Abstract
For the past several decades, tremendous efforts have been made to decrease the complications of diabetes, including diabetic retinopathy. New diagnostic modalities like ultrawide field fundus fluorescein angiography and spectral domain optical coherence tomography has allowed more accurate diagnosis of early diabetic retinopathy and diabetic macular edema. Antivascular endothelial growth factors are now extensively used to treat diabetic retinopathy and macular edema with promising results. There remains uncertainty over the long term effects and the socioeconomic costs of these agents.
Keywords: Diabetic retinopathy, Epidemiology, Vascular endothelial growth factor
INTRODUCTION
Diabetic retinopathy (DR) is the most common complication of diabetes mellitus (DM) and is a leading cause of blindness among working-age people worldwide [1]. Globally, it has been estimated that about 30% of people with DM have DR [2]. The number of people with DM in Korea is predicted to be 3.51 million by the year 2010 (7.08% of the expected population according to the Korea National Statistical Office) and 8.97% (4.55 million cases) by 2020 [3,4]. Park et al. [5] reported that overall prevalence of any DR was 19%, and the prevalence of vision-threatening DR was 5%.
The presence of DR is strongly related to the duration of diabetes [5]. In the Seoul Metropolitan City-Diabetes Prevention Program study, participants with duration of 10 years or greater, retinopathy was found in 55.2% compared with 12.6% in those with diabetes for a duration of 10 years or less [5]. In addition, there was an approximate 3-fold increase in vision-threatening DR in those who had diabetes for 10 years or more compared with those with diabetes for 10 years or less [5].
Recent advances in DR research results have shown the important role of vascular endothelial growth factor (VEGF) in pathogenesis of DR. Furthermore, changes of diagnosis and treatment for DR have included the now almost universal use of optical coherent tomography (OCT) for diagnosis of diabetic macular edema (DME), and the shift of treatment paradigm from traditional laser treatment to intraocular delivery of agents that have anti-VEGF effects. To maximize the impact of these recent advances in DR management, it is important to introduce recent advances in DR not only to ophthalmologists, but also to physicians involved in diabetes managements. This article will therefore focus on recent developments and advances in DR in terms of the epidemiology of the disease, and new concepts in diagnosis and treatment.
CURRENT CONCEPTS IN EPIDEMIOLOGY
Emerging data regarding the epidemiology of DR include temporal trend of DR prevalence and expanding knowledge regarding new risk factors. Recent study results showed that the prevalence and incidence of severe DR may be decreasing in people with recently diagnosed with type 1 diabetes [6,7,8,9,10,11]. A decrease in visual impairment related to DR has been reported in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) and other studies of persons with type 1 diabetes was attributed to a declining incidence of proliferative diabetic retinopathy (PDR) and clinically significant macular edema (CSME), likely resulting from improved glycemic control and more aggressive treatment of blood pressure sooner after diagnosis of diabetes [6,7,8,9,10,11]. The declining prevalence of visual impairment is consistent with declines in the estimated annual incidence of visual impairment in people with type 1 diabetes [12]. In the WESDR, estimated annual incidence of any visual impairment over a 25-year period was markedly lower in the most recent period of the study (an annualized rate of 0.28 between the 1995 to 1996 and 2005 to 2007 examinations compared with an annualized rate of 0.65 between the 1980 to 1982 and 1990 to 1992 examinations) [12].
But, in type 2 diabetes, based on data from the U.S. National Health and Nutrition Examination Survey (NHANES), the prevalence of visual impairment was significantly higher in 2005 to 2008 than in 1999 to 2002 [13]. However, while the number of persons with diabetes reporting visual impairment grew, the age-adjusted percentage of adults with diagnosed diabetes who reported visual impairment declined significantly, from 23.7% in 1997 to 16.7% in 2010. These findings are likely related to changes in medical management of type 2 diabetes [14,15,16]. In NHANES, there was increased use of more than 1 oral hypoglycemic agent from the predominant use of 1 type of oral agent [14,15]. This resulted decrease of the mean glycated hemoglobin (HbA1c) levels and increase of maintaining HbA1c levels of less than 7.0% in 41% and 58% of those with type 2 in 1999 to 2000 and 2005 to 2006, respectively [17].
The inconsistency of results among type 2 diabetes can be explained by several factors [18]. First, the ethnic difference among study population was significant. Most of the participants in the WESDR and Beaver Dam Eye Study (BDES) are white. But in persons with type 2 diabetes participating in the 2005 to 2008 NHANES, Mexican American individuals had the highest prevalence of DR and vision-threatening DR in the United States [10,18]. Second, the WESDR and BDES did not include individuals with maturity-onset diabetes of youth [18,19]. Third, the cause of visual impairment was not ascertained in the NHANES. While DR is inferred as the cause, other underlying causes such as cataract and glaucoma may explain some of the differences in the current NHANES findings and other studies [20,21,22,23]. Furthermore, the decreased prevalence of severe levels of DR was documented in the Blue Mountains Eye Study [21]. This result may be explained by increase of type 2 diabetes population but improvement of blood glucose management among them.
In the Korean population, although there are no directly comparable data on the temporal changes of DR prevalence, there appears to be no documented temporal changes of DR prevalence during past three decades [5,22]. This requires more attention to health care needs and costs and developing a comprehensive public health program to slow or prevent the development of visual impairment associated with ocular problems related to diabetes.
In addition to traditional risk factors such as blood glucose level and duration of diabetes, several new potential risk factors for DR have been identified (Table 1). Patients with a lower concentration of insulin or with insulin resistance have been reported to have less DR after adjustment for age, gender, duration of diabetes, blood pressure, and blood glucose concentration [5]. This implies that insulin resistance may be a risk factor for the progression of DR, independent of other metabolic risk factors. Also, high prevalence of PDR in diabetic patients was reported in patients with low pancreatic β-cell capacity [23]. The possible mechanisms of insulin resistance and β-cell function in the development of DR in type 2 diabetic patients have been explained by a delay in insulin reaching extravascular target sites. Lower pancreatic β-cell insulin secretory capacity may be a risk factor for severe DR [5,24,25,26]. Better β-cell function may promote better long-term metabolic control with lower and more physiological peripheral insulin concentrations. This in turn may result in delayed or reduced development of DR. However, longitudinal investigation is needed to further elucidate the clinical importance of insulin resistance and DR.
Table 1.
Summary of diabetic retinopathy risk factors
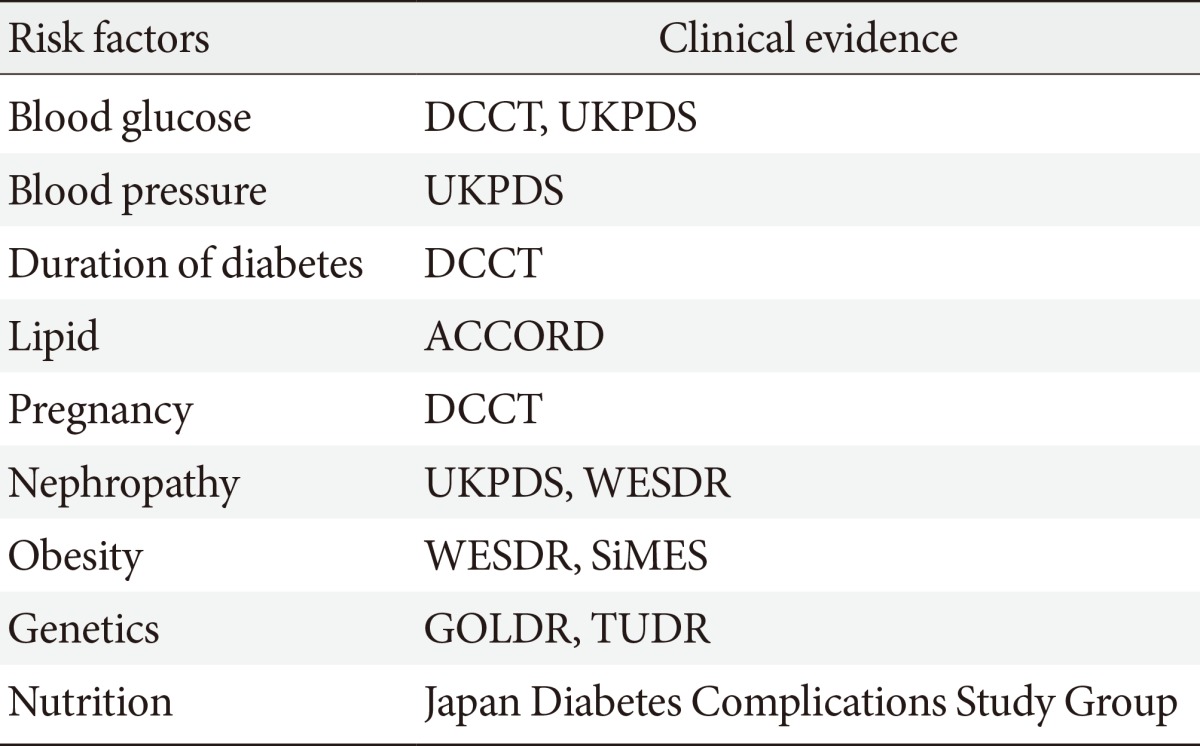
DCCT, diabetes control and complications trial; UKPDS, UK prospective diabetes study; ACCORD, action to control cardiovascular risk in diabetes trial; WESDR, Wisconsin epidemiologic study of diabetic retinopathy; SiMES, Singapore Malay eye study; GOLDR, genetics of Latino diabetic retinopathy study; TUDR, Taiwan-US diabetic retinopathy study.
There have been constant research efforts to understand the genetics of DR. The genetic associations of DR are useful research tool in identifying patients at a high risk of developing DR. A large number of genes and genetic variants have been reported [27,28,29,30,31,32,33,34]. However, these results have not been replicated and yet no genes have been accepted as a high risk gene of DR. Recently, the genome-wide analysis association data for severe DR found a genetic association for susceptibility to DR in five chromosomal regions and PLXDC2 and ARHGAP22, the latter two of which are genes implicated in endothelial cell angiogenesis and increased capillary permeability in Taiwanese population [27]. And most recently, in the Chinese population, three potential susceptibility loci, on chromosomes 13q22.2, 2q31.1, and 2q37.2, for DR and the risk alleles in these regions are possibly associated with DR were reported [28]. However, further studies to confirm these associations are needed [29]. Addition to the well-known risk factors, increasing attention is assigned to obesity and interrelationship with type 2 diabetes. Obesity intensifies the risk of type 2 diabetes, its macrovascular complications, and reduces life. An increase in body mass index (BMI) also correlated significantly with the deterioration of HbA1c, a decrease in high density lipoprotein cholesterol, an increase in triglycerides as well as a higher prevalence of hypertension [30,31,32,33,34]. Both metabolic syndrome and increased oxidative stress due to their association with obesity and DR have also been suggested as possible pathophysiological mechanisms. Most studies have reported a significant association between high BMI and obesity with DR [30,31,32]. But other studies have reported an association between low BMI and DR suggesting a possible protective role for higher BMI in the development of DR [33,34]. These inconstant results may be partly explained by methodological differences, and racial or ethnic differences.
Medical nutritional treatment is important in prevention of diabetes complications, but the preventive effect of nutrition on DR is not well understood.
Observational study done in Japanese type 2 diabetic patients showed that increased fruit intake was associated with reduced incident DR among patients with a low-fat energy-restricted diet [35]. The mechanisms whereby fruits exert preventive effects on DR are not clear, but a high fruit-vegetable intervention is known to increase carotene and vitamin C levels in plasma. Another possibility is that the preventive effects of fruits are mediated through glycemic control. Fruits are low glycemic-index foods rich in dietary fiber, which can slow glucose response after ingestion [36].
CURRENT CONCEPTS IN DIAGNOSIS
Among the important advances of DR diagnosis over past decade is the increasing role of ultrawide field fundus fluorescein angiography (UWFA) and OCT (Figs. 1 and 2).
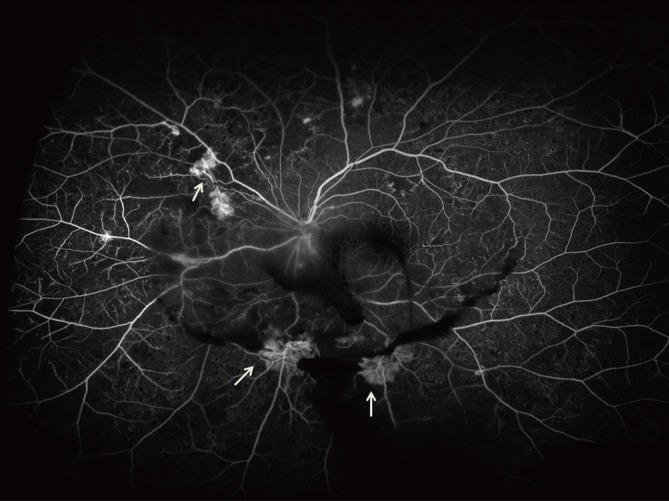
Fig. 1.
Ultrawide-field fluorescein angiogram demonstrates peripheral neovascularization and capillary nonperfusion (arrows) in eye with proliferative diabetic retinopathy.
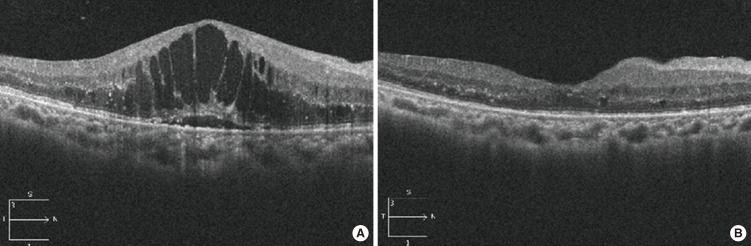
Fig. 2.
Decreased of cystoid diabetic macular edema after intravitreal bevacizumab injection documented by spectral domain optical coherence tomography. (A) Severe cystoid diabetic macular edema with intraretinal cystoid spaces. Vision with 0.12. (B) Decrease of macular thickness with disappearance of intraretinal cystic space after injection. Vision improved to 0.5.
The importance of imaging the peripheral retina in patients with DR has been recognized for many years. Wide-field imaging has been increasing used in the diagnosis and management of DR, initially, primarily as a screening device [37,38]. UWFA allows visualization of the up to 200° of the peripheral retina captured in a single frame. The report by Friberg et al. [39] suggested that wide-field imaging could be used to study the relationship between peripheral capillary nonperfusion and the development of neovascularization, a precursor to PDR. Recently, a study reported that ultrawide-field imaging visualizes 3.2 times more retinal surface area than the conventional seven standard fields [40]. In this report, the UWFA imaged 3.9 times more nonperfusion, 1.9 times more neovascularization, and 3.8 times more panretinal photocoagulation than the conventional seven standard fields [40]. Improved visualization of the retinal periphery through UWFA may have significant implications for the treatment of patients.
OCT has revolutionized the clinical practice of ophthalmology [41,42,43,44,45,46]. It is a noninvasive imaging technique that provides high-resolution, cross-sectional images of the retina, retinal nerve fiber layer and the optic nerve head. In 2006, the first commercially available spectral domain OCT (SD-OCT) system was introduced. SD-OCT employs detection of the light echoes simultaneously by measuring the interference spectrum, using an interferometer with a high-speed spectrometer. This technique achieves scan rates of 20,000 to 52,000 A-scans per second and a resolution of 5 to 7 µm in tissue. Although concerns were made with using central retinal thickness measured with OCT as a gold standard test to diagnose CSME and decide the treatments, attempts to classify CSME characteristics by OCT to aid in diagnosis and establish treatment strategies are made by several studies [42,43,44]. Also associations with visual prognosis of DME with characteristic features of individual retinal layer were studied. The presence of hyper-reflective foci in the outer retina is closely associated with a disrupted inner segment/outer segment line on SD-OCT images and decreased visual acuity (VA) in DME. Addition to SD-OCT findings, other ocular or systemic factors should be investigated that together with the retinal state can serve as prognostic factors for the visual outcome of specific therapies.
CURRENT CONCEPTS IN TREATMENT
This section will focus mainly on recent randomized controlled trials (RCTs) results for DR and DME. However, it should be emphasized that optimal management of systemic risk factors is the most important factor for primary prevention of DR. Intensive control of hyperglycaemia, hypertension, and possibly hyperlipidemia can delay the onset and progression of DR. There are 541 clinical trials listed in the registry of clinical trials (www.clinicaltrials.gov) regarding DR treatment and most of these trials are aimed at treatment of DME. Only selected results from trial results are included in this section (Table 2).
Table 2.
Overview of randomized controlled trials listed in the review for treatment of diabetic macular edema
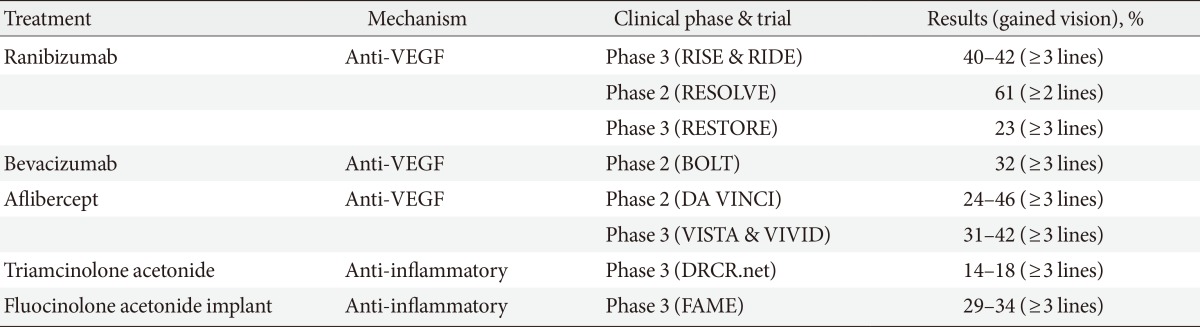
VEGF, vascular endothelial growth factor; RIDE/RISE, a study of ranibizumab injection in subjects with CSDME with center involvement secondary to diabetes mellitus; RESOLVE, safety and efficacy of ranibizumab in diabetic macular edema; RESTORE, a 12-month core study to assess the efficacy and safety of ranibizumab intravitreal injections; BOLT, bevacizumab or laser therapy; DA VINCI, DME and VEGF Trap-Eye investigation of clinical impact study; VISTA, study of intravitreal administration of VEGF Trap-Eye in patients with diabetic macular edema; VIVID, intravitreal alfibercept injection in vision impairment due to DME; DRCR.net, diabetic retinopathy clinical research network; FAME, fluocinolone acetonide in diabetic macular edema.
Ranibizumab
A study of ranibizumab injection in subjects with clinically significant macular edema with center involvement secondary to diabetes mellitus (RISE) & A study of ranibizumab injection in subjects with clinically significant macular edema with center involvement secondary to diabetes mellitus (RIDE) [47,48]
These were phase 3, randomized, multicenter, double-masked, 3-year trials, sham injection-controlled for 2 years. Patients with DME (n=759) were randomized equally to monthly 0.5 or 0.3 mg ranibizumab or sham injection. In the third year, sham patients, while still masked, were eligible to cross over to monthly 0.5 mg ranibizumab. VA outcomes seen at 24 months in ranibizumab groups were consistent through 36 months; the proportions of patients who gained ≥15 letters from baseline at 36 months in the sham/0.5, 0.3, and 0.5 mg ranibizumab groups were 19.2%, 36.8%, and 40.2%, respectively, in RIDE and 22.0%, 51.2%, and 41.6%, respectively, in RISE. In the ranibizumab arms, reductions in central foveal thickness seen at 24 months were, on average, sustained through 36 months. The strong VA gains and improvement in retinal anatomy achieved with ranibizumab at 24 months were sustained through 36 months.
Safety and efficacy of ranibizumab in diabetic macular edema with center involvement (RESOLVE) [49]
RESOLVE was a phase 2 double-masked, sham-controlled RCT evaluating the efficacy and safety of ranibizumab compared with sham treatment over 12 months. Patients (n=151) with VA 20/40 to 20/160 and central retinal thickness (CRT) ≥300 µm were randomly assigned to ranibizumab 0.3 or 0.5 mg, or sham injections. At the end of the study, a mean average change in best-corrected visual acuity (BCVA) by +7.8 letters from baseline was observed in the ranibizumab groups compared to -0.1 letters in the sham group (P<0.0001). Mean CRT reduction was parallel to mean VA improvement. More than three times the proportion of patients who were treated with ranibizumab gained ≥10 and ≥15 letters compared the gain in those receiving sham injections.
A 12-month core study to assess the efficacy and safety of ranibizumab (intravitreal injections) in patients with visual impairment due to diabetic macular edema and a 24 month open-label extension study (RESTORE) [50]
RESTORE consisted with core study (12 months) and 24 months open label extension study. It was a phase III study that aimed to confirm the efficacy and safety of ranibizumab (0.5 mg) as adjunctive therapy when added to laser photocoagulation and/or monotherapy in patients with visual impairment due to DME.
A total of 345 patients with visual impairment 20/32 to 20/160 were enrolled in the study. Significant improvement in BVCA was seen with ranibizumab alone (+6.1 letters) and combined with laser (+5.9 letters) compared with laser monotherapy (+0.8 letter) at 12 months. The proportion of patients who gained ≥10 and ≥15 letters was two to three times greater in the ranibizumab groups compared with laser (37.4% vs. 15.5% and 22.6% vs. 8.2%, respectively). Approximately 9.2% more patients experienced ≥10 letter loss with laser than with ranibizumab. Ranibizumab monotherapy and combined with laser provided superior VA gain over standard laser in patients with visual impairment due to DME. At 1 year, no differences were detected between the ranibizumab and ranibizumab +laser arms. Of the 303 patients who completed the randomized RESTORE 12-month core study, 240 entered the extension study. In patients treated with ranibizumab during the core study, consecutive individualized ranibizumab treatment during the extension study led to an overall maintenance of BCVA and central retinal subfield thickness (CRST) observed at month 12 over the 2-year extension study (prior ranibizumab: +8.0 letters, -142.1 µm; prior ranibizumab+laser: +6.7 letters, -145.9 µm from baseline at month 36) with a median of 6.0 injections (mean, 6.8; prior ranibizumab) and 4.0 injections (mean, 6.0; prior ranibizumab+laser). In the prior laser group, a progressive BCVA improvement (+6.0 letters) and CRST reduction (-142.7 µm) at month 36 were observed after allowing ranibizumab during the extension study, with a median of 4.0 injections (mean, 6.5 injections). Ranibizumab was effective in improving and maintaining BCVA and CRST outcomes with a progressively declining number of injections over 3 years of individualized dosing.
Bevacizumab
A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema [51]
This study purpose was to report the findings at 1 year of a study comparing repeated intravitreal bevacizumab and modified Early Treatment of Diabetic Retinopathy Study macular laser therapy in patients with persistent clinically significant DME.
Subjects were randomized to either intravitreal bevacizumab (6 weekly; minimum of three injections and maximum of nine injections in the first 12 months) or macular laser therapy (4 monthly; minimum of one treatment and maximum of four treatments in the first 12 months).
The primary end point was the difference in early treatment diabetic retinopathy study (ETDRS) BCVA at 12 months between the bevacizumab and laser arms. The bevacizumab group gained a median of eight ETDRS letters, whereas the laser group lost a median of 0.5 ETDRS letters (P=0.0002). The odds of gaining > or =10 ETDRS letters over 12 months were 5.1 times greater in the bevacizumab group than in the laser group (adjusted odds ratio, 5.1; 95% confidence interval, 1.3 to 19.7; P=0.019). Improvements in BCVA and central macular thickness seen with bevacizumab at 1 year were maintained over the second year with a mean of four injections.
Steroid & laser
Intravitreal triamcinolone acetonide versus laser for diabetic macular edema [52,53]
This was phase 3 study to evaluate the efficacy and safety of 1- and 4-mg doses of preservative-free intravitreal triamcinolone in comparison with focal/grid photocoagulation for the treatment of DME. Eight hundred forty study eyes with DME were randomized to focal/grid photocoagulation (n=330), 1 mg intravitreal triamcinolone (n=256), or 4 mg intravitreal triamcinolone (n=254). Retreatment was given for persistent or new edema at 4-month intervals. At 4 months, mean VA was better in the 4-mg triamcinolone group than in either the laser group (P<0.001) or the 1-mg triamcinolone group (P=0.001). By 1 year, there were no significant differences among groups in mean VA. At the 16-month visit and extending through the primary outcome visit at 2 years, mean VA was better in the laser group than in the other two groups (at 2 years, P=0.02 comparing the laser and 1-mg groups, P=0.002 comparing the laser and 4-mg groups, and P=0.49 comparing the 1- and 4-mg groups). Over a 2-year period, focal/grid photocoagulation is more effective and has fewer side effects than 1- or 4-mg doses of preservative-free intravitreal triamcinolone for most patients with DME.
Aflibercept
The DA VINCI study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema [53]
This was multicenter, randomized, double-masked, phase 2 clinical trial designed to examine the effects of intravitreal aflibercept compared to standard laser treatment. Patients (n=221) with CSME were randomized to receive VEGF Trap-Eye of different dosage and schedules or laser treatment. Results at 52 weeks showed greater VA gains for aflibercept- (9.7 to 12.0 letters) than for laser treatment (-1.3 letters). Patients who were treated with VEGF Trap-Eye were more likely to experience ≥10 and ≥15 letter gains compared to those who received laser treatment (45% to 71% vs. 30% and 23.8% to 45.5% vs. 11.4%, respectively).
Study of intravitreal administration of VEGF Trap-Eye in patients with diabetic macular edema (VISTA DME) & intravitreal alfibercept injection in vision impairment due to DME (VIVID DME) [54]
VISTA and VIVID were 2 similarly designed, double-masked, randomized, phase 3 trials to determine the efficacy of intravitreally administered VEGF Trap-Eye on the BCVA assessed by the ETDRS chart in patients with DME with central involvement.
VISTA study was conducted in the United States and the VIVID study was conducted across Europe, Japan, and Australia. Eight hundred seventy-two eyes with type 1 or 2 diabetes mellitus who presented with DME with central involvement received either intravitreal aflibercept injection (IAI) 2 mg every 4 weeks, IAI 2 mg every 8 weeks after 5 initial monthly doses, or macular laser photocoagulation. At week 52, IAI demonstrated significant superiority in functional and anatomic endpoints over laser, with similar efficacy in the every 4 weeks and every 8 weeks groups despite the extended dosing interval in the every 8 weeks group.
Others
A multicenter study to compare multiple doses of intravitreal microplasmin versus sham injection for treatment of patients with diabetic macular edema (MIVI-II)
This was phase 2 randomized, sham injection-controlled, double-masked, ascending-dose, dose-range finding trial of microplasmin intravitreal injection for nonsurgical posterior vitreous detachment (PVD) induction for treatment of DME.
The primary efficacy variable was the proportion of patients with total PVD 14 days after the 25, 75, 125 µg of ocriplasmin, and sham injection. According to study results posted at clinicaltrials. gov, there were no statistically significant differences of PVD induction between 25, 75, or 125 µg versus the sham group.
Fluocinolone acetonide implant compared to sham injection in patients with diabetic macular edema (FAME) [55,56,57,58]
The FAME study, a 36-months, double-blind, sham-controlled, phase 3 study, examined the efficacy and safety profile of fluocinolone acetonide (FA) compared with sham injection in patients with persistent or recurrent DME. DME patients (n=956) were randomized to receive FA intravitreal inserts 0.2, 0.5 µg or sham injection. Treatment efficacy was similar with both low- and high-dose FA but the benefit-to-risk profile was more favourable with the low-dose. At month 36, the percentage of patients who gained ≥15 in letter score using the last observation carried forward method was 28.7% (low dose) and 27.8% (high dose) in the FAc insert groups compared with 18.9% (P=0.018) in the sham group, and considering only those patients still in the trial at month 36, it was 33.0% (low-dose) and 31.9% (high-dose) compared with 21.4% in the sham group (P=0.030). Almost all phakic patients in the FAc insert groups developed cataract, but their visual benefit after cataract surgery was similar to that in pseudophakic patients. The incidence of incisional glaucoma surgery at month 36 was 4.8% in the low-dose group and 8.1% in the high-dose insert group.
The role of surgical treatment for DR especially for DME, have been decreased due to wide spread use of locally delivered anti-VEGF therapy. But recent developments in transconjunctival microincision vitrectomy with 23- or 25-gauge instrumentation have provided potential advantages over traditional 20-gauge vitrectomy, like faster wound healing, decreased surgical time, and early visual recovery [57,58,59]. And there is no doubt that DME with tractional components and vitreous hemorrhage or tractional retinal detachments from PDR needs surgical approach. But until now, most of study results are from small nonrandomized trials and large RCT with long term follow-up are needed to establish a role for this treatment approach.
CONCLUSIONS
Korea has one of the fastest growing diabetes populations and DR is the major cause of vision loss in patients with diabetes. Patients with DR need lifelong attention with versatile treatment approaches. Recent advances in imaging and diagnosis of DR, PDR and DME and the new clinical trials on the use of intravitreal anti-VEGF therapy has improved management of this major clinical and public health problem.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY Meta-Analysis for Eye Disease (META-EYE) Study Group. META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998-2005. Diabetes Care. 2009;32:2016–2020. doi: 10.2337/dc08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korean Diabetes Association; Health Insurance Review & Assessment Service. Report of Task Force Team for basic statistical study of Korean diabetes mellitus: diabetes in Korea 2007. Seoul: Goldfishery; 2008. [Google Scholar]
- 5.Park CY, Park SE, Bae JC, Kim WJ, Park SW, Ha MM, Song SJ. Prevalence of and risk factors for diabetic retinopathy in Koreans with type II diabetes: baseline characteristics of Seoul Metropolitan City-Diabetes Prevention Program (SMC-DPP) participants. Br J Ophthalmol. 2012;96:151–155. doi: 10.1136/bjo.2010.198275. [DOI] [PubMed] [Google Scholar]
- 6.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, Binder C, Parving HH. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859–1868. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordwall M, Bojestig M, Arnqvist HJ, Ludvigsson J Linkoping Diabetes Complications Study. Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of type 1 diabetes-the Linkoping Diabetes Complications Study. Diabetologia. 2004;47:1266–1272. doi: 10.1007/s00125-004-1431-6. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE. Are individuals with diabetes seeing better?: a long-term epidemiological perspective. Diabetes. 2010;59:1853–1860. doi: 10.2337/db09-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2010;117:63–70. doi: 10.1016/j.ophtha.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko F, Vitale S, Chou CF, Cotch MF, Saaddine J, Friedman DS. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999-2002 and 2005-2008. JAMA. 2012;308:2361–2368. doi: 10.1001/jama.2012.85685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh DC, Choi IS, Plauschinat C, Kwon J, Baron M. Impact of comorbid conditions and race/ethnicity on glycemic control among the US population with type 2 diabetes, 1988-1994 to 1999-2004. J Diabetes Complications. 2010;24:382–391. doi: 10.1016/j.jdiacomp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999-2004. Ann Epidemiol. 2008;18:222–229. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122:443–453. doi: 10.1016/j.amjmed.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 16.Mann DM, Woodward M, Ye F, Krousel-Wood M, Muntner P. Trends in medication use among US adults with diabetes mellitus: glycemic control at the expense of controlling cardiovascular risk factors. Arch Intern Med. 2009;169:1718–1720. doi: 10.1001/archinternmed.2009.296. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Klein BE. Is the prevalence of visual impairment rising or falling in the people with diabetes mellitus? It depends on who you study. JAMA Ophthalmol. 2013;131:948–950. doi: 10.1001/jamaophthalmol.2013.4023. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1995;2:49–55. doi: 10.3109/09286589509071451. [DOI] [PubMed] [Google Scholar]
- 20.Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes. The Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–1177. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 21.Cugati S, Kifley A, Mitchell P, Wang JJ. Temporal trends in the age-specific prevalence of diabetes and diabetic retinopathy in older persons: population-based survey findings. Diabetes Res Clin Pract. 2006;74:301–308. doi: 10.1016/j.diabres.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim HK, Kim CH, Kim SW, Park JY, Hong SK, Yoon YH, Lee KU. Development and progression of diabetic retinopathy in Koreans with NIDDM. Diabetes Care. 1998;21:134–138. doi: 10.2337/diacare.21.1.134. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki K, Watanabe K, Motegi T, Kajinuma H. High prevalence of proliferative retinopathy in diabetic patients with low pancreatic B-cell capacity. Diabetes Res Clin Pract. 1989;6:45–52. doi: 10.1016/0168-8227(89)90056-9. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Lee KE, Knudtson MD, Gangnon RE, Klein BE. Changes in visual impairment prevalence by period of diagnosis of diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2009;116:1937–1942. doi: 10.1016/j.ophtha.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JH, Tung TH, Tsai ST, Chou P, Chuang SY, Chen SJ, Lee FL, Shih HC, Li WL. A community-based epidemiologic study of gender differences in the relationship between insulin resistance/ beta-cell dysfunction and diabetic retinopathy among type 2 diabetic patients in Kinmen, Taiwan. Ophthalmologica. 2006;220:252–258. doi: 10.1159/000093080. [DOI] [PubMed] [Google Scholar]
- 26.Maneschi F, Mashiter K, Kohner EM. Insulin resistance and insulin deficiency in diabetic retinopathy of non-insulin-dependent diabetes. Diabetes. 1983;32:82–87. doi: 10.2337/diab.32.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Huang YC, Lin JM, Lin HJ, Chen CC, Chen SY, Tsai CH, Tsai FJ. Genome-wide association study of diabetic retinopathy in a Taiwanese population. Ophthalmology. 2011;118:642–648. doi: 10.1016/j.ophtha.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Sheu WH, Kuo JZ, Lee IT, Hung YJ, Lee WJ, Tsai HY, Wang JS, Goodarzi MO, Klein R, Klein BE, Ipp E, Lin SY, Guo X, Hsieh CH, Taylor KD, Fu CP, Rotter JI, Chen YD. Genome-wide association study in a Chinese population with diabetic retinopathy. Hum Mol Genet. 2013;22:3165–3173. doi: 10.1093/hmg/ddt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132:96–107. doi: 10.1001/jamaophthalmol.2013.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastelan S, Tomic M, Gverovic Antunica A, Ljubic S, Salopek Rabatic J, Karabatic M. Body mass index: a risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm. 2013;2013:436329. doi: 10.1155/2013/436329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 32.Dirani M, Xie J, Fenwick E, Benarous R, Rees G, Wong TY, Lamoureux EL. Are obesity and anthropometry risk factors for diabetic retinopathy? The diabetes management project. Invest Ophthalmol Vis Sci. 2011;52:4416–4421. doi: 10.1167/iovs.11-7208. [DOI] [PubMed] [Google Scholar]
- 33.Rooney D, Lye WK, Tan G, Lamoureux EL, Ikram MK, Cheng CY, Kumari N, Zheng YF, Mitchell P, Wang JJ, Wong TY, Sabanayagam C. Body mass index and retinopathy in Asian populations with diabetes mellitus. Acta Diabetol. doi: 10.1007/s00592-014-0602-2. Epub 2014 Jun 1. DOI: http://dx.doi.org/10.1007/s00592-014-0602-2. [DOI] [PubMed] [Google Scholar]
- 34.Klein R, Klein BE, Moss SE. Is obesity related to microvascular and macrovascular complications in diabetes? The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 1997;157:650–656. [PubMed] [Google Scholar]
- 35.Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, Ohashi Y, Araki A, Ito H, Akanuma Y, Yamada N, Yamashita H, Sone H Japan Diabetes Complications Study Group. Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology. 2013;24:204–211. doi: 10.1097/EDE.0b013e318281725e. [DOI] [PubMed] [Google Scholar]
- 36.Djuric Z, Ren J, Mekhovich O, Venkatranamoorthy R, Heilbrun LK. Effects of high fruit-vegetable and/or low-fat intervention on plasma micronutrient levels. J Am Coll Nutr. 2006;25:178–187. doi: 10.1080/07315724.2006.10719530. [DOI] [PubMed] [Google Scholar]
- 37.Manivannan A, Plskova J, Farrow A, McKay S, Sharp PF, Forrester JV. Ultra-wide-field fluorescein angiography of the ocular fundus. Am J Ophthalmol. 2005;140:525–527. doi: 10.1016/j.ajo.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 38.Wessel MM, Aaker GD, Parlitsis G, Cho M, D'Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785–791. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 39.Friberg TR, Pandya A, Eller AW. Non-mydriatic panoramic fundus imaging using a non-contact scanning laser-based system. Ophthalmic Surg Lasers Imaging. 2003;34:488–497. [PubMed] [Google Scholar]
- 40.Kaines A, Oliver S, Reddy S, Schwartz SD. Ultrawide angle angiography for the detection and management of diabetic retinopathy. Int Ophthalmol Clin. 2009;49:53–59. doi: 10.1097/IIO.0b013e31819fd471. [DOI] [PubMed] [Google Scholar]
- 41.Virgili G, Menchini F, Murro V, Peluso E, Rosa F, Casazza G. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2011;(7):CD008081. doi: 10.1002/14651858.CD008081.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Bolz M, Lammer J, Deak G, Pollreisz A, Mitsch C, Scholda C, Kundi M, Schmidt-Erfurth U Diabetic Retinopathy Research Group Vienna. SAVE: a grading protocol for clinically significant diabetic macular oedema based on optical coherence tomography and fluorescein angiography. Br J Ophthalmol. 2014;98:1612–1617. doi: 10.1136/bjophthalmol-2013-304564. [DOI] [PubMed] [Google Scholar]
- 43.Murakami T, Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabetes Res. 2013;2013:920713. doi: 10.1155/2013/920713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda-Arakawa N, Muraoka Y, Yoshimura N. Segmentational analysis of retinal thickness after vitrectomy in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53:6668–6674. doi: 10.1167/iovs.12-9934. [DOI] [PubMed] [Google Scholar]
- 45.Bolz M, Ritter M, Schneider M, Simader C, Scholda C, Schmidt-Erfurth U. A systematic correlation of angiography and high-resolution optical coherence tomography in diabetic macular edema. Ophthalmology. 2009;116:66–72. doi: 10.1016/j.ophtha.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 46.Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C Diabetic Retinopathy Research Group Vienna. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116:914–920. doi: 10.1016/j.ophtha.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 48.Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP, Ehrlich JS, Hopkins JJ RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 49.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 52.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–1449. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, Vitti R, Ruckert R, Sandbrink R, Stein D, Yang K, Beckmann K, Heier JS. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118:1819–1826. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Brown DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J, Kapik B, Billman K, Kane FE FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–635. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 56.Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C, Reichel E, Soubrane G, Kapik B, Billman K, Kane FE, Green K FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–2132. doi: 10.1016/j.ophtha.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 57.Farouk MM, Naito T, Sayed KM, Nagasawa T, Katome T, Radwan G, Abdallah A, Elagouz M. Outcomes of 25-gauge vitrectomy for proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:369–376. doi: 10.1007/s00417-010-1506-7. [DOI] [PubMed] [Google Scholar]
- 58.Park DH, Shin JP, Kim SY. Comparison of clinical outcomes between 23-gauge and 20-gauge vitrectomy in patients with proliferative diabetic retinopathy. Retina. 2010;30:1662–1670. doi: 10.1097/IAE.0b013e3181d95261. [DOI] [PubMed] [Google Scholar]
- 59.Ozone D, Hirano Y, Ueda J, Yasukawa T, Yoshida M, Ogura Y. Outcomes and complications of 25-gauge transconjunctival sutureless vitrectomy for proliferative diabetic retinopathy. Ophthalmologica. 2011;226:76–80. doi: 10.1159/000328407. [DOI] [PubMed] [Google Scholar]