ABSTRACT
Scratching and skin barrier dysfunctions are pivotal features and therapeutic targets of atopic dermatitis (AD); however, time-of-day-dependent variations of these characteristics remain unclear. NC/Tnd mice have been shown to exhibit severe scratching behavior and skin barrier disruption together with the development of spontaneous atopic dermatitis when they are raised under air-uncontrolled environment. In the present study, time-of-day-dependent variations of scratching behavior and transepidermal water loss (TEWL) were evaluated in NC/Tnd mice that developed moderate to severe AD. Analysis of the mice for 24 hr revealed that scratching frequency and duration were increased from in the afternoon to the nocturnal period when locomotor activity was low, and scratching behavior was decreased in the morning. The highest scratching frequency and duration were 3.8- and 4.1-fold increases in the lowest scratching frequency and duration, respectively. In addition, TEWL on the dorsal skin lesion was decreased in the diurnal period, while that was increased in the nocturnal period. The highest TEWL was a 1.3-fold increase in the lowest TEWL. Significant daily variations were detected in scratching frequency and duration and TEWL. These results indicate that NC/Tnd mice are an appropriate mouse model to investigate time-of-day-dependent variations of scratching behavior and skin barrier dysfunctions associated with AD.
Keywords: animal model, atopic dermatitis, itch, time-of-day-dependent variations, transepidermal water loss
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disorder that affects approximately 10–20% of children and 1–3% of adults [4]. A typical clinical sign in patients with AD is scratching induced by itch [3]. Chronic and intensive scratching elicits inflammation in the skin, releasing a variety of inflammatory mediators and neuromediators from immune cells and keratinocytes. These mediators can activate sensory nerves in the skin, leading to further itch. The process of “itch-scratch cycle” has been shown to induce and exacerbate clinical signs and symptoms of AD [7]. Thus, itch-induced scratching has been considered a crucial clinical sign that can be a therapeutic target in AD.
NC/Tnd mice exhibit AD-like skin lesions and severe scratching behavior spontaneously when they are raised under air-unregulated conventional circumstances, but not specific pathogen free conditions [5, 8]. As clinical signs and symptoms develop in NC/Tnd mice, many inflammatory cells, including lymphocytes, eosinophils and mast cells, infiltrate into the skin and total serum IgE increases [5]. Furthermore, skin barrier functions in NC/Tnd mice were shown to be impaired, because of the decreased ceramide contents in the skin [1]. These features found in NC/Tnd mice are quite similar to those in human patients with AD; thus, NC/Tnd mice have been widely used as a mouse model for human AD.
An increase in itch-induced scratching is commonly observed during a nocturnal period in human patients with AD [2, 9]. However, time-of-day-dependent variations of scratching have not been fully characterized in AD. In the present study, therefore, we evaluated whether NC/Tnd mice showed time-of-day-dependent variations of scratching behavior and skin barrier functions.
MATERIALS AND METHODS
Mice: NC/Tnd mice, an inbred subline of NC/Nga mice, maintained by inbreeding at Tokyo University of Agriculture and Technology [8] were used in this study. NC/Tnd mice were kept in clear acryl cages under air-uncontrolled conventional conditions and fed with regular chow and water ad libitum. Temperature and humidity of an animal room for mice were maintained at 24 ± 1°C and 35 ± 10%, respectively. Lights were turned on at 07:00 hr and off at 19:00 hr automatically. All procedures were approved by the Institutional Animal Care and Use Committee in Tokyo University of Agriculture and Technology.
Clinical skin severity score: Clinical skin severity of dermatitis in 34 NC/Tnd mice (male, n=24; female, n=10) between 6 and 24 weeks of age was scored according to the previously established method [5]. Five different clinical features of AD-like lesions (itch, erythema/hemorrhage, edema, excoriation/erosion and scaling/dryness) were graded as 0 (none), 1 (mild), 2 (moderate) and 3 (severe), with a total score being 15.
Analysis of scratching behavior: Scratching frequency and duration of mice were recorded and analyzed by using SCLABA–Real system (Noveltec Inc., Kobe, Japan) [8]. In brief, mice without any marking were put into the acryl cage and adapted to the condition for at least 3 hr before measurement in the same animal room for keeping mice. Scratching behavior of four mice was simultaneously recorded with a high-speed digital camera from the top of the cage. Since near-infrared light was utilized in SCLABA-Real system, scratching behavior of mice could be detected under the dark condition. The frequency and duration of scratching behavior were then quantified with SCLABA-Real software on the basis of the algorithm that was specific for scratching behavior in mice. To evaluate correlation between clinical skin severity score and scratching behavior, scratching frequency and duration were recorded for 1 hr at 19:00 hr in the 34 NC/Tnd mice described above. To measure time-of-day-dependent variations of scratching behavior, scratching frequency and duration of 36 NC/Tnd mice (male, n=16; female, n=20) with moderate to severe dermatitis between 12 and 24 weeks of age were recorded for 24 hr. To avoid dehydration during 24 hr measurement, each mouse was given agar that was usually used for transport of mice (transport agar, Oriental Yeast Co., Ltd., Tokyo, Japan).
Analysis of locomotor activity: SCLABA-Real system can measure scratching behavior and moving distance of mice inside the cage at the same time. To analyze locomotor activity of NC/Tnd mice during 24 hr, the moving distance of the mice was also calculated with SCLABA-Real software.
Measurement of transepidermal water loss (TEWL): TEWL was measured on the dorsal skin lesion of 10 NC/Tnd mice (male, n=6; female, n=4) with moderate to severe dermatitis between 12 and 24 weeks of age every 2 hr during 24 hr by using a tewameter® TM300 (Courage + Khazaka Electronic GmbH, Cologne, Germany). TEWL was assessed three times under anesthesia with isoflurane inhalation, and the average value of three measurements was regarded as the data at each time point.
Statistical analysis: Correlation between scratching frequency and clinical skin severity score and that between scratching duration and clinical score were examined by Spearman’s rank correlation coefficient, as data were not normally distributed (Shapiro-Wilk test, P<0.05). Because variances of scratching frequency, duration and moving distance during 24 hr were not equal (Levene test, P<0.05), time-of-day-dependent differences of these parameters were analyzed by Friedman test. TEWL value during 24 hr was equal variance (Levene test, P>0.05); therefore, a time-of-day-dependent difference of TEWL was tested by one-way repeated measures ANOVA. All the data were expressed as the mean ± SEM. Statistical analysis was performed by Ekuseru-Toukei 2008 software (Social Survey Research Information Co., Ltd., Tokyo, Japan) and JMP 10.0.2 software (SAS institute Inc., Cary, NC, U.S.A.). P<0.05 was considered statistically significant.
RESULTS
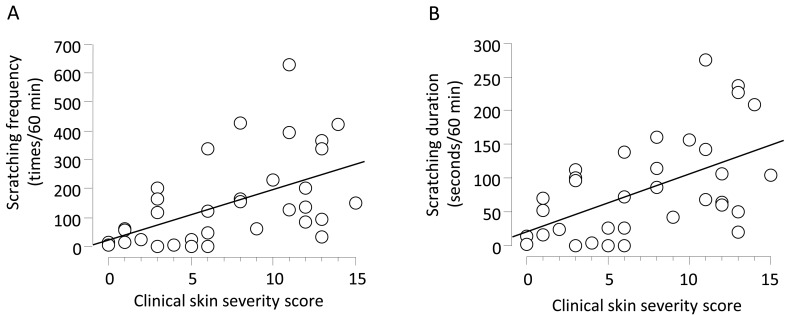
Correlations between scratching behavior and AD: To determine whether NC/Tnd mice showed scratching behavior along with development of AD, we examined correlations between scratching behavior and clinical skin severity score. Analysis by SCLABA-real system revealed that scratching frequency and duration increased as clinical skin severity deteriorated in NC/Tnd mice (Fig. 1A and 1B). Significant positive correlations were detected between scratching frequency and clinical score (spearman’s ρ: 0.5632, P<0.01) and between scratching duration and clinical score (spearman’s ρ: 0.5114, P<0.01). These results indicated that NC/Tnd mice were an adequate mouse model to examine scratching behavior in AD.
Fig. 1.
Correlations between clinical skin severity score and scratching frequency (a) or scratching duration (b). Scratching frequency and duration of 34 NC/Tnd mice (male, n=24; female, n=10) between 6 and 24 weeks of age were recorded for 1 hr at 19:00 hr. Clinical skin severity was scored before measurement of scratching behavior. Linear regression line is shown.
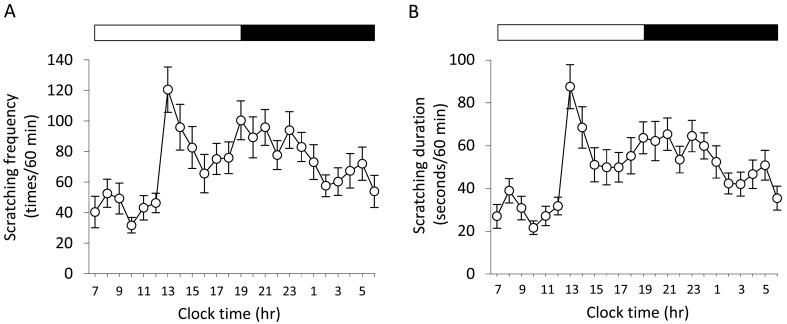
Analysis of scratching behavior for 24 hr: To evaluate daily variations of scratching behavior, we analyzed scratching frequency and duration in NC/Tnd mice for 24 hr. As shown in Fig. 2A and 2B, scratching frequency and duration were increased from in the afternoon to the nocturnal period, and those were decreased in the morning. Time-of-day-dependent variations were detected in scratching frequency and duration (P<0.05). The highest scratching frequency (13:00 hr) and duration (13:00 hr) were 3.8- and 4.1-fold increases in the lowest scratching frequency (10:00 hr) and duration (10:00 hr), respectively (Fig. 2).
Fig. 2.
Time-of-day-dependent variations of scratching behavior in NC/Tnd mice. Scratching frequency (a) and duration (b) of 36 NC/Tnd mice (male, n=16; female, n=20) with moderate to severe dermatitis between 12 and 24 weeks of age were recorded for 24 hr. The white and black bars above the graphs indicate the light and dark periods, respectively. Data represent the mean of 36 mice ± SEM. Friedman test detected significant difference of data among the different times in scratching frequency and duration (P<0.05).
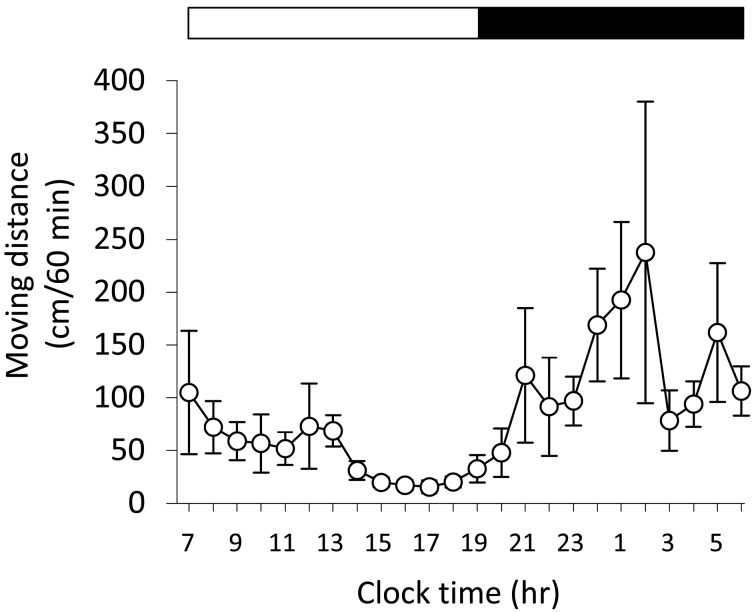
Analysis of locomotor activity for 24 hr: To investigate the locomotor activity of NC/Tnd mice with moderate to severe AD that showed scratching behavior, we assessed moving distance of the mice for 24 hr by SCLABA-Real system at the same time of scratching behavior analysis. As revealed in Fig. 3, moving distance was short in the diurnal period; that was long in the nocturnal period. A time-of-day-dependent difference was detected in moving distance (P<0.05). Similar to other strains of mice, NC/Tnd mice that developed moderate to severe AD were shown to be active during the nocturnal period.
Fig. 3.
A time-of-day-dependent variation of locomotor activity in NC/Tnd mice. As locomotor activity, moving distance of 36 NC/Tnd mice (male, n=16; female, n=20) with moderate to severe dermatitis between 12 and 24 weeks of age were recorded for 24 hr at the same time of scratching behavior analysis. The white and black bars above the graph indicate the light and dark periods, respectively. Data represent the mean of 36 mice ± SEM. Friedman test detected significant difference of data among the different times (P<0.05).
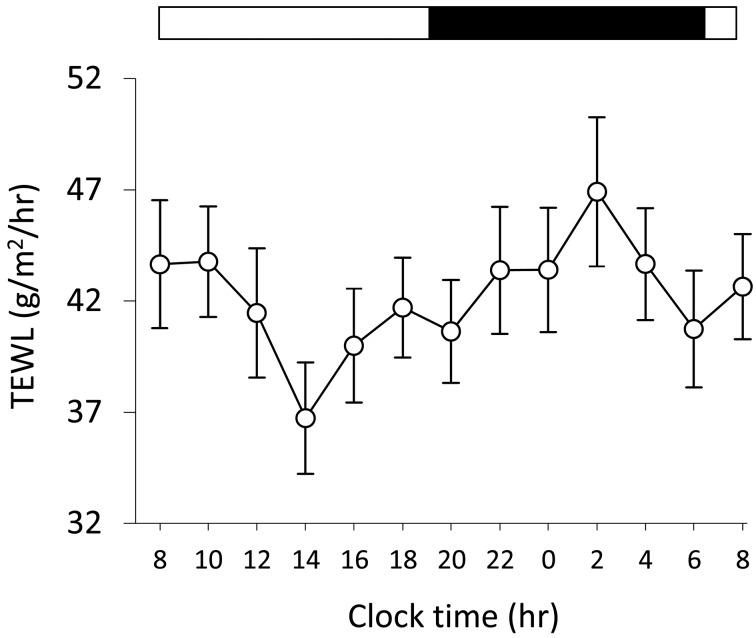
Analysis of TEWL for 24 hr: TEWL is one of the indices of skin barrier functions; an increase in TEWL reflects skin barrier disruption. To assess a time-of-day-dependent variation of skin barrier functions, we measured TEWL in NC/Tnd mice for 24 hr. TEWL value on the dorsal skin lesion was decreased in the diurnal period, while that was increased in the nocturnal period (Fig. 4). A time-of-day-dependent difference was demonstrated in TEWL (P<0.05). The highest TEWL (2:00) was a 1.3-fold increase in the lowest TEWL (14:00) (Fig. 4).
Fig. 4.
A time-of-day-dependent variation of transepidermal water loss (TEWL) in NC/Tnd mice. TEWL on the dorsal skin lesion of 10 NC/Tnd mice (male, n=6; female, n=4) with moderate to severe dermatitis between 12 and 24 weeks of age were recorded every 2 hr for 24 hr. The white and black bars above the graph indicate the light and dark periods, respectively. Data represent the mean of 10 mice ± SEM. One-way repeated measures ANOVA detected significant difference of data among the different times (P<0.05).
DISCUSSION
The present study revealed significant time-of-day-dependent variations of scratching behavior in NC/Tnd mice that developed moderate to severe AD. Scratching frequency and duration were increased from in the afternoon to the nocturnal period when locomotor activity of the mice was decreased. SCLABA–Real system can count scratching frequency and duration of mice without any treatment and marking by using near-infrared light that detects behavior and movement of mice even under the dark period [8]. The system also measures both scratching behavior and moving distance of mice during a certain period, simultaneously. It is therefore conceivable that scratching behavior and locomotor activity of NC/Tnd were assessed under very natural conditions.
Skin barrier dysfunction is one of the dermatological characteristics of AD [3]. The current study demonstrated a significant daily variation of TEWL in the damaged skin of NC/Tnd mice that developed moderate to severe AD. Scratching is closely associated with skin barrier dysfunctions in AD; therefore, it might be suggested that scratching behavior could have induced an increase in TEWL in NC/Tnd mice. Another possible assumption is that skin barrier disruption might have enhanced scratching behavior, since the damaged skin barrier has been considered as a factor leading to itch sensation [3]. Further studies are warranted to investigate the time-of-day-dependent variations between scratching behavior and skin barrier dysfunction.
NC/Tnd mice have been widely used as a mouse model for human AD. To evaluate efficacy of treatment interventions for AD by using NC/Tnd mice, scratching behavior and TEWL have been often measured as dermatological parameters. In the present study, we found significant daily variations of scratching behavior and TEWL in NC/Tnd mice. The highest scratching frequency, duration and TEWL were 3.8-, 4.1- and 1.3-fold increases in the lowest scratching frequency, duration and TEWL, respectively. Our findings indicate that investigators should take into account time-of-day-dependent variations of scratching behavior and TEWL when they analyze NC/Tnd mice.
Multiple factors, including skin temperature, corticosteroids, autonomic nervous system, opioids and cytokines, are assumed to comprise daily variations of scratching and TEWL [6]. To clarify the mechanisms regulating time-of-day-dependent variations of clinical features in AD, NC/Tnd mice could be an appropriate mouse model.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science, Japan. We thank Dr. Hiroshi Matsuda and Dr. Akane Tanaka (Tokyo University of Agriculture and Technology) for their help.
REFERENCES
- 1.Aioi A., Tonogaito H., Suto H., Hamada K., Ra C., Ogawa H., Maibach H., Matsuda H.2001. Impairment of skin barrier function in NC/Nga Tnd mice as a possible model for atopic dermatitis. Br. J. Dermatol. 144: 12–18. doi: 10.1046/j.1365-2133.2001.03946.x [DOI] [PubMed] [Google Scholar]
- 2.Chrostowska-Plak D., Salomon J., Reich A., Szepietowski J. C.2009. Clinical aspects of itch in adult atopic dermatitis patients. Acta Derm. Venereol. 89: 379–383. doi: 10.2340/00015555-0676 [DOI] [PubMed] [Google Scholar]
- 3.Kabashima K.2013. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J. Dermatol. Sci. 70: 3–11. doi: 10.1016/j.jdermsci.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 4.Leung D. Y., Boguniewicz M., Howell M. D., Nomura I., Hamid Q. A.2004. New insights into atopic dermatitis. J. Clin. Invest. 113: 651–657. doi: 10.1172/JCI21060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuda H., Watanabe N., Geba G. P., Sperl J., Tsudzuki M., Hiroi J., Matsumoto M., Ushio H., Saito S., Askenase P. W., Ra C.1997. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 9: 461–466. doi: 10.1093/intimm/9.3.461 [DOI] [PubMed] [Google Scholar]
- 6.Patel T., Ishiuji Y., Yosipovitch G.2007. Nocturnal itch: why do we itch at night? Acta Derm. Venereol. 87: 295–298. doi: 10.2340/00015555-0280 [DOI] [PubMed] [Google Scholar]
- 7.Steinhoff M., Bienenstock J., Schmelz M., Maurer M., Wei E., Bíró T.2006. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J. Invest. Dermatol. 126: 1705–1718. doi: 10.1038/sj.jid.5700231 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A., Amagai Y., Oida K., Matsuda H.2012. Recent findings in mouse models for human atopic dermatitis. Exp. Anim. 61: 77–84. doi: 10.1538/expanim.61.77 [DOI] [PubMed] [Google Scholar]
- 9.Yosipovitch G., Goon A. T., Wee J., Chan Y. H., Zucker I., Goh C. L.2002. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. Int. J. Dermatol. 41: 212–216. [DOI] [PubMed] [Google Scholar]