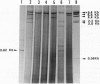
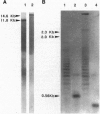
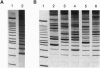
Abstract
It has been shown that the mtDNA of the parasitic trematode Schistosoma mansoni is hypervariable in size. We report here that this length variation is due to a large polymorphic minisatellite composed of two types of repeated sequences of 558 bp and 62 bp. Each minisatellite repeat is made up of a large 558-bp component and a variable tandem array of the small 62-bp unit. Of more fundamental interest was the finding that both the 558-bp and 62-bp components have significant homology with a gene, SM750, previously identified in the nuclear genome of S. mansoni. The small 62-bp unit is identical to the nuclear polymorphic repeat element, which is apparently spread throughout the nuclear genome and is abundant among transcripts, in addition to being present in five tandem copies in SM750. The presence, in the S. mansoni mtDNA, of fragments of genes that are present in and transcribed from the nuclear genome raises the question of the origin of these sequences. The arrangement and the variability that the mtDNA minisatellite embodies were explored as an identity test for S. mansoni based on the use of PCR for tallying the relative abundance of the several repeat numbers of the tandem arrays of the 62-bp unit within the minisatellite structure.
Full text
PDF
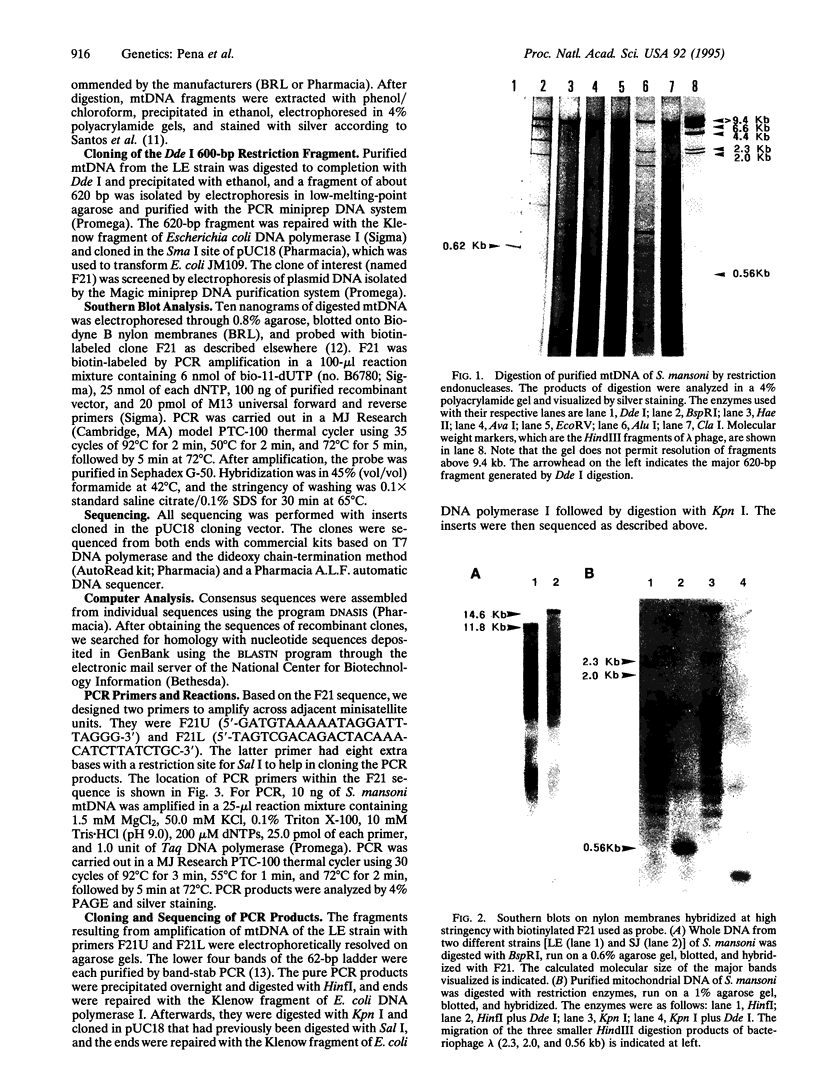


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G. Animal mitochondrial DNA: an extreme example of genetic economy. Int Rev Cytol. 1985;93:93–145. doi: 10.1016/s0074-7696(08)61373-x. [DOI] [PubMed] [Google Scholar]
- Bentzen P., Leggett W. C., Brown G. G. Length and restriction site heteroplasmy in the mitochondrial DNA of american shad (alosa sapidissima). Genetics. 1988 Mar;118(3):509–518. doi: 10.1093/genetics/118.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjourson A. J., Cooper J. E. Band-stab PCR: a simple technique for the purification of individual PCR products. Nucleic Acids Res. 1992 Sep 11;20(17):4675–4675. doi: 10.1093/nar/20.17.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce T. M., Zwick M. E., Aquadro C. F. Mitochondrial DNA in the bark weevils: size, structure and heteroplasmy. Genetics. 1989 Dec;123(4):825–836. doi: 10.1093/genetics/123.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. P., 3rd, Muralidhar M. G., McKerrow J. H., Wang C. C. Evidence for a class of very small introns in the gene for hypoxanthine-guanine phosphoribosyltransferase in Schistosoma mansoni. Nucleic Acids Res. 1989 Feb 25;17(4):1635–1647. doi: 10.1093/nar/17.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densmore L. D., Wright J. W., Brown W. M. Length variation and heteroplasmy are frequent in mitochondrial DNA from parthenogenetic and bisexual lizards (genus Cnemidophorus). Genetics. 1985 Aug;110(4):689–707. doi: 10.1093/genetics/110.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P., Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990 Apr 20;212(4):599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Despres L., Imbert-Establet D., Combes C., Bonhomme F., Monnerot M. Isolation and polymorphism in mitochondrial DNA from Schistosoma mansoni. Mol Biochem Parasitol. 1991 Jul;47(1):139–141. doi: 10.1016/0166-6851(91)90156-z. [DOI] [PubMed] [Google Scholar]
- Desprès L., Imbert-Establet D., Monnerot M. Molecular characterization of mitochondrial DNA provides evidence for the recent introduction of Schistosoma mansoni into America. Mol Biochem Parasitol. 1993 Aug;60(2):221–229. doi: 10.1016/0166-6851(93)90133-i. [DOI] [PubMed] [Google Scholar]
- Dias Neto E., de Souza C. P., Rollinson D., Katz N., Pena S. D., Simpson A. J. The random amplification of polymorphic DNA allows the identification of strains and species of schistosome. Mol Biochem Parasitol. 1993 Jan;57(1):83–88. doi: 10.1016/0166-6851(93)90246-t. [DOI] [PubMed] [Google Scholar]
- Ellis J. Promiscuous DNA--chloroplast genes inside plant mitochondria. Nature. 1982 Oct 21;299(5885):678–679. doi: 10.1038/299678a0. [DOI] [PubMed] [Google Scholar]
- Farrelly F., Butow R. A. Rearranged mitochondrial genes in the yeast nuclear genome. Nature. 1983 Jan 27;301(5898):296–301. doi: 10.1038/301296a0. [DOI] [PubMed] [Google Scholar]
- Franco G. R., Adams M. D., Soares M. B., Simpson A. J., Venter J. C., Pena S. D. Identification of new Schistosoma mansoni genes by the EST strategy using a directional cDNA library. Gene. 1995 Jan 23;152(2):141–147. doi: 10.1016/0378-1119(94)00747-g. [DOI] [PubMed] [Google Scholar]
- Gellissen G., Bradfield J. Y., White B. N., Wyatt G. R. Mitochondrial DNA sequences in the nuclear genome of a locust. Nature. 1983 Feb 17;301(5901):631–634. doi: 10.1038/301631a0. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Posakony J. W., Grula J. W., Roberts J. W., Xin J. H., Britten R. J., Davidson E. H. Mitochondrial DNA sequences in the nuclear genome of Strongylocentrotus purpuratus. J Mol Biol. 1983 Apr 25;165(4):609–632. doi: 10.1016/s0022-2836(83)80270-8. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., MacLeod A., Tamaki K., Neil D. L., Monckton D. G. Minisatellite repeat coding as a digital approach to DNA typing. Nature. 1991 Nov 21;354(6350):204–209. doi: 10.1038/354204a0. [DOI] [PubMed] [Google Scholar]
- La Roche J., Snyder M., Cook D. I., Fuller K., Zouros E. Molecular characterization of a repeat element causing large-scale size variation in the mitochondrial DNA of the sea scallop Placopecten magellanicus. Mol Biol Evol. 1990 Jan;7(1):45–64. doi: 10.1093/oxfordjournals.molbev.a040586. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Simpson A. J., Mullins J. A., Sher A., Nash T. E., Lewis F., Richards C. Differentiation of schistosomes by species, strain, and sex by using cloned DNA markers. Proc Natl Acad Sci U S A. 1984 Feb;81(3):889–893. doi: 10.1073/pnas.81.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte F., Gueride M., Champagne A. M., Mounolou J. C. Direct repeats in the non-coding region of rabbit mitochondrial DNA. Involvement in the generation of intra- and inter-individual heterogeneity. Eur J Biochem. 1990 Dec 12;194(2):561–571. doi: 10.1111/j.1432-1033.1990.tb15653.x. [DOI] [PubMed] [Google Scholar]
- Okimoto R., Chamberlin H. M., Macfarlane J. L., Wolstenholme D. R. Repeated sequence sets in mitochondrial DNA molecules of root knot nematodes (Meloidogyne): nucleotide sequences, genome location and potential for host-race identification. Nucleic Acids Res. 1991 Apr 11;19(7):1619–1626. doi: 10.1093/nar/19.7.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R., Macfarlane J. L., Clary D. O., Wolstenholme D. R. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics. 1992 Mar;130(3):471–498. doi: 10.1093/genetics/130.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena S. D., Macedo A. M., Gontijo N. F., Medeiros A. M., Ribeiro J. C. DNA bioprints: simple nonisotopic DNA fingerprints with biotinylated probes. Electrophoresis. 1991 Feb-Mar;12(2-3):146–152. doi: 10.1002/elps.1150120209. [DOI] [PubMed] [Google Scholar]
- Rand D. M., Harrison R. G. Molecular population genetics of mtDNA size variation in crickets. Genetics. 1989 Mar;121(3):551–569. doi: 10.1093/genetics/121.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORT R. B., MENZEL M. Y. Chromosomes of nine species of schistosomes. J Parasitol. 1960 Jun;46:273–287. [PubMed] [Google Scholar]
- Santos F. R., Pena S. D., Epplen J. T. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum Genet. 1993 Feb;90(6):655–656. doi: 10.1007/BF00202486. [DOI] [PubMed] [Google Scholar]
- Simpson A. J., Sher A., McCutchan T. F. The genome of Schistosoma mansoni: isolation of DNA, its size, bases and repetitive sequences. Mol Biochem Parasitol. 1982 Aug;6(2):125–137. doi: 10.1016/0166-6851(82)90070-6. [DOI] [PubMed] [Google Scholar]
- Spotila L. D., Hirai H., Rekosh D. M., Lo Verde P. T. A retroposon-like short repetitive DNA element in the genome of the human blood fluke, Schistosoma mansoni. Chromosoma. 1989 May;97(6):421–428. doi: 10.1007/BF00295025. [DOI] [PubMed] [Google Scholar]
- Spotila L. D., Rekosh D. M., LoVerde P. T. Polymorphic repeated DNA element in the genome of Schistosoma mansoni. Mol Biochem Parasitol. 1991 Sep;48(1):117–120. doi: 10.1016/0166-6851(91)90172-3. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Lonsdale D. M. Mitochondrial and chloroplast genomes of maize have a 12-kilobase DNA sequence in common. Nature. 1982 Oct 21;299(5885):698–702. doi: 10.1038/299698a0. [DOI] [PubMed] [Google Scholar]
- Thorsness P. E., Fox T. D. Escape of DNA from mitochondria to the nucleus in Saccharomyces cerevisiae. Nature. 1990 Jul 26;346(6282):376–379. doi: 10.1038/346376a0. [DOI] [PubMed] [Google Scholar]
- Walker T. K., Simpson A. J., Rollinson D. Differentiation of Schistosoma mansoni from S. rodhaini using cloned DNA probes. Parasitology. 1989 Feb;98(Pt 1):75–80. doi: 10.1017/s0031182000059709. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. S., Chapman A. M. Length and sequence variation in evening bat D-loop mtDNA. Genetics. 1991 Jul;128(3):607–617. doi: 10.1093/genetics/128.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. F., Ma D. P., Wilson R. K., Roe B. A. DNA sequence of the Xenopus laevis mitochondrial heavy and light strand replication origins and flanking tRNA genes. Nucleic Acids Res. 1983 Jul 25;11(14):4977–4995. doi: 10.1093/nar/11.14.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaart P., Samallo J., Agsteribbe E. Similar genes for a mitochondrial ATPase subunit in the nuclear and mitochondrial genomes of Neurospora crassa. Nature. 1982 Jul 8;298(5870):187–189. doi: 10.1038/298187a0. [DOI] [PubMed] [Google Scholar]