ABSTRACT
Thirty-four fowl adenoviruses (FAdVs) isolated from chickens with gizzard erosion (GE) from 1999 to 2010 were characterized phylogenetically together with foreign isolates. The phylogenetic analysis based on part of the hexon gene classified these 34 FAdV isolates into 3 groups: FAdV-1, −8a and 8b, thereby suggesting that FAdVs associated with GEs in chickens are diverse. All 30 FAdV-1 isolates were genetically identical, and they were also identical with FAdV-1 isolates from GEs in chickens in European countries (Germany, Poland, Austria, Hungary and Italy). Thus, the same type of FAdV-1 has been associated with outbreaks of GE in Japanese chickens for the past 10 years, which may have spread from a common ancestor, although the epidemiological relationship is unknown.
Keywords: fowl adenovirus, gizzard erosion, phylogeny
Gizzard erosion (GE) in slaughtered commercial broiler chickens has been observed frequently in several countries, including Japan [2, 6, 8, 14,15,16]. In most cases, the affected chickens exhibit no apparent clinical signs: thus, GE is confirmed mostly based on inspections at slaughterhouses. By virologic examination, fowl adenovirus (FAdV) was isolated from affected gizzard in many cases [2, 8, 16]. Furthermore, GE was experimentally reproduced in chickens inoculated with the isolated virus [12, 13]. However, conventional FAdV-1 strains, such as Ote type [11], fail to induce GE. This suggests that the pathogenicity of FAdVs is diverse.
In general, adenoviruses are non-enveloped icosahedral viruses with a diameter of approximately 70–100 nm [1]. The major structural proteins are hexon and fiber, and the hexon protein is the major capsid protein of the non-enveloped icosahedral virion where the type, group and subgroup-specific determinants are located. At present, FAdVs are classified based on their serological relationships, and 11 fowl serotypes have been recognized to date [1]. These 11 serotypes have been grouped into 5 species (FAdV A−E).
In many cases of GE in chickens, the isolated FAdVs belonged to serotype 1, although serotype 8 has also been isolated on occasions. However, the genetic relationships among FAdV isolates have not been fully analyzed. Recently, GE outbreaks caused by FAdV have been reported in foreign countries, and the genetic information for the causative viruses has been deposited in GenBank [2, 5, 6]. Here, we performed a phylogenetic analysis of the viruses isolated from these cases and Japanese cases to gain insights into the epidemiology of FAdV associated with GE in Japan.
We used 34 Japanese FAdV samples, which were obtained during from 1999 to 2010 (Table 1). Primary chicken kidney cell (CKC) cultures were used routinely for the isolation and propagation of the viruses. Initially, the presence of FAdV in CKC cultures was determined using FAdV-specific polymerase chain reaction (PCR), as described previously [9].
Table 1. Fowl adenoviruses isolated from chickens with gizzard erosion in this study.
| Virus | Prefecture | Year of isolation | Type | Reference |
|---|---|---|---|---|
| 99ZH | Hokkaido | 1999 | 1 | Ono et al. [16] |
| G0054 | Gifu | 2000 | 8b | Okuda et al. [10] |
| JP/Tokushima/2000GE | Tokushima | 2000 | 1 | Mase et al. [9] |
| JP/Kagawa/2000GE | Kagawa | 2000 | 1 | Mase et al. [9] |
| ZK-1 | Miyazaki | 2000 | 1 | Ono et al. [15] |
| ZK-2 | Miyazaki | 2000 | 1 | Ono et al. [15] |
| ZK-3 | Kagoshima | 2000 | 1 | Ono et al. [15] |
| ZK-4 | Miyazaki | 2000 | 8b | Ono et al. [15] |
| ZK-5 | Iwate | 2000 | 1 | Ono et al. [15] |
| ZK-6 | Gunma | 2000 | 8a | Ono et al. [15] |
| ZK-7 | Gifu | 2000 | 1 | Ono et al. [15] |
| ZK-8 | Gunma | 2000 | 1 | Ono et al. [15] |
| ZK-9 | Shizuoka | 2000 | 1 | Ono et al. [15] |
| ZK-10 | Shizuoka | 2000 | 1 | Ono et al. [15] |
| ZK-11 | Miyazaki | 2000 | 1 | Ono et al. [15] |
| ZK-12 | Miyazaki | 2000 | 1 | Ono et al. [15] |
| M013 | Miyazaki | 2001 | 8b | Okuda et al. [10] |
| JP/Oita/2001GE | Oita | 2001 | 1 | Mase et al. [9] |
| ZK-13 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| ZK-14 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| ZK-15 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| ZK-16 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| ZK-17 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| ZK-18 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| ZK-19 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| ZK-20 | Miyazaki | 2001 | 1 | Ono et al. [15] |
| JP/Shimane/2007GE | Shimane | 2007 | 1 | This study |
| JP/Shimane/2008GE | Shimane | 2008 | 1 | This study |
| JP/Niigata/2008-1GE | Niigata | 2008 | 1 | This study |
| JP/Niigata/2008-2GE | Niigata | 2008 | 1 | This study |
| JP/Niigata/2008-3GE | Niigata | 2008 | 1 | This study |
| JP/Niigata/2008-4GE | Niigata | 2008 | 1 | This study |
| Mie/IC-01G/2010 | Mie | 2010 | 1 | This study |
| JP/Niigata/2010GE | Niigata | 2010 | 1 | This study |
To examine the genetic relationships among the FAdV isolates associated with GE in chickens (GE-FAdV), we determined partial nucleotide sequences of the hexon gene from the GE-FAdV isolates. Viral DNA was extracted from infected culture fluids using a QIAamp DNA Micro Kit (Qiagen Inc., Valencia, CA, U.S.A.). The primers were designed based on conserved sequences identical to a region of the reported hexon protein gene of group I-III avian adenovirus (AAV) that included the L1 region, which contained diagnostically relevant sequences that can be used to identify the group and type of AAVs [9]. The following primer set was used: HexF1: 5′- GAYRGYHGGRTNBTGGAYATGGG −3′ and HeXR1: 5′- TACTTATCNACRGCYTGRTTCCA-3′. PCR amplification, sequencing and phylogenetic analysis were performed as described previously [9].
The primer pair used for PCR successfully amplified products of approximately 800 bp from the DNA samples of all the GE-FAdV isolates used in this study.
These GE-FAdVs were classified into 3 types, 1, 8a and 8b, according to a method described previously (Table 1) [9]. The nucleotide sequences of all the GE-FAdV-1 isolates used in this study were almost identical (100%, index isolate: JP/Oita/2001GE). This suggests that the FAdV-1 isolates obtained from chickens with GE in Japan share a common ancestor. Compared with the sequence obtained from the reference FAdV-1 strain (Ote), the sequences obtained from the GE-FAdV-1 isolates shared 99.2% sequence similarity. However, the ZK-6 (8a) and M013 (8a) isolates obtained from chickens with GE in Japan shared 98.9% and 100% sequence similarity with the TR-59 strain (reference FAdV-8a strain), respectively. On the other hand, the ZK-4 (8b) and G0054 (8b) isolates shared 99.6% and 99.6% sequence similarity with the 764 strain (reference FAdV-8b strain), respectively.
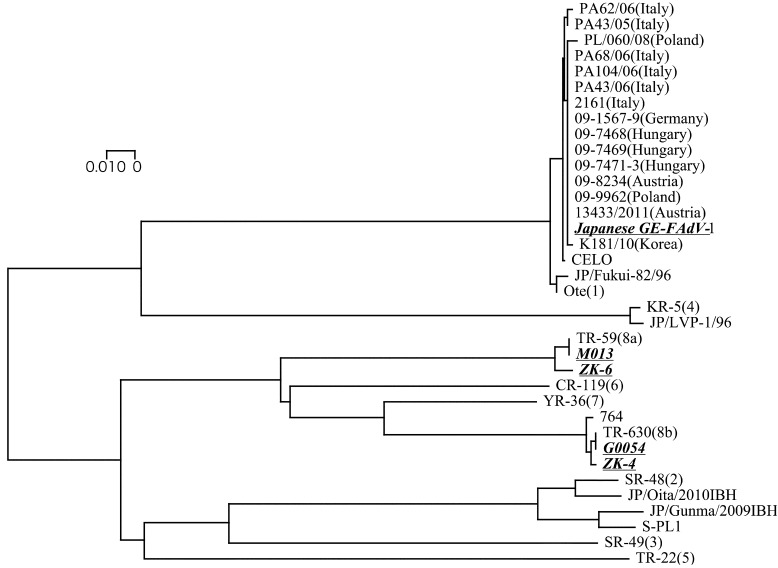
The phylogenetic analysis with foreign FAdV isolates showed that the Japanese GE-FAdV-1 was also identical with the FAdV-1 viruses isolated from chickens with GE in European countries (Germany, Poland, Austria, Hungary and Italy) (Fig. 1). Furthermore, Korean GE-FAdV (K181/10) strain [5] was also genetically similar to these GE-FAdV-1 isolates. This suggests that GE-FAdV-1 may have spread from a common ancestor, although the epidemiological relationship is unknown. On the other hand, the FAdV-8a and FAdV-8b isolates were not reported in chickens with GE in such foreign countries. This suggests that these types may not have spread in chickens in foreign countries.
Fig. 1.
Phylogenetic tree based on the hexon gene of fowl adenovirus (FAdV). Nucleotides 18632−19155 from the complete genome of FAdV-A (GenBank Accession No. U46933) were used in the phylogenetic analysis. The horizontal distances are proportional to the minimum number of nucleotide differences required to join nodes and sequences. The viruses employed in this study are shown in bold italics and underlines. The numbers in parentheses for the reference strains indicate the FAdV serotypes.
To confirm the GE-FAdV serotypes, a virus neutralization assay was performed using micro methods with representative isolates (JP/Oita/2001GE, ZK-6, and ZK-4) from each type (1, 8a and 8b). Briefly, the constant virus and varying serum technique was employed, using 200 50% tissue culture infected doses (TCID50) of virus isolates with 2-fold dilutions of antisera starting from 1:20. The plates were kept at 37°C in a CO2 incubator and observed daily for 7 days to detect cytopathic effects, and the neutralization titers were calculated. Antisera for each of the nine conventional FAdV reference serotype strains (Ote, SR-48, SR-49, KR-5, TR-22, CR-119, YR-36, TR-59 and TR630) prepared by Kawamura et al. [4] were used. As expected, FAdV-1 was neutralized by antisera against Ote (serotype 1), whereas FAdV-8a and 8b were neutralized by antisera against TR-59 (serotype 8a) and TR630 (serotype 8b) (Table 2).
Table 2. Results of the virus neutralization tests using fowl adenoviruses (FAdV) strains.
| Antiserum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Viruses | Ote (1a) | SR-48 (2) | SR-49 (3) | KR-5 (4) | TR-22 (5) | CR-119 (6) | YR-36 (7) | TR-59 (8a) | TR-630 (8b) |
| JP/Oita/2001GE (1) | 320b) | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| ZK-6 (8a) | <20 | <20 | <20 | <20 | <20 | 160 | <20 | 5,120 | 2,560 |
| ZK-4 (8b) | <20 | <20 | <20 | <20 | <20 | 40 | <20 | 640 | 10,240 |
| Homologous Titer | 640 | 5,120 | 10,240 | 1,280 | 20,480 | 20,480 | 10,240 | 10,240 | 20,480 |
a) FAdV Serotype. b) Titer is expressed as the reciprocal of that of the serum dilution.
Most reported GE-FAdV isolates belong to serotype 1, although serotype 8 has also been isolated occasionally in Japan [10, 15]. However, the phylogenetic relationships among the GE-FAdV-1 isolates and the isolates from other countries are not clear.
Here, we performed a phylogenetic analysis of GE-FAdV isolates in Japan. All of the GE-FAdV-1 isolates in Japan were identical, thereby suggesting that all of the isolates were derived from a common ancestor. Furthermore, the GE-FAdV-1 isolates in Japan were also identical with those in European countries [7, 8, 17]. These results suggest that these GE-FAdV-1 isolates might have spread from a common ancestor, although the epidemiological relationship is unknown.
On the other hand, the GE-FAdV-8 isolates were divided into types FAdV-8a and FAdV-8b, thereby suggesting that the FAdV-8 types associated with GE in chickens are diverse. According to the recent ICTV definition [1, 18], FAdV-8a is the former serotype EU8 (US6), and 8b is EU9 (US7) [18]. Both 8a and 8b are neutralized by antiserum against serotype 8 (TR-59 and TR630), and thus, these previously different serotypes cross-react. Indeed, this cross reactivity was observed in the present study. In foreign countries, however, cases of GE caused by FAdV-8a and 8b have not been reported, thereby suggesting that such cases are less frequent than those due to FAdV-1.
The transmission route of GE-FAdVs is still unknown. Previously, Ono et al. demonstrated that the GE-FAdV-1 (99ZH) strain spreads rapidly through direct contact with inoculated chickens [13]. In addition, the vertical transmission of FAdV has also been reported [3]. Many cases of GE are detected in slaughtered chickens, but the actual frequency in the field is unknown. Thus, a continued surveillance of FAdV will be required to understand the epidemiology of the virus associated with GE.
Acknowledgments
We thank the Institute of Animal Health of JA Zen-noh (National Federation of Agricultural Co-operative Associations) for providing the ZK1-20 isolates. We also thank the veterinary officials of Niigata, Tokushima, Kagawa, Oita, Mie and Shimane Prefectures for providing the viral samples. We wish to thank Mr. T. Inoue for his technical assistance in this study.
REFERENCES
- 1.Adair B. M., Fitzgerald S. D.2008. Group I adenovirus infections. pp. 252–266. In: Diseases of Poultry, 12th ed. (Saif, Y. M., Barners, H. J., Glisson, J. R., Fadly, A. M., McDougald, L. R. and Swayne, D. E. eds.), Iowa State Press, Ames. [Google Scholar]
- 2.Domanska-Blicharz K., Tomczyk G., Smietanka K., Kozaczynski W., Minta Z.2011. Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult. Sci. 90: 983–989. doi: 10.3382/ps.2010-01214 [DOI] [PubMed] [Google Scholar]
- 3.Grgić H., Philippe C., Ojkic D., Nagy E.2006. Study of vertical transmission of fowl adenoviruses. Can. J. Vet. Res. 70: 230–233. [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamura H., Shimizu F., Tsubahara H.1964. Avian Adenovirus: Its Properties and Serological Classification. Natl. Inst. Anim. Health Q. (Tokyo) 4: 183–193. [PubMed] [Google Scholar]
- 5.Lim T. H., Kim B. Y., Kim M. S., Jang J. H., Lee D. H., Kwon Y. K., Lee J. B., Park S. Y., Choi I. S., Song C. S.2012. Outbreak of gizzard erosion associated with fowl adenovirus infection in Korea. Poult. Sci. 91: 1113–1117. doi: 10.3382/ps.2011-02050 [DOI] [PubMed] [Google Scholar]
- 6.Manarolla G., Pisoni G., Moroni P., Gallazzi D., Sironi G., Rampin T.2009. Adenoviral gizzard erosions in Italian chicken flocks. Vet. Rec. 164: 754–756. doi: 10.1136/vr.164.24.754 [DOI] [PubMed] [Google Scholar]
- 7.Marek A., Gunes A., Schulz E., Hess M.2010. Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. J. Virol. Methods 170: 147–154. doi: 10.1016/j.jviromet.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 8.Marek A., Schulz E., Hess C., Hess M.2010. Comparison of the fibers of Fowl adenovirus A serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains. J. Vet. Diagn. Invest. 22: 937–941. doi: 10.1177/104063871002200613 [DOI] [PubMed] [Google Scholar]
- 9.Mase M., Mitake H., Inoue T., Imada T.2009. Identification of group I-III avian adenovirus by PCR coupled with direct sequencing of the hexon gene. J. Vet. Med. Sci. 71: 1239–1242. doi: 10.1292/jvms.71.1239 [DOI] [PubMed] [Google Scholar]
- 10.Okuda Y., Ono M., Shibata I., Sato S.2004. Pathogenicity of serotype 8 fowl adenovirus isolated from gizzard erosions of slaughtered broiler chickens. J. Vet. Med. Sci. 66: 1561–1566. doi: 10.1292/jvms.66.1561 [DOI] [PubMed] [Google Scholar]
- 11.Okuda Y., Ono M., Shibata I., Sato S., Akashi H.2006. Comparison of the polymerase chain reaction-restriction fragment length polymorphism pattern of the fiber gene and pathogenicity of serotype-1 fowl adenovirus isolates from gizzard erosions and from feces of clinically healthy chickens in Japan. J. Vet. Diagn. Invest. 18: 162–167. doi: 10.1177/104063870601800204 [DOI] [PubMed] [Google Scholar]
- 12.Okuda Y., Ono M., Yazawa S., Shibata I., Sato S.2001. Experimental infection of specific-pathogen-free chickens with serotype-1 fowl adenovirus isolated from a broiler chicken with gizzard erosions. Avian Dis. 45: 19–25. doi: 10.2307/1593007 [DOI] [PubMed] [Google Scholar]
- 13.Ono M., Okuda Y., Shibata I., Sato S., Okada K.2007. Reproduction of adenoviral gizzard erosion by the horizontal transmission of fowl adenovirus serotype 1. J. Vet. Med. Sci. 69: 1005–1008. doi: 10.1292/jvms.69.1005 [DOI] [PubMed] [Google Scholar]
- 14.Ono M., Okuda Y., Yazawa S., Imai Y., Shibata I., Sato S., Okada K.2003. Adenoviral gizzard erosion in commercial broiler chickens. Vet. Pathol. 40: 294–303. doi: 10.1354/vp.40-3-294 [DOI] [PubMed] [Google Scholar]
- 15.Ono M., Okuda Y., Yazawa S., Shibata I., Sato S., Okada K.2003. Outbreaks of adenoviral gizzard erosion in slaughtered broiler chickens in Japan. Vet. Rec. 153: 775–779. [PubMed] [Google Scholar]
- 16.Ono M., Okuda Y., Yazawa S., Shibata I., Tanimura N., Kimura K., Haritani M., Mase M., Sato S.2001. Epizootic outbreaks of gizzard erosion associated with adenovirus infection in chickens. Avian Dis. 45: 268–275. doi: 10.2307/1593040 [DOI] [PubMed] [Google Scholar]
- 17.Pizzuto M. S., De Battisti C., Marciano S., Capua I., Cattoli G.2010. Pyrosequencing analysis for a rapid classification of fowl adenovirus species. Avian Pathol. 39: 391–398. doi: 10.1080/03079457.2010.510499 [DOI] [PubMed] [Google Scholar]
- 18.Steer P. A., Kirkpatrick N. C., O’Rourke D., Noormohammadi A. H.2009. Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. J. Clin. Microbiol. 47: 311–321. doi: 10.1128/JCM.01567-08 [DOI] [PMC free article] [PubMed] [Google Scholar]