Abstract
Objective
Neural tissue transplantation has been a promising strategy for the treatment of Parkinson's disease (PD). However, transplantation has the disadvantages of low-cell survival and/or development of dyskinesia. Transplantation of cell aggregates has the potential to overcome these problems, because the cells can extend their axons into the host brain and establish synaptic connections with host neurons. In this present study, aggregates of human brain-derived neural stem cells (HB-NSC) were transplanted into a PD animal model and compared to previous report on transplantation of single-cell suspensions.
Methods
Rats received an injection of 6-OHDA into the right medial forebrain bundle to generate the PD model and followed by injections of PBS only, or HB-NSC aggregates in PBS into the ipsilateral striatum. Behavioral tests, multitracer (2-deoxy-2-[18F]-fluoro-D-glucose ([18F]-FDG) and [18F]-N-(3-fluoropropyl)-2-carbomethoxy-3-(4-iodophenyl)nortropane ([18F]-FP-CIT) microPET scans, as well as immunohistochemical (IHC) and immunofluorescent (IF) staining were conducted to evaluate the results.
Results
The stepping test showed significant improvement of contralateral forelimb control in the HB-NSC group from 6-10 weeks compared to the control group (p<0.05). [18F]-FP-CIT microPET at 10 weeks posttransplantation demonstrated a significant increase in uptake in the HB-NSC group compared to pretransplantation (p<0.05). In IHC and IF staining, tyrosine hydroxylase and human β2 microglobulin (a human cell marker) positive cells were visualized at the transplant site.
Conclusion
These results suggest that the HB-NSC aggregates can survive in the striatum and exert therapeutic effects in a PD model by secreting dopamine.
Keywords: Parkinson's disease, Cell transplantation, Human brain-derived neural stem cells, Cell aggregates, Rat model, [18F]-FP-CIT microPET
INTRODUCTION
Parkinson's disease (PD) is neurodegenerative disorder characterized by progressive loss of substantia nigra pars compacta (SNpc) neurons1,4,5,20). The progressive loss of cells leads to motor dysfunctions including tremor, rigidity, and bradykinesia6,10,28).
Neural tissue transplantation has been a promising strategy for PD because the transplanted tissues can produce dopamine in the striatum, which has a therapeutic effect in PD patients2,15). However, tissue grafts have the disadvantages of low rates of cell survival and/or development of dyskinesia9). Stem-cell transplantation was recently tried, but the effects were controversial due to possible tumorigenesis8), poor differentiation into dopaminergic cells, or low survival rates19). The transplantation of spheroids, or cell aggregations, has been proposed to overcome these problems, because the cell aggregates can extend their axons into the host brain and establish synaptic connections with host neurons following transplantation into specific brain areas13).
In our previous study, human brain-derived neural stem cells (HB-NSC) which were injected into the striatum of a PD rat model as single-cell suspensions showed partial differentiation to dopaminergic cells, but without therapeutic effect30). We hypothesized that the injected single HB-NSC cell suspensions could partially differentiate into dopaminergic cells, which produced dopamine within the cell, but were unable to secrete it. Therefore, in the present study, we transplanted HB-NSC as cell aggregates form into the striatum of PD rats to determine their efficacy compared to single-cell transplantations. We expected that the cell aggregates have the advantage of a pre-established cell network which reported in transplantation of spheroid form13) that might promote high rates of survival and differentiation. After the transplantation, we evaluated the results by behavioral tests, PET study and histological study.
MATERIALS AND METHODS
Animal and 6-OHDA lesion procedures
Twenty two adult female Sprague-Dawley rats (Orient Bio lnc., Seongnam, Korea) weighing 180-210 g were used in this study. All animals were housed individually in cages, with free access to food and water in a climate-controlled room. This study was approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Science. At the beginning of the study, each animal was anesthetized with Zoletil 50 (Virbac S.A, Carros, France) and Rompun (Bayer, Leverkusen, Germany) at doses of 37.5 mg/kg and 6 mg/kg, respectively, using an intraperitoneal (i.p.) injection. A total of 22 rats were administered unilateral injections of 14 µg 6-OHDA hydrochloride (Sigma, St. Louis, MO, USA) in 4 µL of 0.9% saline with 0.1% ascorbic acid delivered into the right medial forebrain bundle at the following coordinates : anteroposterior -4.4 mm, lateral +1.2 mm relative to the bregma and ventral -7.8 mm from the dura by Paxinos's rat atlas, with the tooth bar set at -2.4 mm. The injections were delivered at a rate of 1 µL/min using a Hamilton syringe (33-gauge) and an automated microsyringe pump (Harvard Apparatus, Holliston, MA, USA). Once the injection was finished, the needle was left in place for 5 minutes to prevent backflow of the solution. Among 22 rats, 18 rats which showed apomorphine-induced rotation exceeded 6 rotations per min after 4 weeks of 6-OHDA injection (success rate 81.8%) were selected for this study. Then, HB-NSC cells were injected in 9 rats (HB-NSC group) and PBS in 9 rats (control group). However, 2 rats were dead during PET study, so the final numbers of experimental animals became 9 in HB-NSC group and 7 in control group.
Transplantation of aggregates of human brain-derived neural stem cells
HB-NSC (San Pedro, CA, USA) were expanded in brain stem-cell growth medium according to the manufacturer's instructions. For formation of homogenous aggregates, polyethylene glycol (PEG) hydrogel microwell arrays with a diameter of 500 µm were produced using micro-fabrication procedures, as reported previously13). The expanded HB-NSC were allowed to form aggregations within the PEG hydrogel microwell arrays. Briefly, HB-NSC at an approximate cell density of 1×106 cells/200 µL were deposited into each microwell array, and then placed in a 5% CO2 humidified incubator for 30 min to allow the cells to settle. Non-adherent cells were carefully removed by washing with PBS, and the adherent cells were allowed to form aggregations within each microwell. The cell aggregations were retrieved from the PEG hydrogel after 5 days of culture in the brain stem-cell growth medium (Celprogen, San Pedro, CA, USA). The cell aggregates are visible with the naked eyes and the diameter of each aggregate is approximately 200 µm. And then, the cell aggregation were administered in sterilized PBS at a concentration of 8×104 cells/8 µL. We could evaluate the concentration of cell aggregation by cell counting with trypan blue viability staining which was partially harvested from cell aggregation solution and transformed into cell suspension.
The cell aggregations transplanted into the right striatum of the PD rat model (HB-NSC group, n=9) using a 22 G Hamilton syringe and a stereotaxic microinjection device (coordinates : AP +0.6 mm from the bregma, ML +3.5 mm from the midline, DV -4.5 mm, nose bar +2.3 mm) 4 weeks after the 6-OHDA injection. The control group (n=7) received 8 µL of PBS (pH 7.4) injected at the same coordinates. All injections used a rate of 1 µL/min, and the needle was held in place for 5 min after the injection, followed by withdrawal at a rate of 1 mm/min. Postoperatively, all rats received daily Cyclosporine A (Chong Kun Dang Pharm, Seoul, Korea) at 10 mg/kg (i.p.) until the animals were sacrificed.
Behavioral tests
The stepping tests were performed six times : prior to any treatment, 1 week after 6-OHDA injection, and 2, 4, 6, and 8 weeks after cell transplantation, as previously described24), with slight modifications. Briefly, both hind limbs were firmly held in one hand of the experimenter, while the other hand was used to hold one of the forelimbs. The anterior end of the animal was lowered onto a treadmill (Jeung Do Bio & Plant Co., Seoul, Korea), which was set at a rate of 0.9 m/5 s. The rat's body remained stationary while the unilateral forelimb was allowed to touch the moving treadmill track for >5 seconds while the treadmill was operating. All of the experiments were video recorded to count the number of adjusted steps taken in the backward direction. Every rat performed the stepping test twice during every session, and the data were averaged. The data are expressed as the percentage of contralateral forelimb steps versus ipsilateral forelimb steps.
Apomorphine-induced rotation tests were performed in transparent cylinders. The rotation tests were conducted 1 week after 6-OHDA injection, and 4 and 8 weeks after cell transplantation, as previously described25), with slight modifications. Briefly, as soon as 0.5 mg/kg apomorphine (Sigma; in sterile water, injected subcutaneously) was administered, the rat was harnessed to an automated rotometer (Panlab, Barcelona, Spain) and its rotational behavior was assessed over 45 minutes. Data are expressed as the net average rotations (contralateral-ipsilateral turns) per minute. 6-OHDA-lesioned rats that demonstrated >6 rotations per minute were selected for inclusion in the present study.
Multitracer [18F]-FDG and [18F]-FP-CIT microPET study
All animals were scanned using the microPET Focus 120 system (Siemens Medical Solutions, Inc., Erlagen, Germany), which includes a 12×12 array of lutetium oxyorthosilicate and 96 detector blocks. Glucose metabolic activity was assessed in the control (n=3) and HB-NSC groups (n=5) using [18F]-FDG at 10 weeks posttransplantation. After fasting for at least 12 h, the rats were injected with 1 mCi [18F]-FDG in 0.2 mL of sterile saline via the tail vein. One hour after the injection, the animals were scanned for 30 min under isoflurane anesthesia.
Dopamine transporter function was assessed with the same animals in the control (n=3) and HB-NSC group (n=5) using [18F]-FP-CIT, which binds to the dopamine transporter with high affinity, at 1 or 2 weeks after 6-OHDA injection (PD status before cell transplantation) and at 5 and 10 weeks after cell transplantation. Rats were injected with 1 mCi [18F]-FP-CIT in 0.4 mL sterile saline via the tail vein, immediately followed by dynamic scanning for 90 minutes.
The [18F]-FDG PET data were reconstructed using a 3-dimensional (3D) maximum a posteriori (MAP) algorithm, and the [18F]-FP-CIT data were reconstructed using a 2D ordered-subset expectation maximization16). PET images were analyzed using the ASIPro software (Siemens Preclinical Solutions, Knoxville, TN, USA) to generate a 3D volume of interest that consisted of three regions of interest (ROI). To produce 3D volume of interest, we selected a coronal image which showed highest uptake of striatum, and then, three consecutive ROIs in axial images which show striatum were taken on the selected coronal image base. Each ROI of axial image was composed of 5×5 pixels30). Data are expressed as the percentage of ipsilateral standardized uptake values based on the mean uptake in the 3D volume of interest against the contralateral standardized uptake values. The equation of standardized uptake values is [tissue concentration (MBq/cc)]/[injected dose (MBq)/body weight (g)].
Immunohistochemical and immunofluorescent tissue staining
Tissue was harvested 12 weeks after cell transplantation. Rats were transcardially and sequentially perfused with 0.9% saline containing 10000 IU heparin (Hanlim Pharm, Seoul, Korea) and 4% paraformaldehyde in PBS. Extracted brain tissues were postfixed overnight in the same fixative, followed by incubation and dehydration in 30% sucrose until they sank. The IHC and IF procedures were performed as previously described11,17). Serial sections from frozen tissue, including the striatum (anteroposterior +2.5 to 0.0 mm) ,were produced and the brain tissue was cut into 40 µm sections on a sliding microtome (HM450; Sliding electric microtome, Thermo Scientific). The sections were stored in 0.08% sodium azide (Sigma-Aldrich, St. Louis, USA) in PBS at 4℃ until analysis.
For IHC, striatal sections were incubated overnight with primary antibodies (anti-TH monoclonal 1 : 5000, Sigma Aldrich, St. Louis, USA) diluted in PBS (pH 7.4) containing 0.5% BSA (bioWORLD, Dublin, USA). These sections were subsequently incubated for 2 h at room temperature with biotinylated secondary antibodies produced in mouse (Vector Laboratories, Burlingame, CA, USA) and diluted 1 : 200 in PBS containing 0.5% BSA. TH staining was detected by adding diaminobenzidine (R&D System, Minneapolis, MN, USA). Sections were mounted on gelatin-coated slides and dried. Diaminobenzidine-treated tissues were dehydrated in a series of alcohol and clearing agents, and a coverslip was applied. TH-positive cells were observed in the coronal sections of the striatum using a Nikon 80i microscope (Nikon, Tokyo, Japan) at 100× magnification using the NIS-Elements F3.0 software (Nikon, Tokyo, Japan).
For IF, tissue sections were incubated with blocking solution containing 1% BSA, 0.2% Triton X-100 (Sigma Aldrich, St. Louis, USA) and 0.05% sodium azide, rinsed in 0.5% BSA in PBS twice and then incubated with anti-TH monoclonal (1 : 5000, Sigma Aldrich, St. Louis, USA) and anti-human β2 microglobulin polyclonal (1 : 800, Dako, Seoul, Korea) antibodies for 1 h at room temperature. The sections were washed three times and incubated for 90 min with Alexa 546-labeled anti-mouse IgG (1 : 200, Invitrogen, Grand Island, NY, USA) and Alexa 488-labeled anti-rabbit IgG (1 : 200, Invitrogen Grand Island, NY, USA). After washing twice, the sections were mounted onto gelatin-coated slides, dried at 50℃ for 30 min, and cover-slipped with VECTOR mounting (Dako, Seoul, Korea) medium for fluorescence. Fluorescent images were obtained using a confocal microscope (TCS-ST2; Leica, Wetzlar, Germany).
Statistical analysis
All data are presented as mean±SD. All statistical analyses were performed using SPSS (version 12.0.1; SPSS Inc., Chicago, IL, USA). Behavioral parameters were analyzed using Mann-Whitney U test, and PET data were analyzed by the paired sample t-test for working out the relative importance of each data which was mat-ched one-on-one. A p-value <0.05 was considered significant.
RESULTS
Behavioral tests
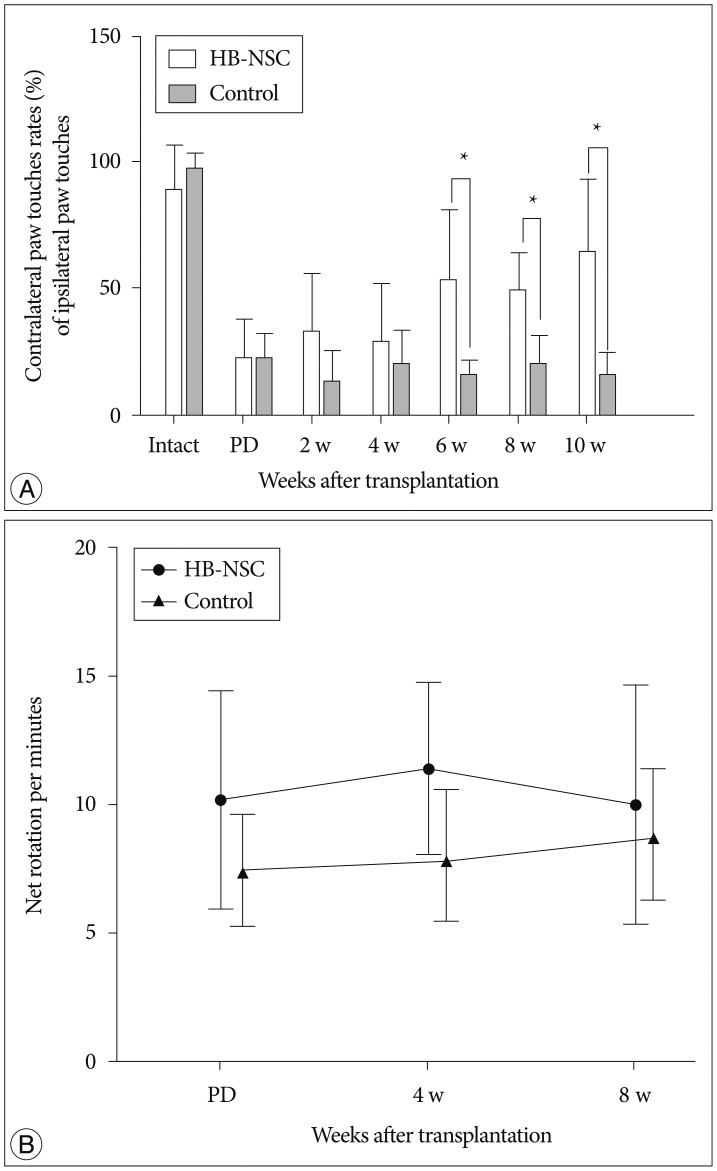
The stepping test showed no significant increase in contralateral paw touches up to 4 weeks posttransplantation; however, at 6 weeks posttransplantation, a significant improvement of 52.9% was measured. At 8 weeks posttransplantation, the increase was 48.9% and this improvement was maintained through to 10 weeks, at which time the increase was 64.2% in the HB-NSC group compared to the control group (Fig. 1A). In apomorphine-induced rotation tests, the HB-NSC group showed a slight decrease in contralateral rotations at week 8, compared to week 4; however, the difference was not statistically significant (Fig. 1B).
Fig. 1.
In the step test, the number of contralateral forelimb touches in the HB-NSC group (n=9) improved significantly, compared to the control group (n=7), beginning at 6 weeks after HB-NSC transplantation. The increase was maintained through 10 week posttransplantation (*p<0.05). In the apomorphine-induced rotation test, a slight decrease was observed at week 8, compared to week 4, in the HB-NSC transplantation group, but the difference was not significant.
Multitracer [18F]-FDG and [18F]-FP-CIT microPET analysis
To examine glucose metabolism, [18F]-FDG microPET scans were used. There was no significant decrease in uptake of [18F]-FDG in either the control or HB-NSC transplantation group, indicating that glucose metabolism was normal, as is characteristic of PD (Fig. 2A). Using [18F]-FP-CIT to quantify dopamine transporter activity, a gradual increase in radioactivity in the lesion-side striatum was detected in the HB-NSC group and, in particular, there was a statistically significant increase in dopamine trans-porter activity by 10 weeks posttransplantation compared to the pretransplantation state (48.5±3.9% vs. 41.0±2.6%, respectively) (Fig. 2B). These microPET data are consistent with the results of the stepping test in which significant improvement was detected from 6 weeks posttransplantation onward.
Fig. 2.
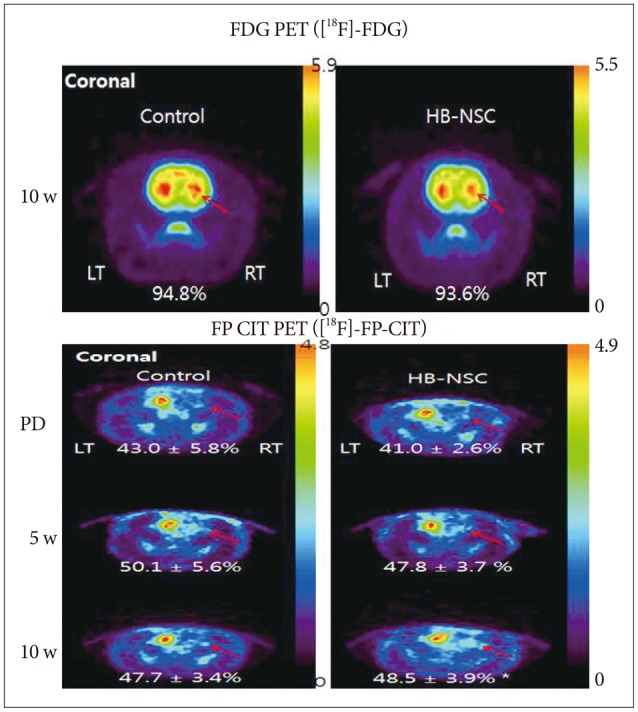
Coronal images of [18F]-FDG and [18F]-FP-CIT PET scans in striatal area are shown, respectively. In the [18F]-FDG PET scan 10 week posttransplantation, the uptake did not show significant differences in bilateral striatum in control group as well as HB-NSC group, which means PD models were created successfully. [18F]-FP-CIT PET scans were performed before transplantation (PD), 5 weeks after transplantation (5 w), and 10 weeks after transplantation (10 w). The HB-NSC group showed a statistically significant recovery in the uptake of [18F]-FP-CIT in the transplanted striatum (arrow) at 10 weeks posttransplantation compared to PD (*p< 0.05) whereas there was no significant improvement of uptake at 5 weeks posttransplantation (p=0.065), suggesting that dopamine is secreted by the transplanted cells. The color bar indicates the relative uptake intensity and shows maximum standardized uptake values in the image.
Immunohistochemistry and immunofluorescence
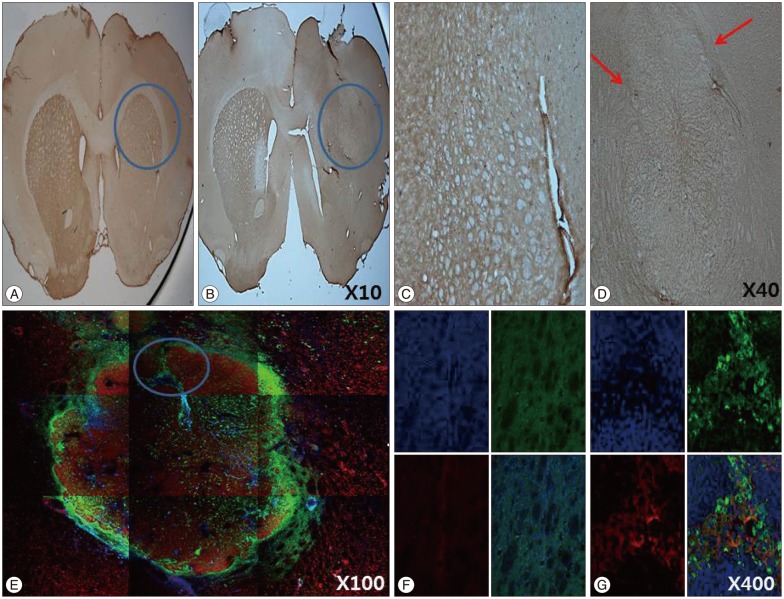
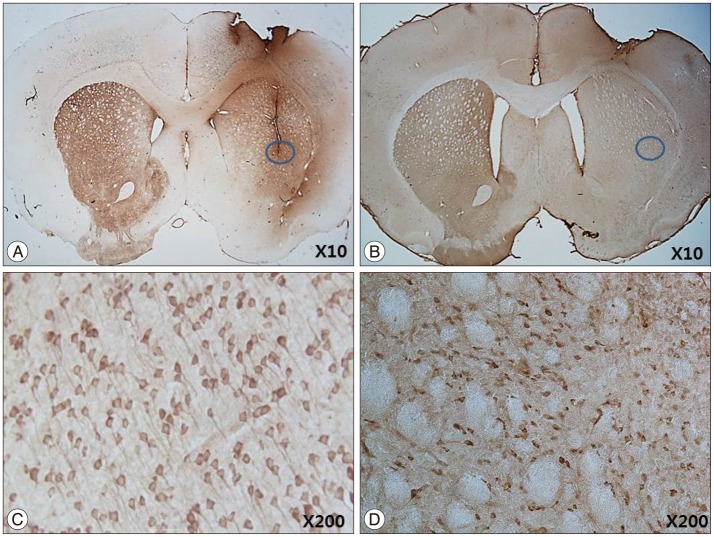
IHC and IF analysis revealed the presence of tyrosine hydroxylase (TH)-positive cells in the ipsilateral striatum of the HB-NSC transplantation group (Fig. 3). High-magnification images showed that the TH-positive cells corresponded to the cells positive for the human β2 microglobulin marker (Fig. 3G), which demonstrates HB-NSC origin. The TH-positive cells in the striatum were surrounded by a round margin that indicated the area of the transplantation (Fig. 3B, D, E). These results indicate that the implanted HB-NSC aggregates successfully differentiated into dopaminergic cells within the host striatal tissue. This finding differs from our previous study using single-cell suspensions for injection which demonstrated abnormal parallel staining of dendrites (Fig. 4A, C). Dendrites of the stained cells extended in various directions, suggesting network formation with the neighboring cells (Fig. 4B, D).
Fig. 3.
The results of immunohistochemistry and immunoflourescence for tyrosin hydroxylase (TH) at 12 weeks posttransplantation in coronal sections of the striatum in the HB-NSC group are shown in B, D, E, G, and the control group in A, C, F. The cell aggregates were TH-positive, and a round margin delineating the border between HB-NSC aggregates and host striatal tissue (red arrows) can been seen in D. The round margin of injected cells in HB-NSC group is shown prominently by IF staining (E), and colocalization of DAPI (blue), TH (red) and human β2 microglobulin (green) positive cells is observed in G (magnification ×400) which is indicated by circle in E. There was no positive staining of IHC (A and C) and IF (F) in control group.
Fig. 4.
IHC staining of TH after 12 weeks ipsilateral cell transplantation with single-cell suspension (A, courtesy of Yoon et al. 2013) and cell aggregates (B). Different patterns of TH-positive staining in transplantation between single-cell suspensions and cell aggregates were detected. In transplantation with single-cell suspensions, abnormal parallel staining of dendrites which considered to be the characterastics of partially differentiated and poorly connected dopaminergic cells was observed (C, magnification ×200, courtesy of Yoon et al. 2013). In transplantation of cell aggregates, dendrites of the stained cells extended in various directions, suggesting the formation of a network with the neighboring cells (D, magnification ×200).
DISCUSSION
In previous studies, neural stem cell transplants in PD had difficulty for surviving and differentiating in the host striatum19). Over the past decade, clinical trials of neural stem cell transplantation in PD patients as well as animal experiments with PD model used intrastriatal grafts because intrastriatal dopaminergic cell grafts not only are capable of reinnervate with the host striatal tissue but also replace the functions of dopaminergic cells of substantia nigra21,23,26,27). On the contrary, fetal dopaminergic neurons transplanted into the substantia nigra have been reported to regrow axons into the striatum in one report22), but another literature showed failure of extending axons into the striatum12). In this regard, we think the transplantation into substantia nigra does not seem to be effective and appropriate compared to striatal injection and also the injection of enough volume of cells might be difficult in substantia nigra compared to the striatal injection. Our previous study on transplantation of HB-NSC as a suspension of single cells showed that the injected cells survived and were demonstrated as positive in TH staining, but behavioral tests and [18F]-FP-CIT scans showed no improvement30). Thus, in this study, aggregation form of HB-NSC were transplanted into the striatum of 6-OHDA rat models and the results were evaluated using behavioral tests, multitracer-microPET scans, IHC, and IF tissue staining.
In the behavioral stepping test, the HB-NSC group showed insignificant increases in contralateral forelimb touches up to 4 weeks after transplantation, but then drastically improved from 6 to 10 weeks posttransplantation. In apomorphine-induced rotation tests, the HB-NSC group exhibited a slight decrease in contralateral rotations by week 8 compared to week 4 posttransplantation, although the difference was not statistically significant. In recent cell transplantation studies, apomorphine-induced rotation tests did not fully reflect the PD status because of apomorphine sensitization18). The significant improvement in the stepping test in the HB-NSC group was not found in the previous single-cell suspension transplant study30). In the stepping test, the drama-tic improvement at 6 weeks posttransplantation suggests an increase in transplanted cell function, previously described as small-world network development by 4 weeks posttransplantation7). The network formed among transplanted HB-NSC or between HB-NSC and host striatal cells could affect cell survival as well as provide therapeutic effects.
Imaging of dopaminergic neurons by techniques such as [18F]-FP-CIT PET scans has been used to diagnose PD3,14). Striatal uptake of [18F]-FP-CIT, a ligand of the dopamine transporter, decreases in PD. This imaging method can diagnose the state of PD more precisely than behavior tests. [18F]-FP-CIT microPET images were useful and consistent indicators of movement disorders in a rat model in our previous reports29,30). In this study, there was a significant increase in [18F]-FP-CIT uptake by 10 weeks posttransplantation compared to the pretransplantation state. These results suggest that the improvements in the behavioral stepping test are the result of dopamine secretion from the transplanted HB-NSC. However, as we described in the methods section, 2 rats in control group were dead during microPET study and comparison between HB-NSC group and control group could not be performed which is a limitation of this study. [18F]-FDG PET scan was performed to evaluate glucose metabolism in the striatum. In typical PD model made by 6-OHDA injection into medial forebrain bundle, glucose metabolism of striatum does not decrease. Therefore, we studied [18F]-FDG PET at 10 week posttransplantation, and the uptake of bilateral striatum did not show significant differences in control group and HB-NSC group, which suggest successful PD models were made.
Transplantation of both single-cell suspensions, reported in our previous study30), and aggregates of HB-NSC results in the appearance of TH-positive cells in striatum. However, improvements in the stepping tests and increasing uptake of [18F]-FP-CIT were demonstrated only after transplantation of HB-NSC aggregates, indicating that dopamine secretion at therapeutic levels only resulted from the trans-plantation of cell aggregates. Although single-cell suspensions transplantation resulted in a wide range of TH-positive cells in the striatum, the transplanted cells showed abnormal parallel staining which considered to be dendrites of partially differentiated and poorly connected dopaminergic cells (Fig. 4C). In addition, no change in [18F]-FP-CIT scans in single-cell suspensions transplanted into the striatum suggests that dopamine secretion from the transplanted cells is absent as a result of incomplete differentiation30). By contrast, cell ag-gregate transplantations showed TH-positive cells only in a small area in the striatum (Fig. 3B, D), but dendrites of the stained cells extended in various directions (Fig. 4D) suggesting the formation of a network with the neighboring cells. From these data, we hypothesize that the cell aggregates adapt well and efficiently secrete neurotransmitter within the host tissue. The number of cells per transplant in this study was less than 10% of the single-cell suspensions used in the previous study (8×104 vs. 1× 106), and this difference in injected cell numbers may explain the small area of TH-positive cells compared to previous study.
This study has the limitation of showing the advantage of pre-established cell network in cell transplantation because we used cell aggregates not spheroid form which is currently difficult to make and transplant deep into the brain. In addition, the phenomenon of marginal TH positive staining in the injection site of cell aggregates could not be explained and quantitative comparison between cell aggregation form and single-cell suspensions was not carried out since the cell numbers of two studies were not similar. These are another limitations of this study.
CONCLUSION
In conclusion, this study showed that cell aggregates of HB-NSC transplanted into the striatum not only survive well, but also exert a therapeutic effect in a rat PD model by secreting dopamine. Such success was not achieved in the transplantation of single-cell suspensions of HB-NSC. Thus, this result suggests that the form of cell transplantation is critical for successful therapy.
Acknowledgements
This work was supported by a "KRCF National Agenda Project", and by an Asan Life Science Institute Grant (11-241) from the Asan Medical Center, Seoul, Republic of Korea.
References
- 1.Agid Y. Parkinson's disease : pathophysiology. Lancet. 1991;337:1321–1324. doi: 10.1016/0140-6736(91)92989-f. [DOI] [PubMed] [Google Scholar]
- 2.Björklund A, Dunnett SB, Stenevi U, Lewis ME, Iversen SD. Reinnervation of the denervated striatum by substantia nigra transplants : functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res. 1980;199:307–333. doi: 10.1016/0006-8993(80)90692-7. [DOI] [PubMed] [Google Scholar]
- 3.Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34:1583–1593. doi: 10.1007/s00259-007-0426-1. [DOI] [PubMed] [Google Scholar]
- 4.Dauer W, Przedborski S. Parkinson's disease : mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 6.Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downes JH, Hammond MW, Xydas D, Spencer MC, Becerra VM, Warwick K, et al. Emergence of a small-world functional network in cultured neurons. PLoS Comput Biol. 2012;8:e1002522. doi: 10.1371/journal.pcbi.1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdö F, Bührle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 9.Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 10.Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson's disease. J Intern Med. 2009;266:358–371. doi: 10.1111/j.1365-2796.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 11.Hwang O, Baker H, Gross S, Joh TH. Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse. 1998;28:140–153. doi: 10.1002/(SICI)1098-2396(199802)28:2<140::AID-SYN4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Bjorklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, et al. Neural transplantation for the treatment of Parkinson's disease. Lancet Neurol. 2003;2:437–445. doi: 10.1016/s1474-4422(03)00442-3. [DOI] [PubMed] [Google Scholar]
- 13.Kato-Negishi M, Tsuda Y, Onoe H, Takeuchi S. A neurospheroid network-stamping method for neural transplantation to the brain. Biomaterials. 2010;31:8939–8945. doi: 10.1016/j.biomaterials.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Kazumata K, Dhawan V, Chaly T, Antonini A, Margouleff C, Belakhlef A, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998;39:1521–1530. [PubMed] [Google Scholar]
- 15.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JS, Lee JS, Im KC, Kim SJ, Kim SY, Lee DS, et al. Performance measurement of the microPET focus 120 scanner. J Nucl Med. 2007;48:1527–1535. doi: 10.2967/jnumed.107.040550. [DOI] [PubMed] [Google Scholar]
- 17.Kim ST, Choi JH, Chang JW, Kim SW, Hwang O. Immobilization stress causes increases in tetrahydrobiopterin, dopamine, and neuromelanin and oxidative damage in the nigrostriatal system. J Neurochem. 2005;95:89–98. doi: 10.1111/j.1471-4159.2005.03342.x. [DOI] [PubMed] [Google Scholar]
- 18.Lei Z, Jiang Y, Li T, Zhu J, Zeng S. Signaling of glial cell line-derived neurotrophic factor and its receptor GFRα1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol. 2011;70:736–747. doi: 10.1097/NEN.0b013e31822830e5. [DOI] [PubMed] [Google Scholar]
- 19.Lund RD, Hauschka SD. Transplanted neural tissue develops connections with host rat brain. Science. 1976;193:582–584. doi: 10.1126/science.959815. [DOI] [PubMed] [Google Scholar]
- 20.McNaught KS, Belizaire R, Jenner P, Olanow CW, Isacson O. Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson's disease. Neurosci Lett. 2002;326:155–158. doi: 10.1016/s0304-3940(02)00296-3. [DOI] [PubMed] [Google Scholar]
- 21.Mehta V, Spears J, Mendez I. Neural transplantation in Parkinson's disease. Can J Neurol Sci. 1997;24:292–301. doi: 10.1017/s0317167100032959. [DOI] [PubMed] [Google Scholar]
- 22.Nikkhah G, Cunningham MG, Cenci MA, McKay RD, Björklund A. Dopaminergic microtransplants into the substantia nigra of neonatal rats with bilateral 6-OHDA lesions. I. Evidence for anatomical reconstruction of the nigrostriatal pathway. J Neurosci. 1995;15(5 Pt 1):3548–3561. doi: 10.1523/JNEUROSCI.15-05-03548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci. 1996;19:102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 24.Paillé V, Henry V, Lescaudron L, Brachet P, Damier P. Rat model of Parkinson's disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov Disord. 2007;22:533–539. doi: 10.1002/mds.21308. [DOI] [PubMed] [Google Scholar]
- 25.Puschban Z, Scherfler C, Granata R, Laboyrie P, Quinn NP, Jenner P, et al. Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience. 2000;95:377–388. doi: 10.1016/s0306-4522(99)00457-1. [DOI] [PubMed] [Google Scholar]
- 26.Rehncrona S. A critical review of the current status and possible developments in brain transplantation. Adv Tech Stand Neurosurg. 1997;23:3–46. doi: 10.1007/978-3-7091-6549-2_1. [DOI] [PubMed] [Google Scholar]
- 27.Tabbal S, Fahn S, Frucht S. Fetal tissue transplantation [correction of transplanation] in Parkinson's disease. Curr Opin Neurol. 1998;11:341–349. doi: 10.1097/00019052-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Williams LN, Seignourel P, Crucian GP, Okun MS, Rodriguez RL, Skidmore FM, et al. Laterality, region, and type of motor dysfunction correlate with cognitive impairment in Parkinson's disease. Mov Disord. 2007;22:141–145. doi: 10.1002/mds.21220. [DOI] [PubMed] [Google Scholar]
- 29.Yoon HH, Lee CS, Hong SH, Min J, Kim YH, Hwang O, et al. Evaluation of a multiple system atrophy model in rats using multitracer microPET. Acta Neurochir (Wien) 2012;154:935–940. doi: 10.1007/s00701-011-1133-z. [DOI] [PubMed] [Google Scholar]
- 30.Yoon HH, Min J, Shin N, Kim YH, Kim JM, Hwang YS, et al. Are human dental papilla-derived stem cell and human brain-derived neural stem cell transplantations suitable for treatment of Parkinson's disease? Neural Regen Res. 2013;8:1190–1200. doi: 10.3969/j.issn.1673-5374.2013.13.004. [DOI] [PMC free article] [PubMed] [Google Scholar]