manner indicative of clathrin endocytosis. By contrast, AKAV infection in bovine-derived cell lines (LB9.K and MDBK cells) is independent of this pathway. Further analyses indicated that AKAV entry into bovine cell lines involves a non-clathrin, non-caveolae endocytic pathway that is dependent on dynamin. We conclude that although both cell types require a low pH for AKAV penetration, AKAV utilizes alternative entry pathways into mammalian cell lines.
Keywords: Akabane virus, caveolae, clathrin, endocytosis
Akabane virus (AKAV), an animal pathogen of the serogroup Simbu, genus Orthobunyavirus, family Bunyaviridae, is an enveloped virus with a tripartite negative-stranded RNA genome (L, M and S segments). The infection is transmitted by arthropod-borne vector Culicoides spp., and mosquitoes can cause abortion, stillbirth, premature birth and congenital arthrogryposis-hydranencephaly in cattle, sheep and goats, resulting in significant economic losses in the livestock industry [12]. Based on the S RNA segment sequences, AKAV isolates can be classified into 4 genogroups (I-IV), which are widely distributed in Asia, Australia, the Middle East and Africa [4, 40]. Currently, viruses in genogroups I and II cause Akabane disease, especially in Japan, Korea and China [15, 16, 24]. Vaccination has reduced the prevalence of Akabane disease; however, cases still occur in areas in which vaccines are administered [13]. It has been suggested that vaccine failure is due to antigenic variation among AKAV strains [2, 16, 25, 40]. Therefore, the development of an effective vaccine and a novel antiviral strategy are required to control this disease.
The mechanism of virus entry into the host cell is an important target for antiviral drug design. It appears that bunyaviruses use receptor-mediated endocytosis for their entry into cells [8]. After binding with the receptor, uptake of the virus into cells is mediated by an endocytic pathway; most of the viruses in this family utilize clathrin-dependent endocytosis [11, 14, 31, 33], although recent reports have shown that some viruses enter cells via another pathway. For example, Rift Valley fever phlebovirus strain MP-12 enters mammalian cell lines via caveolae-dependent endocytosis [10], and Uukuniemi phlebovirus and Andes hantavirus enter via clathrin-independent endocytosis [19, 30].
Because there is little information on the entry pathway of AKAV, in this study, we first analyzed AKAV entry pathway into mammalian cell lines. Here, we determined that AKAV infection can employ alternative endocytic routes by comparative analyses between non-bovine-derived cell lines and bovine-derived cell lines using chemical inhibitors of different endocytic pathways. These observations increase our understanding of the entry mechanisms of AKAV.
MATERIALS AND METHODS
Cells and viruses: Bovine kidney cells (LB9.K), African green monkey kidney cells (Vero), Madin-Darby bovine kidney cells (MDBK) and hamster lung cells (HmLu-1) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% or 5% fetal calf serum (FCS), at 37°C in a humidified 5% CO2 atmosphere. Baby hamster kidney cells (BHK) were maintained in Eagle’s minimal essential medium (EMEM) supplemented with 5% FCS under the same conditions. AKAV OBE-1 strain [3] and Iriki strain [22] were propagated in HmLu-1 in medium without FCS and titrated in HmLu-1 cells for the plaque assay [26]. Bovine viral diarrhea virus (BVDV) KS86-1 cp strain was propagated as described previously [23].
Reagents and antibodies: Chlorpromazine (CPZ), Nystatin (Nys), methyl-β-cyclodextrin (MβCD), Bafilomycin A1 (BafA1) and Dynasore (Dyn) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Sucrose and ammonium chloride (NH4Cl) were purchased from Wako (Osaka, Japan). Anti-AKAV N mouse monoclonal antibody (5F11) was described previously [2]. Anti-clathrin heavy chain (CHC) rabbit polyclonal antibody (P1663) and anti-caveolin-1 (CAV-1) rabbit monoclonal antibody (D46G3) were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). The secondary antibodies Alexa Flour 488- or 546-labeled goat anti-mouse immunoglobulin G (IgG) and Alexa Flour 546-labeled goat anti-rabbit IgG were purchased from Invitrogen (Carlsbad, CA, U.S.A.). Transferrin conjugated with Texas red (T2875) and cholera toxin B subunit (CTB) conjugated with FITC (C1655) were purchased from Invitrogen and Sigma-Aldrich, respectively.
Drug treatments for the AKAV and BVDV endocytic pathways: Each confluent cell line was treated with CPZ, sucrose, Nys, MβCD, Dyn, NH4Cl and BafA1 for 1 hr at 37°C. For the control, cells were added to medium with or without DMSO for 1 hr at 37°C. AKAV OBE-1, Iriki strain or BVDV at a multiplicity of infection (MOI) of 5 was added to the drug-treated or drug-untreated cell plate and incubated for 1 hr at 4°C, and then, the cells were incubated at 37°C in the presence of drugs until 1.5 hr or 6 hr post-infection (p.i.). Cell membrane-bound virus was removed with 100 µg/mL heparin sodium (MP Biomedicals, Santa Ana, CA, U.S.A.) for 10 min at 37°C. The AKAV antigen was detected by an immunofluorescence assay (IFA) at 1.5 hr p.i., and the AKAV or BVDV internalization was determined by comparing viral RNAs using real-time qRT-PCR at 6 hr p.i. relative to the viral RNAs of drug-untreated virus-infected cells.
Immunofluorescence microscopy assay (IFA): Virus-infected cells with or without drug treatment were fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.1% FCS and 0.3% Triton X-100 and blocked with PBS containing 5% FCS for 30 min. Cells were incubated with primary antibodies for 1 hr, followed by incubation with fluorescent secondary antibodies for 1 hr. After washings, cells were mounted on coverslips with fluorescent mounting medium (Dako, Tokyo, Japan) and visualized using a Carl Zeiss LSM 510 confocal microscope (Carl Zeiss, Tokyo, Japan).
Quantitative real-time RT-PCR (real-time qRT-PCR): Virus-infected cells were washed with phosphate-buffered saline (PBS), scraped and diluted with cell lysis solutions from the SV total RNA Isolation System (Promega, Madison, WI, U.S.A.). Viral RNA was extracted according to the manufacturer’s protocol. cDNA was synthesized from purified RNA using the PrimeScript RT Reagent Kit (Perfect Real time) (Takara, Otsu, Japan) following the manufacturer’s protocol. A quantitative real-time PCR assay was performed using the Thermal Cycler Dice System (Takara) as described previously [41]. The primers used to amplify AKAV mRNA were designed to recognize the AKAV S segment as described previously [35]. The primers used to amplify BVDV mRNA were described previously [38]. The amount of RNA was normalized to the amount of 18S ribosomal RNA (18S rRNA) in each sample. The relative amounts of each mRNA were analyzed using Thermal Cycler Dice Software Ver.1.02 (Takara).
Colocalization of AKAV internalization and endocytic coated vesicle markers: Cells were seeded in 4-well chamber slides. After 24 hr, cells were infected with AKAV at an MOI of 2 for 1 hr at 4°C for binding and then incubated for 2 min at 37°C. Every 2 min, the colocalization of AKAV and endocytic coated vesicle markers was determined by an LSM 510 confocal microscope. Briefly, after unbound virus was removed, cells were washed, fixed, permeabilized and blocked as described above. Cells were double immunostained with mouse anti-AKAV N 1:500 and rabbit anti-CHC or -CAV-1 1:500 for 1 hr at room temperature. Cells were washed 3 times, followed by incubation with Alexa-488 labeled goat anti-mouse IgG1 and Alexa-546 labeled goat anti-rabbit for 1 hr. Then, cells were washed 5 times. Cells were mounted on coverslips with fluorescent mounting medium (Dako) and visualized using a confocal microscope.
Transferrin and cholera toxin subunit B uptake assay: Cells were pretreated with CPZ (10 µg/mL), Nys (50 µg/mL), MβCD (2.5 mM) or Dyn (100 µM), or without drugs (with or without DMSO) for 1 hr at 37°C. Then, transferrin conjugated with Texas red (10 µg/mL) or CTB conjugated with FITC (2 µg/mL) was added, and cells were incubated for 30 min at 37°C. At 30 min p.i., cells were incubated with heparin for 10 min to remove cell membranes and unbound dye, washed with PBS, fixed with 4% paraformaldehyde and observed with a confocal microscope.
Cell viability test: To determine the cytotoxicity of all drugs, a trypan blue exclusion test was used to determine cell viability. Adherent LB9.K cells and HmLu-1 cells were pretreated with drugs at various concentrations for 6 hr at 37°C and then tested as described previously [36]. Approximately 300–500 cells were counted in each treatment. The results were described in percentage (%) values of viable cells.
RESULTS
Drugs did not affect cellular viability: The effect of the inhibitors on cellular viability was examined. More than 90% of treated cells remained viable after 6 hr of treatment with all inhibitors; however, high concentrations of CPZ (20 µg/mL), MβCD (5 mM), Nys (100 µg/mL) and Dyn (150 µM) reduced cell viability to approximately 80% (data not shown). Altogether, the results confirmed that endocytic inhibitors did not produce unfavorable effects in terms of cellular viability.
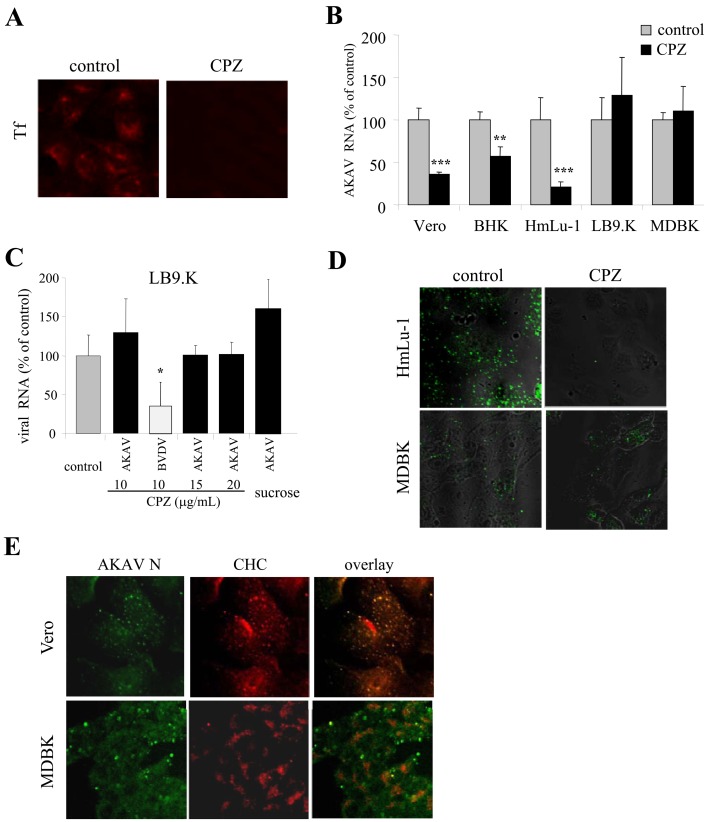
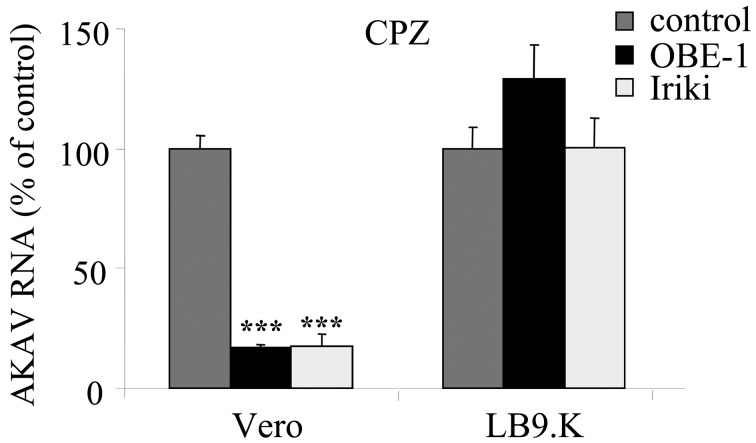
AKAV entry required clathrin-dependent endocytosis in non-bovine-derived cell lines, but not in bovine-derived cell lines: To analyze AKAV entry using clathrin inhibitors, CPZ and sucrose were chosen [9, 39]. We first determined whether CPZ and sucrose are effective at inhibiting the clathrin-dependent endocytic pathway by observing transferrin uptake into cells, as transferrin (Tf) is a ligand known to enter cells by the clathrin-dependent endocytic pathway. The results showed that the uptake of Tf was inhibited in CPZ- (Fig. 1A) and sucrose- (data not shown) treated LB9.K cells. Next, we investigated the effect of CPZ treatment on AKAV entry in different mammalian cell lines. Real-time qRT-PCR results revealed that the AKAV RNA level decreased significantly in non-bovine-derived cell lines (Vero, BHK and HmLu-1 cells) treated with CPZ. By contrast, the viral RNA levels were not altered in bovine-derived cell lines, i.e., LB9.K and MDBK cells (Fig. 1B). We also tested various concentrations of CPZ and 0.45 M sucrose as another clathrin inhibitor in bovine-derived LB9.K cells (Fig. 1C). There were no significant differences in AKAV RNA levels in any tests, whereas LB9.K cells treated with CPZ exhibited significantly decreased BVDV RNA levels. BVDV is known to enter via classical clathrin-dependent endocytosis [18]. The clathrin-dependency of AKAV entry was confirmed by IFA. HmLu-1 and MDBK cells were pretreated with CPZ for 1 hr, followed by infection with AKAV OBE-1 strain, fixed for 1.5 hr p.i. and immunostained with AKAV N. The results showed that N staining was detected in CPZ-treated MDBK cells, while N staining was not detected after CPZ treatment in HmLu-1 cells (Fig. 1D). To further confirm involvement of the clathrin-dependent endocytic pathway in AKAV infection in non-bovine-derived and bovine-derived cell lines, we observed the colocalization of AKAV and clathrin-coated pits using a confocal microscope. AKAV N colocalized with a clathrin heavy chain (CHC) marker in Vero cells. However, the majority of AKAV N did not colocalize with CHC; only a few instances of overlap (~25%) were observed in MDBK cells (Fig. 1E). The similar results were obtained in other non-bovine (HmLu-1) and bovine (LB9.K) cell lines (data not shown). Altogether, these results indicated that AKAV enters non-bovine-derived cell lines through the clathrin-dependent endocytic pathway, while bovine-derived cell lines do not utilize primarily a clathrin-dependent endocytic pathway for AKAV entry.
Fig. 1.
Requirement of clathrin-endocytosis in AKAV entry. (A) LB9.K cells were treated with CPZ or without drug (control) for 1 hr following incubation with transferring (Tf) conjugated with Texas red. (B) Various cell lines were pretreated with CPZ or without drug (control) and infected with AKAV. Intracellular AKAV RNAs at 6 hr post-infection (p. i.) were measured by real-time qRT-PCR. (C) LB9.K cells were pretreated with various concentrations of CPZ or sucrose (0.45M), followed by infection with AKAV or BVDV. Then, AKAV RNAs were quantified. (D) Cells were pretreated with CPZ or without drug (control), followed by AKAV infection. At 1.5 hr p.i., cells were fixed and immunostained for AKAV N protein (green). (E) Localization of AKAV N (green) and clathrin heavy chain (CHC) marker (red) in various cell lines was immunostained. qRT-PCR values to measure AKAV RNAs in drug-treated cells are expressed as percentages relative to drug-untreated control cells. Values represent means and standard deviation (SD) of three independent experiments. Statistical significance is indicated by * (P<0.05), ** (P<0.01) or *** (P<0.005) (Student’s t-test).
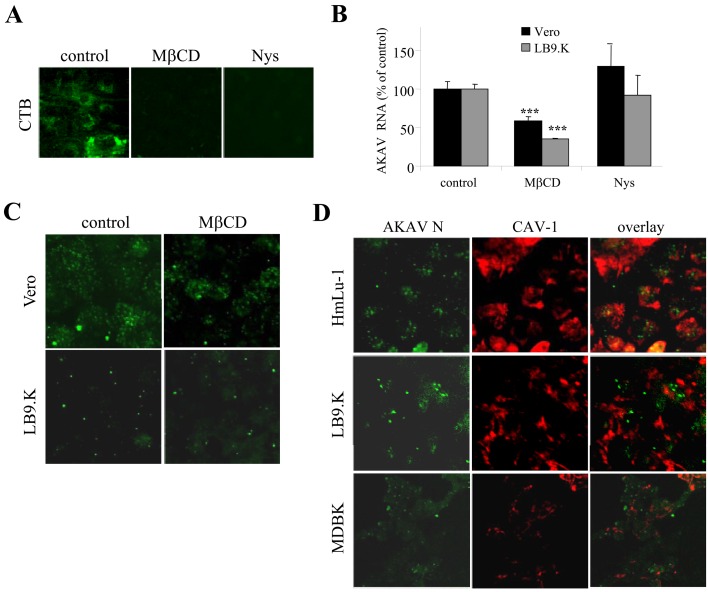
AKAV entry did not require the caveolae/lipid raft endocytic pathway: To explore the clathrin-independent endocytosis of AKAV infection in bovine-derived cells, a caveolae/lipid raft-dependent endocytic pathway was analyzed next. Lipid rafts are associated with the trafficking of cytokines, glycosylphosphatidylinositol (GPI) proteins, bacteria toxins and viruses into cells. Lipid rafts primarily contain cholesterol and sphingolipids [5]. To determine whether AKAV entry in bovine cells involves this pathway, we chose MβCD and Nys as inhibitors [27, 32]. Because BHK cells resist the disruption of lipid rafts by MβCD [1], we used cell lines other than BHK cells in this experiment. The effectiveness of MβCD (2.5 mM) and Nys (50 µg/mL) on the caveolae/lipid raft entry pathway was verified by the complete blockage of a control CTB in LB9.K cells (Fig. 2A). Next, LB9.K and Vero cells were pretreated with the same concentrations of MβCD and Nys for 1 hr and then infected with AKAV OBE-1 strain. At 6 hr p.i., AKAV RNAs were quantified by real-time qRT-PCR. In Nys-treated cells, there was no significant difference in AKAV RNAs, whereas there was a significant decrease in AKAV RNA in MβCD-treated cells compared with untreated control cells (Fig. 2B). However, the IFA result revealed that the internalization of N proteins remained substantially unchanged in MβCD (Fig. 2C), suggesting a limited effect of MβCD-treatment on AKAV entry. Moreover, a caveolae marker (CAV-1) and AKAV N were not colocalized in various cell lines (Fig. 2D). Together, these data indicate that caveolae/lipid rafts are involved in AKAV entry neither in bovine-derived cell lines nor non-bovine-derived cell lines.
Fig. 2.
Requirement of caveolae/lipid raft endocytosis in AKAV entry. (A) Cells were pretreated with MβCD or Nys, or without drugs (control) for 1 hr, followed by incubation with cholera toxin B subunit (CTB) conjugated with FITC. (B) Vero and LB9.K cells were pretreated with MβCD or Nys for 1 hr and infected with AKAV. Cells were incubated in the presence of drugs for 6 hr. qRT-PCR values to measure AKAV RNAs are expressed as percentages relative to untreated virus-infected control cells. Values represent means and SD of three independent experiments. Statistical significance is indicated by *** (P<0.005) (Student’s t-test). (C) Cells were treated with MβCD for 1 hr following AKAV infection in the presence of drug. At 1.5 hr p.i., cells were fixed and immunostained for AKAV N (green). (D) Localization of AKAV N protein (green) and caveolae marker (CAV-1) (red) in various cell was immunostained following AKAV infection at 1.5 hr p. i..
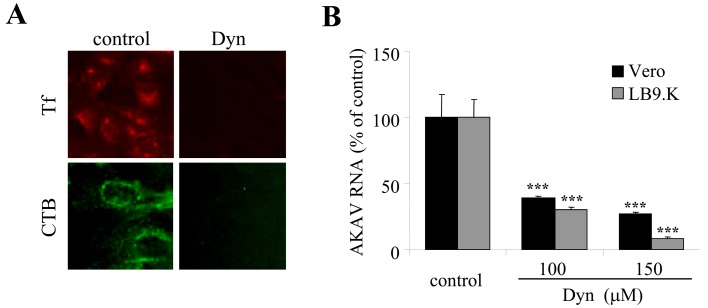
AKAV entry required dynamin as a cellular factor in endocytic pathways: Dynamin, a hydrolase enzyme in the GTPase family, is an essential cellular component for vesicle formation in many endocytic pathways. Dynamin acts during the scission of vesicles from the cell membrane, which is a required step in many endocytic pathways, such as clathrin-, caveolae-, interleukin-2 (IL-2)- and phagocytosis-mediated endocytosis [21]. To determine whether dynamin is required for AKAK entry, Dynasore (Dyn), a GTPase inhibitor that targets dynamin, was used for the assay [20]. Tf and CTB were used as positive controls and were blocked by Dyn treatment in LB9.K cells (Fig. 3A). LB9.K and Vero cells were treated with Dyn (100 and 150 µM) for 1 hr, followed by infection with AKAV OBE-1 strain. At 6 hr p.i., AKAV RNAs were quantified by real-time qRT-PCR and compared to the viral RNAs of untreated virus-infected control cells (treated with DMSO). The results showed that AKAV RNA levels decreased significantly after Dyn treatment of Vero cells and LB9.K cells (Fig. 3B). The results confirm that AKAV entry into non-bovine cell lines requires dynamin as a cellular factor in the clathrin-dependent endocytic pathway, while AKAV entry into bovine cell lines requires dynamin as a cellular factor in its non-clathrin, non-caveolae endocytic pathway.
Fig. 3.
Requirement of dynamin in AKAV entry as a cellular factor in endocytic pathways. (A) LB9.K cells were pretreated with Dyn or without drug (control) for 1 hr followed by incubation with transferrin (Tf) conjugated with Texas red (red) or CTB conjugated with FITC (green). (B) LB9.K and Vero cells were pretreated with Dyn. After 1 hr of treatment, cells were infected with AKAV in the presence of drug for 6 hr. Real-time qRT-PCR values to measure intracellular AKAV RNAs are expressed as percentages relative to untreated virus-infected control cells. Values represent the mean and SD of three independent experiments. Statistical significance is indicated by *** (P<0.005) (Student’s t-test).
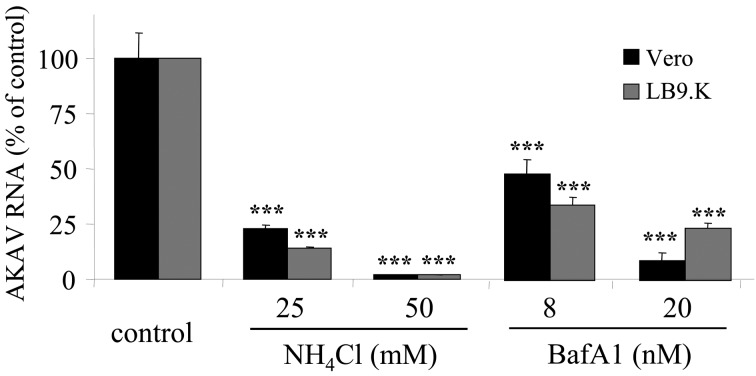
AKAV penetration requires low pH: To determine whether a low pH is required for AKAV entry, NH4Cl and BafA1 were used in this experiment. LB9.K and Vero cells were treated with NH4Cl and BafA1 for 1 hr, followed by infection with AKAV OBE-1 strain. AKAV RNAs were significantly decreased by treatment with both reagents after 6 hr p.i. (Fig. 4). Furthermore, AKAV N was not detected by the immunostaining method in the treatment groups (data not shown). These experiments suggest that AKAV requires a low pH environment to enter both cell lines.
Fig. 4.
Low pH requirement of AKAV penetration. LB9.K and Vero cells were pretreated with NH4Cl or BafA1. After 1 hr of treatment, cells were infected with AKAV and were incubated in the presence of drugs for 6 hr. Real-time qRT-PCR values to measure intracellular AKAV RNAs are expressed as percentages relative to untreated virus-infected cells. Values represent the mean and SD of three independent experiments. Statistical significance is indicated by *** (P<0.005) (Student’s t-test).
AKAV genogroup strains utilize a similar endocytic pathway: To determine whether the pathogenic AKAV genogroups I and II employ different endocytic pathways, Vero cells and LB9.K cells were pretreated with CPZ, followed by infection with either the Iriki strain, the prototype strain of genogroup I, or OBE-1, the prototype strain of genogroup II. No significant difference was observed between these 2 strains, suggesting that both genogroup strains utilize the same endocytic pathway, that is, a clathrin-dependent pathway in HmLu-1 cells and a clathrin-independent pathway in LB9.K cells (Fig. 5).
Fig. 5.
Requirement of clathrin-endocytosis in entry of AKAV strains. LB9.K and Vero cells were pretreated with CPZ for 1 hr, followed by infection with either AKAV OBE-1 (genogroup II) or Iriki (genogroup I) strains in the presence or absence (control) of drug for 6 hr. Real-time qRT-PCR values to measure intracellular AKAV RNAs are expressed as percentages relative to untreated virus-infected cells. Values represent the mean and SD of three independent experiments. Statistical significance is indicated by *** (P<0.005) (Student’s t-test).
DISCUSSION
In this study, we investigated the entry of AKAV via endocytic pathways in mammalian cell lines. Currently, many endocytic pathways are known to be employed by cells, depending on the sizes of the cargo and the various cellular components involved, including clathrin, caveolae, macropinocytosis, lipid rafts, IL-2, GEEC, flotillin, Arf6, phagocytosis and unidentified endocytic pathways [21]. Because of the diversity of endocytic pathways, we focused on the most commonly observed pathways for virus entry, which are the clathrin-dependent and caveolae-dependent endocytic pathways. We found that AKAV entry involves alternative endocytic pathways in different mammalian cell lines. The studies showed that AKAV entry into non-bovine-derived cell lines is regulated via the classical clathrin-dependent endocytic pathway, whereas its entry into bovine cell lines involves a non-clathrin, non-caveolae endocytic pathway (s) that require dynamin.
Alternative entry pathways have been reported in viruses that have a broad cell- and host-tropism, such as influenza and dengue viruses. The reason why these viruses, as well as AKAV, use different pathways is still unknown. One of the possible causes may come from the different receptor usages of viruses that depend on cell-specific properties of infected hosts. The different entry pathways may affect the viral infectivity, and for AKAV, all cell lines used in this experiment are known to possess sensitivity to AKAV infection [17]. Although AKAV naturally infects cattle, sheep and goats, suckling mice and hamster lung cell lines, such as HmLu-1 cells, are commonly used to propagate AKAV in laboratories because AKAV replicates more rapidly in these cell lines than in bovine cells [7]. It is known that viruses are delivered via clathrin-dependent endocytosis from the cell surface to the early endosome only in 1–2 min [21]. This timeframe might explain, in part, why AKAV replicates rapidly in hamster lung cell lines. To better understand the infectivity of this neurovirulent and mosquito-borne virus, we will investigate the AKAV entry mechanism into neuronal and insect cells in further experiments.
It is clear from our data that AKAV, like other bunyaviruses, requires low pH for its entry and penetration [8, 14]. Low pH plays a role in virus entry and endosome formation. Many receptors and viral glycoproteins require low pH to function via conformational changes. Alteration of the pH has been shown to affect the biochemical properties of receptors and viruses. By endosome formation after viral uptake into cells, the virus particle will fuse with the endosomal membrane and gain access to a low pH environment, which is required for the release of the viral genome into the cytosol. Here, we found that AKAV entry involves multiple endocytic pathways, but uses the same endosome vesicle for uncoating. How bunyavirus nucleocapsids are released from endosomal compartments still remains unclear. Colocalization studies of GTPase and viruses reveals that Uukuniemi phelbovirus and Oropouche orthobunyavirus nucleocapsids are released from the late endosomal compartment less than 1 hr post-infection [19, 31], whereas that of La Crosse orthobunyavirus is released from the early endosomal compartment [11]. Interestingly, because the late endosomal compartment contains cholesterol-rich microdomains [34], our evidence that the removal of cholesterol by MβCD decreases AKAV from 1.5 hr to 6 hr p.i. might indicate that AKAV is released from the late endosomal compartment. Precise elucidation of this AKAV penetration mechanism may be useful for the design of antiviral drugs, as has been the case for adamantanes, which acts against influenza virus [28], or U186666A, a late endosomal inhibitor candidate, which acts against dengue virus [29].
The pathogenesis of AKAV includes a wide variety of clinical symptoms. AKAV genogroup II strains have been reported to affect only pregnant ruminants causing reproductive and nervous system symptoms, while AKAV genogroup I strains cause not only reproductive symptoms in pregnant ruminants but also encephalitis in calves and adults [37]. A previous reverse genetic study of AKAV revealed that the M segment of AKAV is responsible for the different pathogenicities between 2 genogroups (Sugiura, personal communication). Like other orthobunyaviruses, the M segment of AKAV encodes two envelope glycoproteins (Gc and Gn) and the nonstructural protein NSm [6]. AKAV Gc and Gn have been reported to be involved in cell receptor binding, while the function of AKAV NSm remains unknown. In this study, we did not observe a difference in entry pathways between the 2 genogroup strains under our experimental settings, providing a possibility that the different pathogenicities of the AKAV strains may come from the functions of the NSm protein, not the G proteins. Identification of alternative entry pathways for AKAV will help us to understand molecular dissection of this virus and to develop treatments for its infection.
Acknowledgments
We would like to thank Yassir Mahgoub Mohamed for providing BVDV KS86-1 cp strain. This work was supported in part by a Research and Development Project for Application in Promoting new Policies in Agriculture, Forestry and Fisheries grant from the Ministry of Agriculture, Forestry and Fisheries.
REFERENCES
- 1.Abrami L., van Der Goot F. G.1999. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J. Cell Biol. 147: 175–184. doi: 10.1083/jcb.147.1.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi H., Inaba Y.1997. Antigenic diversity of Akabane virus detected by monoclonal antibodies. Virus Res. 47: 187–196. doi: 10.1016/S0168-1702(96)01415-3 [DOI] [PubMed] [Google Scholar]
- 3.Akashi H., Kaku Y., Kong X., Pang H.1997. Antigenic and genetic comparisons of Japanese and Australian Simbu serogroup viruses: evidence for the recovery of natural virus reassortants. Virus Res. 50: 205–213. doi: 10.1016/S0168-1702(97)00071-3 [DOI] [PubMed] [Google Scholar]
- 4.Akashi H., Kaku Y., Kong X. G., Pang H.1997. Sequence determination and phylogenetic analysis of the Akabane bunyavirus S RNA genome segment. J. Gen. Virol. 78: 2847–2851. [DOI] [PubMed] [Google Scholar]
- 5.Duncan M. J., Shin J. S., Abraham S. N.2002. Microbial entry through caveolae: variations on a theme. Cell. Microbiol. 4: 783–791. doi: 10.1046/j.1462-5822.2002.00230.x [DOI] [PubMed] [Google Scholar]
- 6.Elliott R. M.1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3: 572–577. [PMC free article] [PubMed] [Google Scholar]
- 7.Eto N., Yamada K., Nagamine K., Haramaki K., Shirahata S., Murakami H.1991. Multiplication of bovine viruses in hamster lung HmLu-1 cells cultured in protein-free medium. Agric. Biol. Chem. 55: 1175–1177. doi: 10.1271/bbb1961.55.1175 [DOI] [PubMed] [Google Scholar]
- 8.Hacker J. K., Hardy J. L.1997. Adsorptive endocytosis of California encephalitis virus into mosquito and mammalian cells: a role for G1. Virology 235: 40–47. doi: 10.1006/viro.1997.8675 [DOI] [PubMed] [Google Scholar]
- 9.Hansen S. H., Sandvig K., van Deurs B.1993. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121: 61–72. doi: 10.1083/jcb.121.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmon B., Schudel B. R., Maar D., Kozina C., Ikegami T., Tseng C. T., Negrete O. A.2012. Rift Valley fever virus strain MP-12 enters mammalian host cells via caveola-mediated endocytosis. J. Virol. 86: 12954–12970. doi: 10.1128/JVI.02242-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollidge B. S., Nedelsky N. B., Salzano M. V., Fraser J. W., González-Scarano F., Soldan S. S.2012. Orthobunyavirus entry into neurons and other mammalian cells occurs via clathrin-mediated endocytosis and requires trafficking into early endosomes. J. Virol. 86: 7988–8001. doi: 10.1128/JVI.00140-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inaba Y., Kurogi H., Omori T.1975. Akabane disease: epizootic abortion, premature birth, stillbirth and congenital arthrogryposis-hydranencephaly in cattle, sheep and goats caused by Akabane virus. Aust. Vet. J. 51: 584–585. doi: 10.1111/j.1751-0813.1975.tb09397.x [DOI] [PubMed] [Google Scholar]
- 13.Inaba Y., Matsumoto M.1990. Akabane virus. pp.467–480. In: Virus Infections of Ruminants (Dinter, Z. and Morein, B. eds.), Elsevier, Amsterdam. [Google Scholar]
- 14.Jin M., Park J., Lee S., Park B., Shin J., Song K. J., Ahn T. I., Hwang S. Y., Ahn B. Y., Ahn K.2002. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 294: 60–69. doi: 10.1006/viro.2001.1303 [DOI] [PubMed] [Google Scholar]
- 15.Jun Q., Qingling M., Zaichao Z., Kuojun C., Jingsheng Z., Minxing M., Chuangfu C.2012. A serological survey of Akabane virus infection in cattle and sheep in northwest China. Trop. Anim. Health Prod. 44: 1817–1820. doi: 10.1007/s11250-012-0168-3 [DOI] [PubMed] [Google Scholar]
- 16.Kamata H., Inai K., Maeda K., Nishimura T., Arita S., Tsuda T., Sato M.2009. Encephalomyelitis of cattle caused by Akabane virus in southern Japan in 2006. J. Comp. Pathol. 140: 187–193. doi: 10.1016/j.jcpa.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 17.Kurogi H., Inaba Y., Takahashi E., Sato K., Omori T., Miura Y., Goto Y., Fujiwara Y., Hatano Y., Kodama K., Fukuyama S., Sasaki N., Matumoto M.1976. Epizootic congenital arthrogryposis-hydranencephaly syndrome in cattle: isolation of Akabane virus from affected fetuses. Arch. Virol. 51: 67–74. doi: 10.1007/BF01317835 [DOI] [PubMed] [Google Scholar]
- 18.Lecot S., Belouzard S., Dubuisson J., Rouille Y.2005. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 79: 10826–10829. doi: 10.1128/JVI.79.16.10826-10829.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozach P. Y., Mancini R., Bitto D., Meier R., Oestereich L., Overby A. K., Pettersson R. F., Helenius A.2010. Entry of bunyaviruses into mammalian cells. Cell Host Microbe 7: 488–499. doi: 10.1016/j.chom.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T.2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10: 839–850. doi: 10.1016/j.devcel.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 21.Mercer J., Schelhaas M., Helenius A.2010. Virus entry by endocytosis. Annu. Rev. Biochem. 79: 803–833. doi: 10.1146/annurev-biochem-060208-104626 [DOI] [PubMed] [Google Scholar]
- 22.Miyazato S., Miura Y., Hase M., Kubo M., Goto Y., Kono Y.1989. Encephalitis of cattle caused by Iriki isolate, a new strain belonging to Akabane virus. Nippon Juigaku Zasshi 51: 128–136. doi: 10.1292/jvms1939.51.128 [DOI] [PubMed] [Google Scholar]
- 23.Nagai M., Sakoda Y., Mori M., Hayashi M., Kida H., Akashi H.2003. Insertion of cellular sequence and RNA recombination in the structural protein coding region of cytopathogenic bovine viral diarrhoea virus. J. Gen. Virol. 84: 447–452. doi: 10.1099/vir.0.18773-0 [DOI] [PubMed] [Google Scholar]
- 24.Oem J. K., Yoon H. J., Kim H. R., Roh I. S., Lee K. H., Lee O. S., Bae Y. C.2012. Genetic and pathogenic characterization of Akabane viruses isolated from cattle with encephalomyelitis in Korea. Vet. Microbiol. 158: 259–266. doi: 10.1016/j.vetmic.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 25.Ogawa Y., Fukutomi T., Sugiura K., Sugiura K., Kato K., Tohya Y., Akashi H.2007. Comparison of Akabane virus isolated from sentinel cattle in Japan. Vet. Microbiol. 124: 16–24. doi: 10.1016/j.vetmic.2007.03.020 [DOI] [PubMed] [Google Scholar]
- 26.Ogawa Y., Sugiura K., Kato K., Tohya Y., Akashi H.2007. Rescue of Akabane virus (family Bunyaviridae) entirely from cloned cDNAs by using RNA polymerase I. J. Gen. Virol. 88: 3385–3390. doi: 10.1099/vir.0.83173-0 [DOI] [PubMed] [Google Scholar]
- 27.Ohtani Y., Irie T., Uekama K., Fukunaga K., Pitha J.1989. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186: 17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x [DOI] [PubMed] [Google Scholar]
- 28.Oxford J. S., Schild G. C.1967. Inhibition of the growth of influenza and rubella viruses by amines and ammonium salts. Br. J. Exp. Pathol. 48: 235–243. [PMC free article] [PubMed] [Google Scholar]
- 29.Poh M. K., Shui G., Xie X., Shi P. Y., Wenk M. R., Gu F.2012. U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral Res. 93: 191–198. doi: 10.1016/j.antiviral.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 30.Ramanathan H. N., Jonsson C. B.2008. New and Old World hantaviruses differentially utilize host cytoskeletal components during their life cycles. Virology 374: 138–150. doi: 10.1016/j.virol.2007.12.030 [DOI] [PubMed] [Google Scholar]
- 31.Santos R. I., Rodrigues A. H., Silva M. L., Mortara R. A., Rossi M. A., Jamur M. C., Oliver C., Arruda E.2008. Oropouche virus entry into HeLa cells involves clathrin and requires endosomal acidification. Virus Res. 138: 139–143. doi: 10.1016/j.virusres.2008.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnitzer J. E., Oh P., Pinney E., Allard J.1994. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127: 1217–1232. doi: 10.1083/jcb.127.5.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon M., Johansson C., Mirazimi A.2009. Crimean-Congo hemorrhagic fever virus entry and replication is clathrin-, pH- and cholesterol-dependent. J. Gen. Virol. 90: 210–215. doi: 10.1099/vir.0.006387-0 [DOI] [PubMed] [Google Scholar]
- 34.Sobo K., Chevallier J., Parton R. G., Gruenberg J., van der Goot F. G.2007. Diversity of raft-like domains in late endosomes. PLoS ONE 2: e391. doi: 10.1371/journal.pone.0000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stram Y., Kuznetzova L., Guini M., Rogel A., Meirom R., Chai D., Yadin H., Brenner J.2004. Detection and quantitation of akabane and aino viruses by multiplex real-time reverse-transcriptase PCR. J. Virol. Methods 116: 147–154. doi: 10.1016/j.jviromet.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 36.Strober W.2001. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. (Appendix): 3B. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K., Murakami T., Sueyoshi M., Tsuda T., Inai K., Acorda J. A., Yamaguchi R., Tateyama S.2000. Detection of Akabane viral antigens in spontaneous lymphohistiocytic encephalomyelitis in cattle. J. Vet. Diagn. Invest. 12: 518–524. doi: 10.1177/104063870001200605 [DOI] [PubMed] [Google Scholar]
- 38.Vilcek S., Herring A. J., Herring J. A., Nettleton P. F., Lowings J. P., Paton D. J.1994. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 136: 309–323.3. doi: 10.1007/BF01321060 [DOI] [PubMed] [Google Scholar]
- 39.Wang L. H., Rothberg K. G., Anderson R. G.1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123: 1107–1117. doi: 10.1083/jcb.123.5.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamakawa M., Yanase T., Kato T., Tsuda T.2006. Chronological and geographical variations in the small RNA segment of the teratogenic Akabane virus. Virus Res. 121: 84–92. doi: 10.1016/j.virusres.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 41.Yamane D., Kato K., Tohya Y., Akashi H.2008. The relationship between the viral RNA level and upregulation of innate immunity in spleen of cattle persistently infected with bovine viral diarrhea virus. Vet. Microbiol. 129: 69–79. doi: 10.1016/j.vetmic.2007.11.004 [DOI] [PubMed] [Google Scholar]