Abstract
The fatty acid composition of animal cells cultured in serum-free medium can be manipulated when the synthesis of endogenous fatty acids is inhibited by a biotin analog and fatty acids are supplied in the medium as detergent esters of Tween. When mouse LM cells were grown in medium supplemented with Tween-19:0 (an ester of Tween and nonadecanoic acid), odd chain fatty acid content of cellular phospholipids and neutral lipids increased from 1% to 75%. Concurrently, the saturated fatty acid content increased from 27% to 85%. Similar alterations in fatty acid content have been observed when BHK21 cells are subjected to the same enrichment regime. The ability to control the fatty acid composition of cultured animal cells is a prerequisite to investigations into the role of the membrane lipid physical state in processes unique to these cells.
Keywords: LM cells, desthiobiotin, serum-free medium, membranes, detergents
Full text
PDF
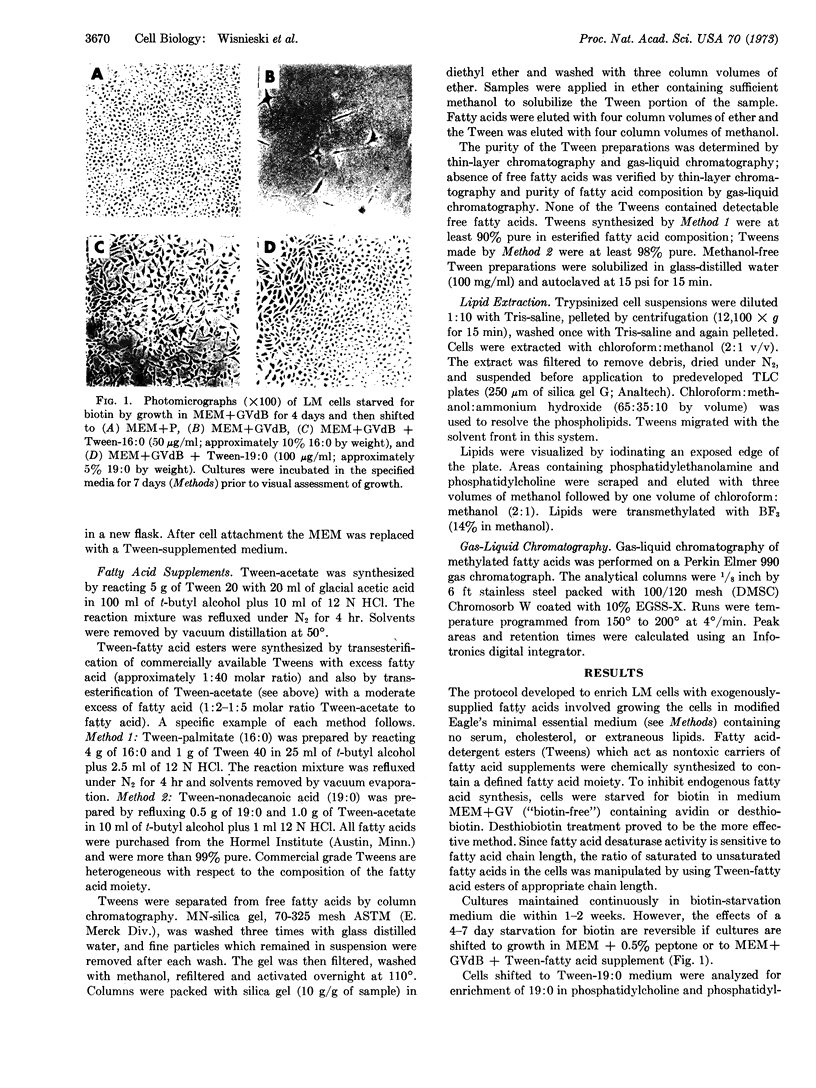



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Cumming R. B., Walton M., Snyder F. Lipid metabolism in cells grown in tissue culture: O-alkyl, O-alk-I-enyl, and acyl moieties of L-M cells. Biochim Biophys Acta. 1969 Apr 29;176(3):491–501. doi: 10.1016/0005-2760(69)90216-1. [DOI] [PubMed] [Google Scholar]
- Brett D., Howling D., Morris L. J., James A. T. Specificity of the fatty acid desaturases. The conversion of saturated to monoenoic acids. Arch Biochem Biophys. 1971 Apr;143(2):535–547. doi: 10.1016/0003-9861(71)90238-4. [DOI] [PubMed] [Google Scholar]
- Brody S., Allen B. The effects of branched chain fatty acid incorporation into Neurospora crassa membranes. J Supramol Struct. 1972;1(2):125–134. doi: 10.1002/jss.400010205. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani M., Crowfoot P. D., Wakil S. J. Molecular organization of lipids in Escherichia coli membranes. II. Effect of phospholipids on succinic-ubiquinone reductase activity. J Biol Chem. 1972 Nov 25;247(22):7251–7256. [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R. P. Uptake and retention of fatty acids by tissue culture cells. Wistar Inst Symp Monogr. 1967;6:33–47. [PubMed] [Google Scholar]
- Levey G. S. Restoration of glucagon responsiveness of solubilized myocardial adenyl cyclase by phosphatidylserine. Biochem Biophys Res Commun. 1971 Apr 2;43(1):108–113. doi: 10.1016/s0006-291x(71)80093-1. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L. J. Mechanisms and stereochemistry in fatty acid metabolism. Biochem J. 1970 Aug;118(5):681–693. doi: 10.1042/bj1180681g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Krans H. M., Kozyreff V., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. VI. Evidence for a role of membrane lipids. J Biol Chem. 1971 Jul 25;246(14):4447–4454. [PubMed] [Google Scholar]
- Rethy A., Tomasi V., Trevisani A., Barnabei O. The role of phosphatidylserine in the hormonal control of adenylate cyclase of rat liver plasma membranes. Biochim Biophys Acta. 1972 Dec 1;290(1):58–69. doi: 10.1016/0005-2736(72)90052-1. [DOI] [PubMed] [Google Scholar]
- Réthy A., Tomasi V., Trevisani A. The role of lipids in the activity of adenylate cyclase of rat liver plasma membranes. Arch Biochem Biophys. 1971 Nov;147(1):36–40. doi: 10.1016/0003-9861(71)90306-7. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Overath P. Lipids containing trans-unsaturated fatty acids change the temperature characteristic of thiomethylgalactoside accumulation in Escherichia coli. J Mol Biol. 1969 Aug 28;44(1):209–214. doi: 10.1016/0022-2836(69)90416-1. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Abortive assembly of the lactose transport system in Escherichia coli. Biochemistry. 1973 Jul 17;12(15):2816–2822. doi: 10.1021/bi00739a007. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Transport system assembly and the mobility of membrane lipids in Escherichia coli. Biochemistry. 1973 Jul 17;12(15):2822–2829. doi: 10.1021/bi00739a008. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]






