Abstract
AIM: To develop a model of stress-induced senescence to study the hepatocyte senescence associated secretory phenotype (SASP).
METHODS: Hydrogen peroxide treatment was used to induce senescence in the human HepG2 hepatocyte cell line. Senescence was confirmed by cytochemical staining for a panel of markers including Ki67, p21, heterochromatin protein 1β, and senescence-associated-β-galactosidase activity. Senescent hepatocytes were characterised by gene expression arrays and quantitative polymerase chain reaction (qPCR), and conditioned media was used in proteomic analyses, a human chemokine protein array, and cell migration assays to characterise the composition and function of the hepatocyte SASP.
RESULTS: Senescent hepatocytes induced classical markers of senescence (p21, heterochromatin protein 1β, and senescence-associated-β-galactosidase activity); and downregulated the proliferation marker, Ki67. Hepatocyte senescence induced a 4.6-fold increase in total secreted protein (P = 0.06) without major alterations in the protein profile. Senescence-induced genes were identified by microarray (Benjamini Hochberg-corrected P < 0.05); and, consistent with the increase in secreted protein, gene ontology analysis revealed a significant enrichment of secreted proteins among inducible genes. The hepatocyte SASP included characteristic factors such as interleukin (IL)-8 and IL-6, as well as novel components such as SAA4, IL-32 and Fibrinogen, which were validated by qPCR and/or chemokine protein array. Senescent hepatocyte-conditioned medium elicited migration of inflammatory (granulocyte-macrophage colony stimulating factor, GM-CSF-derived), but not non-inflammatory (CSF-1-derived) human macrophages (P = 0.022), which could contribute to a pro-inflammatory microenvironment in vivo, or facilitate the clearance of senescent cells.
CONCLUSION: Our novel model of hepatocyte senescence provides insights into mechanisms by which senescent hepatocytes may promote chronic liver disease pathogenesis.
Keywords: Cell aging, Chemokines, Hepatocytes, Inflammation, Liver diseases, Macrophages
Core tip: Hepatocyte senescence is observed in all chronic liver diseases of hepatocellular origin, even at early stages of disease progression. Although widely studied in cancer biology, the role of cellular senescence in the pathogenesis of inflammatory diseases is not known. We developed a novel model of stress-induced hepatocyte senescence and used it to demonstrate that senescent human hepatocytes adopt a hyper-secretory phenotype, which is likely to condition their microenvironment and contribute to disease pathogenesis. We used microarray and proteomic analysis to characterise senescent hepatocytes and identify candidate mediators; and confirmed the functional relevance of senescence-associated secretory phenotype by demonstrating that conditioned media from senescent hepatocytes elicits inflammatory macrophage migration.
INTRODUCTION
In chronic liver diseases such as viral infection and non-alcoholic fatty liver disease (NAFLD), hepatocyte regenerative capacity is impaired due to increased cell turnover and injury by DNA damage or oxidative stress[1,2]. Growth-arrested hepatocytes may evolve to a senescent state, characterized by cell cycle arrest and resistance to growth factor stimulation, despite continued metabolic activity. Senescent cells in liver sections have been detected using various methods, including histochemical staining for β-galactosidase activity (an enzymatic marker associated with replicative senescence) or positive staining for the cyclin-dependent kinase inhibitor, p21, in hepatocyte nuclei. Other cytochemical techniques assess telomere shortening or the formation of senescence-associated heterochromatin foci (SAHF). Using one or more of these techniques, several groups have reported the presence of increased numbers of senescent hepatocytes in chronic hepatitis and cirrhosis[3-5]. Senescent hepatocytes were preferentially located in periportal or periseptal areas[3,6,7], particularly adjacent to areas with prominent mononuclear cell infiltration[8] and bile ductular reaction[9].
Senescence arrests the growth of cells at risk of neoplastic transformation, and it has most frequently been studied in the context of tumour suppression[10]. However, the role of senescence in tumorigenesis is complex, since factors secreted by senescent cells can also promote malignant phenotypes in neighbouring cells, such as proliferation and invasion[11]. The contribution of senescence to non-cancer pathologies has rarely been studied, although it is recognised that senescent cells can have deleterious effects on the tissue microenvironment. Accumulation of senescent cells in chronic liver diseases may contribute to ongoing injury, altered tissue repair and fibrogenesis[11,12]. Recent studies in human alcoholic liver disease and NAFLD demonstrated that, in addition to a strong association between hepatocyte p21 expression and fibrosis stage, there was an independent relationship between the proportion of senescent hepatocytes and an adverse clinical outcome (including hepatocellular cancer, liver transplantation and liver-related death)[13,14]. The mechanistic basis for the pathogenic effects of senescence in chronic liver disease remains unclear, although it is recognized that senescent cells can adversely affect their microenvironment via the adoption of specific “secretory” phenotypes (including cytokines, chemokines, growth factors and proteases), which typically have a pro-inflammatory effect on surrounding cells[11]. The senescence-associated secretory phenotype (SASP)[15] has been extensively characterised in other cells, most notably fibroblasts. It is known that different cell types express distinct but overlapping SASPs in response to different triggers of senescence[15], but the SASP of senescent hepatocytes has not been investigated.
The aim of this study was to develop an in vitro model to investigate senescence-induced changes in hepatocyte gene expression and secretory profile. The chemokine profile and chemotactic capacity of cultured senescent hepatocytes was specifically examined, in view of their location in diseased tissue adjacent to areas with prominent mononuclear cell infiltration,
MATERIALS AND METHODS
Cell culture and preparation of senescent cell conditioned media
The human hepatoma-derived cell line, HepG2, was purchased from American Type Culture Collection. Unless otherwise indicated, cells were cultured in “complete medium” comprising Dulbecco’s modified eagle’s medium supplemented with 10% foetal bovine serum (FBS, Invitrogen), 100 U/mL penicillin/100 μg/mL streptomycin/2 mmol GlutaMAX/20 μmol non-essential amino acids, and maintained at 37 °C/5% CO2. For dose response assays, HepG2 cells were cultured for 48 h in the presence of 150-600 μmol/L hydrogen peroxide (H2O2, Sigma). Cell viability and metabolic activity was determined using the CellTiter96® Assay (Promega). The degree of cell lysis was determined by assaying for lactate dehydrogenase in culture medium (Sigma). Fully lysed HepG2 cells (treated with 0.25% Triton-X 100) were used as a positive control. To induce senescence, HepG2 cells were seeded at 2 × 105/well in 6 well plates and treated with a single dose of H2O2 (300 μmol/L) each day for the first 2 d to induce replicative arrest. Senescence was assessed following culture for a further 7 d (without H2O2 treatment), with the media refreshed every 3 d. Due to the proliferative disadvantage of non-senescent cells, control cultures were seeded at 5 × 104 cells/well and cultured in parallel to senescent cultures for 7 d. Cells for immunostaining were seeded on glass coverslips and treated identically. To facilitate study of conditioned media, in some experiments cells were subsequently cultured for 24 h in serum free medium prior to harvest of cells and conditioned medium on day (D) 10. Conditioned culture media was clarified by centrifugation at 600 g, filtered (0.22 μm micropore filters; Corning) and stored in single-use aliquots at -80 °C. The protein concentration of conditioned culture media was quantified by Bradford Assay (Bio-Rad). To generate human monocyte-derived macrophages (HMDM), CD14+ monocytes were isolated from human buffy coats, obtained from the Australian Red Cross Blood Service. Ficoll-Paque Plus (GE Healthcare) density gradient separation was used to isolate Peripheral Blood Mononuclear Cells followed by positive selection of CD14+ monocytes (Miltenyi Biotech). Isolated CD14+ monocytes (> 90% purity by flow cytometry) were cultured for 7 d in Iscove’s Modified Dulbecco’s Medium with 10% heat inactivated FBS/50 U/mL Penicillin/50 μg/mL Streptomycin in the presence of human macrophage colony stimulating factor (M-CSF) (1 × 104 U/mL) or granulocyte macrophage colony stimulating factor (GM-CSF) (10 ng/mL) to generate HMDM.
Assays for cellular senescence
Replicative arrest was assessed by p21WAF1/Cip1 (EA10, Invitrogen) and Ki-67 staining (SP6, Cell Marque), with DAPI counter-staining for cell nuclei. Senescence was determined by staining for HP1β (1MOD-1A9, Millipore) and senescence-associated β-galactosidase (SA-β-Gal) activity (Sigma). Cells in a single 400 × high power field (HPF) were quantified by counting the number of positive cells as a proportion of total DAPI-stained cells (10 × HPF/treatment). Results were expressed as the proportion of positively-stained nuclei in treated and untreated cell cultures. All experiments were performed three times and results pooled for analysis.
Polyacrylamide gel electrophoresis and mass spectrometry
Standard polyacrylamide gel electrophoresis (PAGE) was performed with the NuPAGE system (10% Bis-Tris gel, MES-SDS running buffer). Two dimensional (2D) gel electrophoresis, in-gel digest and protein identification by tandem mass spectrometry were performed as previously described[16]. To focus on small molecular weight proteins, 12% SDS-PAGE and pH3-11 non-linear IPG gradients were used. Spectrum Mill software (Agilent Technologies) was used for database searching, using the SwissProt database (species Homo sapiens, with carbamidomethylated cysteine as fixed modification and oxidized methionine as variable), a maximum missed cleavage of 2, precursor mass tolerance of ± 20 and product mass tolerance of ± 50. Results were filtered by protein score of > 11.0, peptide score of > 10, and % scored peak intensity of > 60.
Microarray analysis of untreated and senescent HepG2 cells
Total RNA was extracted from untreated HepG2 cells cultured for 2 d, as well as control and senescent cells cultured for 10 d (including 24 h in serum free medium), using TRI reagent (Sigma). RNA quality was assessed with an Agilent 2100 BioAnalyser and only samples with a RNA integrity number above 8.0 were included. cRNA was generated from 500 ng total RNA using the Illumina TotalPrep cRNA Amplification Kit (Applied Biosciences) and hybridised to Human HT-12_V3 Expression BeadChips (Illumina). Array data were processed using Illumina GenomeStudio software and imported into Genespring (Agilent) for analysis. Each array was normalised to the 50th percentile probe expression (per array normalisation), and each probe was normalised to its average expression in control, untreated HepG2 cells at day 10. Data were filtered to remove probes that did not reach an Illumina detection score of 1 in at least one sample. A senescence-associated gene expression signature was identified by selecting probes that were differentially expressed between control and senescent cells (D10, ≥ 1.5 ×, P < 0.05, t-test with Benjamini Hochberg multiple testing correction), and subtracting probes that were differentially expressed between untreated HepG2 cells at baseline (D2) and D10 (≥ 1.5 ×, Benjamini Hochberg-corrected P < 0.05). Gene ontology (GO) analysis was performed using DAVID[17] with the Illumina HT12 genome as background. Significantly enriched (P < 0.05) GO terms, KEGG pathways and sequence features were hand-curated to remove redundant terms.
Real-time polymerase chain reaction
RNA was reverse-transcribed to cDNA using SuperScriptIII Reverse Transcriptase (Invitrogen). Quantitative real-time PCR (qPCR) for genes of interest was performed using SYBR green (ABI) on an HT9000 cycler with default cycle settings. Relative expression was analysed using HPRT as housekeeping gene and the delta-Ct method. Primer sequences used in this study were: HPRT, Forward: TCAGGCAGTATAATCCAAAGATGGT; HPRT, Reverse: AGTCTGGCTTATATCCAACACTTCG; IL6, Forward: ATG CAA TAA CCA CCC CTG AC; IL6, Reverse: AAA GCT GCG CAG AAT GAG AT; SAA4, Forward: CCA AAG CCA GCA GAG GTA CCA AC; SAA4, Reverse: ACG AAC GCC AGC TTT CAC TGG; ECADH, Forward: ATT GCA AAT TCC TGC CAT TC; ECADH, Reverse: GCT GGC TCA AGT CAA AGT CC; IL32, Forward: AGA CAG TGG CGG CTT ATT ATG AG; IL32, Reverse: GCA CCG TAA TCC ATC TCT TTC TTT; IL8, Forward/Reverse: proprietary (Applied Biosystems).
Human chemokine array and macrophage migration assays using conditioned media
The human chemokine antibody array (RD Systems, ARY017) was performed according to the manufacturers’ instructions. Macrophage migration was assessed using the xCELLigence (RTCA DP) system (ACEA Biosciences) in 16 well CIM-Plates. The upper chamber of each well of the plate was coated with 0.1% Fibronectin from human plasma (Sigma-Aldrich). The lower chamber was filled with 160 μL control or senescent conditioned media (CM). Thirty microlitres of complete media was added to the top chamber and the set-up was allowed to equilibrate at 37 °C for 30 min. 1 × 105 HMDM resuspended in complete media without differentiation factors were added to the top chamber. Impedance readings were collected every 5 min over a 24 h period. xCELLigenece assays were conducted using CM from 4 independent senescence cultures with M-CSF- and GM-CSF-derived HMDM from 2 independent blood donors. Transwell assays were conducted using 24 well transwell culture plates (Corning) with control or senescent conditioned medium in the lower chamber. After 24 h incubation, cells were fixed in paraformaldehyde, and stained with DAPI. Five random images per transwell membrane were captured and nuclei were counted, blinded as to sample identity. Transwell assays were conducted using CM from 4 independent senescence cultures with M-CSF- and GM-CSF-derived HMDM from 1 blood donor.
Statistical analysis
Where not elsewhere described, statistical analyses were conducted in GraphPad Prism, using the Mann-Whitney U test to compare between treatment conditions. For independent biological replicates data were expressed as mean ± SE where n > 3, or mean ± range where n = 2. For technical replicates data were expressed as mean ± SD.
RESULTS
Oxidative stress causes replicative arrest and senescence in HepG2 cells
We sought to establish an in vitro model of oxidative stress-induced senescence in the human hepatocyte cell line, HepG2 by exposure to an acute (48 h), sub-lethal dose of H2O2, an oxidant produced in normal cellular aerobic metabolism and inflammation. H2O2 treatment was previously reported to induce premature senescence in fibroblasts, and has been used as a model of aging[18]. We first performed a H2O2 dose response to select a dose that did not affect hepatocyte viability. Treatment with 300 μmol/L H2O2 for 48 h did not affect HepG2 metabolic activity (Figure 1A), or survival (lactate dehydrogenase release into culture medium was 8% ± 0.1% and 8.5% ± 0.1% of the lysed cell control for untreated and H2O2-treated HepG2 cells respectively). Following treatment, cells were cultured for a further 7 d in the absence of H2O2, to allow time for the SASP to develop[11], and 1 d in serum free media (to minimise interference of serum proteins in conditioned media studies). Cells were thus routinely analysed on D10 of the experimental protocol. H2O2-treated HepG2 cells adopted a flattened, elongated morphology, typical of senescent cells (Figure 1B). Senescence was confirmed by reduced expression of the proliferation-associated protein Ki67, together with increases in β-galactosidase activity, expression of the cell cycle inhibitor p21, and appearance of nuclear heterochromatin protein-1-beta (HP1β) positive nuclear foci (Figure 1C, D, P < 0.01). The involvement of both p21 and SAHF suggests the involvement of both the p53- and p16/Rb-dependent senescence pathways in hepatocytes, consistent with reports of p16/p21 co-expression in senescent liver cells in vivo[19].
Figure 1.
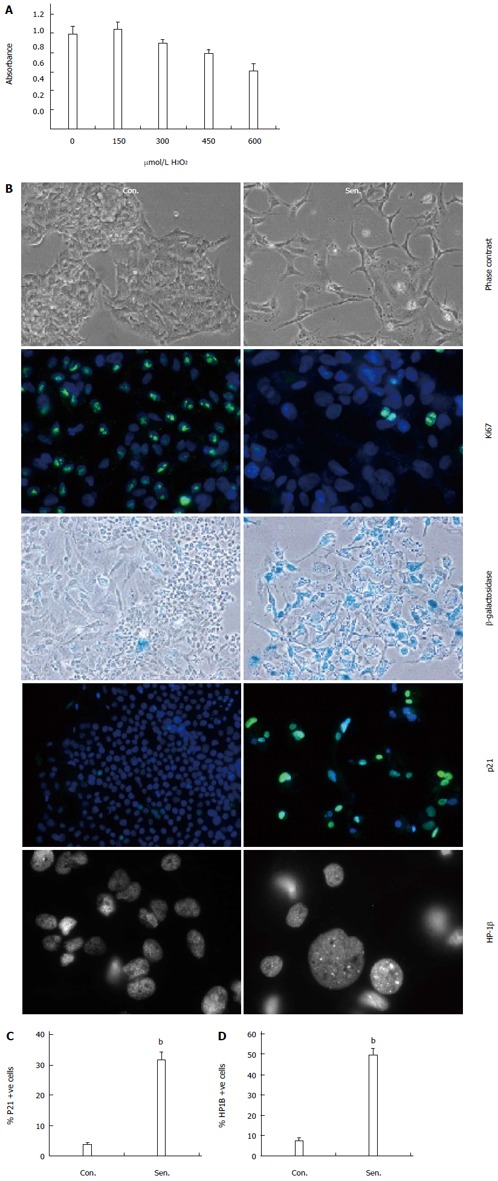
Stress-induced senescence in human hepatocytes. A: HepG2 cells were treated with the indicated doses of hydrogen peroxide (H2O2) and survival assessed using a tetrazolium reduction assay; B: Phase contrast, Ki67 immunocytochemistry, β-galactosidase activity, p21 immunocytochemistry, and heterochromatin protein 1 beta (HP1β) immunocytochemistry in control (Con.) and H2O2-treated senescent (Sen.) HepG2 7 d after release from H2O2 treatment. All panels magnification × 200, except HP-1β magnification × 630; C: Quantification of p21+ nuclei; D: Quantification of cells with HP1β+ foci. Data are representative of at least 3 independent experiments. bP < 0.01 vs control group.
Senescent hepatocyte secretory profile
One of the cardinal features associated with cellular senescence is an increase in secretory activity[15]. Senescent hepatocytes secreted more protein than control cells on a per cell basis (mean 4.6-fold increase per cell, P = 0.06). SDS PAGE analysis of proteins secreted by an equivalent number of control and senescent cells illustrated this increase in protein production, and suggested a similar repertoire of secreted proteins in senescent and control cells (Figure 2A). Analysis of an equivalent amount of protein from control and senescent CM by 2D PAGE further demonstrated that the gross hepatocyte secretory profile in control and senescent hepatocyte conditioned medium was similar, suggesting a generalised upregulation of normal hepatocyte secretory products (Figure 2B, C). Senescent hepatocytes may even express a more limited repertoire of proteins than non-senescent cells, at least for abundant proteins visible by coomassie staining (compare Figure 2B, C). One region of the 2D gel (indicated in Figure 2) that was clearly different between control and senescent CM was excised and subjected to mass spectrometry. 14 proteins in this region were identified by ≥ 2 unique peptides in senescent CM, compared to 31 in control CM. The most abundant proteins in senescent CM were alpha-fetoprotein and albumin (21/19 spectra respectively, compared to 8/6 spectra in control CM). Complement C3 was the only protein uniquely detected in senescent CM. Since few differences between control and senescent secretory profiles were evident in our preliminary proteomics analysis, and because many SASP factors (such as cytokines and chemokines) may be expressed at low levels, we adopted a transcriptomic approach to further characterise senescent hepatocytes.
Figure 2.
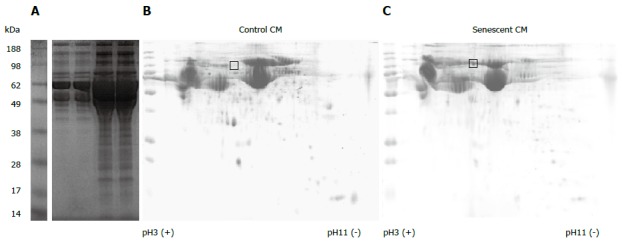
Secretory profile of control and senescent hepatocytes. Proteins in conditioned media (CM) derived from an equivalent number of control (Con.) and senescent (Sen.) hepatocytes were separated by 1-dimensional polyacrylamide gel electrophoresis (PAGE) (A). 150 μg protein from control (B) and senescent (C) hepatocyte conditioned media was separated by 2-dimensional PAGE. Gels were stained with colloidal coomassie. The gel region excised for liquid chromatography mass spectrometry services analysis is indicated. Gel images are representative of 2 independent experiments.
Transcriptome-based characterisation of the human hepatocyte SASP
Previous studies of cellular senescence have demonstrated that SASP-associated proteins are generally regulated at the level of transcription[15]. In order to gain insight into the hepatocyte SASP we used microarrays to identify changes in gene expression associated with hepatocyte senescence. Senescent and control untreated HepG2 cells were harvested on D10 of the experimental protocol for Illumina expression profiling. Untreated HepG2 cultures harvested on D2 were considered as baseline expression. Both untreated and H2O2-treated cells showed significant changes in gene expression after 10 d culture (Figure 3A), however, senescence induced unique mRNA expression changes in HepG2 cells (326 probes, including CDKN1A (which encodes p21). Probes that were uniquely regulated in senescent cultures are clustered in Figure 3B, whereas commonly regulated probes are clustered in Figure 3C. As is apparent from Figure 3B, senescence was primarily associated with gene induction. Not surprisingly, GSTA1 and GSTA2, encoding glutathione S-transferase enzymes that protect cells against oxidative stress, were among the most highly induced transcripts, although these enzymes have not been associated with senescence in other cell types. Gene ontology enrichment analysis of senescence-induced probes confirmed the increased expression of genes encoding secreted proteins (Table 1). Consistent with other senescent cell models, inflammatory/defence response genes were also highly enriched in the senescent cells, however the enrichment of lipid and drug metabolism pathways has not been previously reported and may be unique to hepatocytes (Table 1). Several gene ontologies were enriched among the 37 genes encoding annotated secreted proteins, which likely contribute to the hepatocyte SASP, including plasma components and proteins involved in the immune response (Table 1). These include the typical SASP component IL8, mRNAs from other functional families typically associated with senescence (e.g., IGFBP7[20], SERPINA6), and acute phase proteins that may be unique to the hepatocyte SASP, including complement 1S, haptoglobin and hemopexin. Commonly regulated genes were broadly associated with cell cycle and metabolism, consistent with a degree of replicative exhaustion and possibly a quiescent state in control cells after prolonged culture (e.g., the proliferation-associated PCNA was downregulated in both control and senescent cells). Nevertheless, the cardinal features of senescence were only observed in H2O2-treated hepatocytes (Figure 1).
Figure 3.
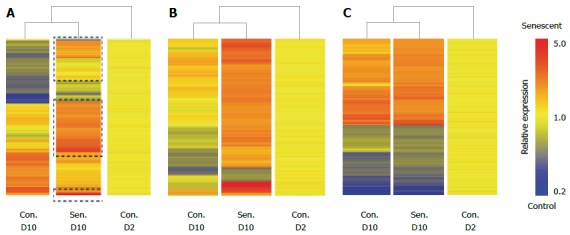
Senescent hepatocyte gene expression signature. Senescent hepatocytes (Sen.) were compared to untreated hepatocytes (Con.) at D (Day) 2 and D10 of the experimental protocol by illumina microarray. A: Hierarchical clustering of probes that were differentially expressed between control and senescent hepatocytes at D10; B: Hierarchical clustering of probes that were uniquely regulated in senescent hepatocytes; C: Hierarchical clustering of probes that were commonly regulated in senescent hepatocytes and untreated hepatocytes between D2 and D10. Probes are clustered by distance correlation with average linkage.
Table 1.
Gene ontology analysis of senescent hepatocyte gene expression signature
| Gene list | Ontology | % list | P value | Fold enrichment |
| A: senescence induced | Steroid metabolism | 8.5 | 5.50E-12 | 7.4 |
| Endoplasmic reticulum | 15.7 | 7.10E-9 | 2.8 | |
| Oxidation/reduction | 12.1 | 8.00E-9 | 3.5 | |
| Drug metabolism | 4.8 | 7.80E-8 | 8.6 | |
| Lysosome | 6.0 | 2.20E-6 | 4.9 | |
| Retinol metabolism | 4.0 | 2.20E-6 | 8.3 | |
| Signal peptide | 27.8 | 1.40E-5 | 1.7 | |
| Iron ion binding | 6.5 | 3.20E-5 | 3.6 | |
| Defence response | 8.5 | 3.00E-4 | 2.5 | |
| Lipid biosynthesis | 5.6 | 5.40E-4 | 3.1 | |
| Lipid catabolism | 4.0 | 7.20E-4 | 4.1 | |
| Secreted | 14.9 | 1.50E-3 | 1.7 | |
| Regulation of apoptosis | 9.3 | 1.90E-3 | 2.0 | |
| Inflammatory response | 4.4 | 1.50E-2 | 2.4 | |
| B: secreted | Disulfide bond | 62.2 | 3.90E-10 | 4.1 |
| Plasma | 16.2 | 9.10E-7 | 32.6 | |
| Glycoprotein | 56.8 | 2.10E-5 | 2.5 | |
| Defence response | 21.6 | 2.10E-4 | 6.0 | |
| Immune response | 16.2 | 1.10E-2 | 4.2 | |
| Lipid binding | 13.5 | 1.40E-2 | 5.0 | |
| Cell adhesion | 13.5 | 5.80E-2 | 3.3 | |
| Regulation of proliferation | 13.5 | 8.30E-2 | 2.9 |
Gene ontology enrichment in (A) senescence-induced genes and (B) senescence-induced genes falling in the “Secreted” ontology was analysed using DAVID.
To confirm the results of the microarray, qPCR was performed to assess the expression of selected genes in senescent HepG2 cells. Genes were chosen based on degree of regulation on the microarray and their potential biological relevance. Figure 4 demonstrates the increased expression of SAA4, ECADH, IL32 and IL8 (the latter a frequent SASP feature[15]) in senescent hepatocytes 8 d after release from H2O2 treatment. We also quantified IL6 mRNA expression by qPCR, since it is a key senescence-associated gene[11], but was not detectable by microarray. IL6 was below the limit of detection in 2/4 control HepG2 cultures, however it was induced in senescent hepatocytes (Figure 4E).
Figure 4.
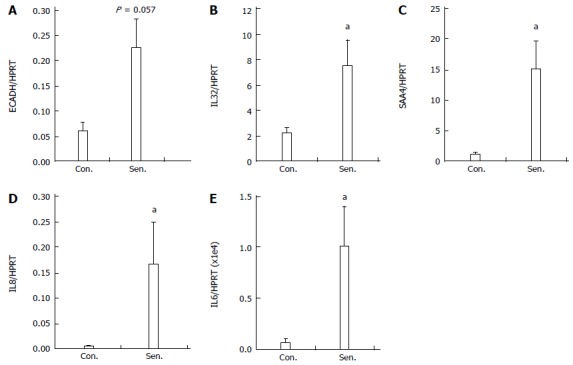
Quantitative polymerase chain reaction validation of microarray. Relative mRNA expression of A: ECADH; B: IL32; C: IL8; D: SAA4 and E: IL6 in control (Con.) and senescent (Sen.) hepatocytes was quantified by real-time polymerase chain reaction PCR. Data represent mean + SEM, n = 4 independent experiments. aP < 0.05 vs control group.
Chemokine profile of senescent hepatocytes
In order to focus on chemokines secreted by senescent hepatocytes, since these low abundance proteins are likely to be masked by abundant secretory products (Figure 2), we used a protein array to investigate the expression of 31 chemokines in control and senescent CM (equivalent volume). Seven chemokines were detectable in hepatocyte CM (Figure 5A, B); prominent among them were CCL20 (also known as liver activation regulated chemokine) and CXCL16. As shown at the mRNA level (Figure 4) IL-8, was present at higher levels in senescent CM (Figure 5A, B). Consistent with the microarray there was also a modest increase in fibrinogen (included on the chemokine array as a sample control due to its broad expression), which showed significant upregulation of FGB in senescent cells. Since senescent cell cultures contained on average 5-fold fewer cells, considering chemokine levels on a per cell basis would further amplify the difference between control and senescent CM. The relatively reduced expression of CXCL16 on the chemokine array is also consistent with the microarray, in which CXCL16 mRNA was induced during culture in control but not senescent cells.
Figure 5.
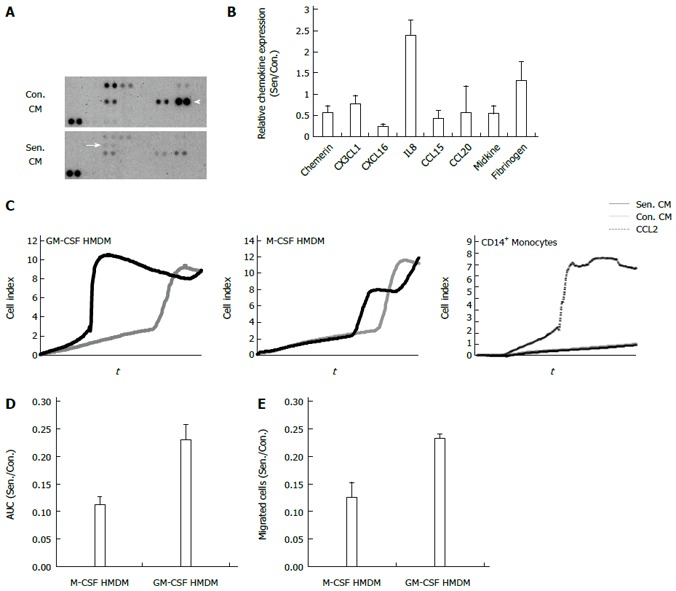
Chemokine profile and chemotactic effects of senescent hepatocyte conditioned medium. A: Representative human chemokine array. Arrow: IL8. Arrow head: CCL20; B: Relative protein expression in control and senescent conditioned medium (CM). Data show the average + range of 2 independent experiments; C: Representative xCELLigence trace, macrophage and monocyte migration in response to control and senescent CM and recombinant CC chemokine ligand 2 (CCL2); D: Relative migration of macrophage colony stimulating factor (M-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF)-derived human monocyte derived macrophages (HMDM) in senescent (Sen.) compared to control (Con.) CM in D: xCELLigence assay quantified by the area under the curve (AUC); E: Transwell assay, quantified by counting migrated nuclei. Data represent mean + SE.
Senescent hepatocyte conditioned medium induces macrophage migration
Senescent hepatocytes have been implicated in fibrogenesis and chronic liver disease progression, both directly and via recruitment of inflammatory cells, especially macrophages[21]. We investigated monocyte and monocyte-derived macrophage migration towards control and senescent HepG2 CM using the xCELLigence system to monitor cell migration in real time (Figure 5C). Primary peripheral blood monocytes did not respond to either CM, but did migrate to the chemokine CCL2, which was used as a positive control. By contrast, HMDM differentiated in the presence of M-CSF or GM-CSF migrated in response to an equivalent volume of control and senescent CM (Figure 5C). Whilst senescent CM did not alter M-CSF-derived macrophage migration, there was a significant increase in GM-CSF-derived macrophage migration towards senescent CM compared to control CM (Figure 5C and 5D, P = 0.022). The contribution of morphological change to the xCELLigence signal cannot be discounted, as this system simply measures electrical impedance. We therefore used conventional transwell assays to confirm differential migration of GM-CSF- but not M-CSF-derived macrophages towards senescent CM (Figure 5E). As discussed above, the increased macrophage migration to senescent cell secretory products is even greater when considered on a per cell basis.
DISCUSSION
Senescent hepatocytes are prominent in chronic liver diseases, but their “secretory phenotype” and contribution to the tissue microenvironment has not been characterized. In this study, premature senescence was induced in HepG2 cells by oxidative stress, providing a novel model to examine hepatocyte senescence. Treatment with a sublethal dose of H2O2 provides a relevant in vitro model of hepatocyte senescence as oxidative stress with the generation of reactive oxygen species, including H2O2, is a key feature of many chronic liver diseases[22], Reactive oxygen species-induced senescence is mediated by both the characterised pathways to senescence - p53/p21 and p16/Rb[10]. In the current model, hepatocytes treated with 300 μmol/L H2O2 for 48 h developed a senescent phenotype, which persisted for at least 7 d after release from treatment. The treated cells acquired several markers of senescence, namely reduced Ki67 expression, the appearance of SAHF and p21 and SA-β-Gal positivity[10]. We used our model to profile the hepatocyte SASP, demonstrating that it shares features with other senescent cells, and that it promotes inflammatory macrophage migration.
Although the focus of this study is hepatocyte senescence and its potential contribution to chronic liver disease, senescence in the liver is clearly a complex issue. Other liver cell types, notably activated hepatic stellate cells[12] and hepatic progenitor cells[19], have been reported to senesce, and the outcomes of senescence may differ depending on cell type and context. Senescence and immune-mediated clearance of activated HSC, for example, was shown to limit fibrosis in mice[12], whilst HSC senescence promoted obesity-associated hepatocellular cancer[23]. Hepatocyte senescence may likewise have protective and pathogenic functions; as defective immune clearance of pre-malignant senescent hepatocytes was associated with early development of liver cancer[21,24], whereas triggering senescence by reactivation of endogenous p53 mediated clearance of liver tumours[25]. Interestingly, HCV core protein specifically targets the p16 senescence response, suggesting hepatocyte senescence is employed as a strategy to limit viral replication[26].
Increasing evidence suggests senescent cells contribute to the pathogenesis of chronic disease by adopting a characteristic secretory phenotype, known as the SASP, which alters the tissue microenvironment[11,15]. This is consistent with the strong ‘secreted protein’ signature we observed upon expression profiling senescent hepatocytes, and their increased protein production compared to control cells. Senescent hepatocytes upregulated expression of inflammatory cytokines/chemokines, matrix-remodeling proteases and growth factors; and also display increased expression of genes accordant with their liver-specific cellular function such as lipoproteins and cholesterol metabolism. Many of these changes in gene expression are seen in other senescent cell types (fibroblasts and epithelial cells), consistent with a conserved core phenotype that may be controlled at a transcriptional level in response to genotoxic stress[15].
IL-8 is one of the most prominent components of the SASP - regardless of cell type and senescence trigger. Several studies in human chronic liver disease suggest a link between IL-8 and liver injury. In chronic hepatitis C and B infection, serum IL-8 levels increase progressively with severity of liver disease[27,28] and development of hepatocellular cancer[27,29]. Although hepatocyte senescence was not examined in these earlier studies, we and others have previously shown that many hepatocytes in liver sections from patients with NAFLD[30,31], HCV[5], and hepatocellular cancer[7] are senescent, as assessed by nuclear p21 or SA-β-Gal expression. The biologic effects of IL-8 in the microenvironment of senescent hepatocytes have not been determined. IL-8 production by senescent cells has been shown to reinforce growth arrest[32], stimulate angiogenesis and promote tumorogenesis[33]. IL-8, together with IL-6, has also been implicated in eliciting EMT in senescent cells, however we did not detect a signature consistent with EMT in senescent HepG2 cells, in fact ECADH was upregulated. IL-8 is also a potent leukocyte chemoattractant, which may play a role in recruiting macrophages or neutrophils to senescent cells. Elevated intrahepatic IL-8 and CXCR1 levels were associated with hepatic macrophage accumulation in hepatocellular, but not cholestatic, chronic liver diseases, in parallel with increased CXCR1 expression on circulating monocytes[28]. We demonstrated that senescent hepatocyte CM enhanced migration of HMDM differentiated in the presence of the inflammatory cytokine GM-CSF, but not the constitutive macrophage growth factor, M-CSF, or monocytes. This finding is consistent with our recent demonstration of elevated IL8RA/CXCR2 mRNA in GM-CSF-derived HMDM, as compared to those derived with M-CSF[34], and suggests senescent hepatocytes modulate the inflammatory milieu by selectively recruiting specific immune cells, via IL-8 and/or other SASP components. Indeed, defective immune surveillance of senescent cells exacerbated liver injury and fibrosis, and tumour development in mouse models of liver disease[12,21]. Increased numbers of senescent hepatocytes were also observed in explant livers from immunosuppressed HCV patients[21].
In summary, we have developed an in vitro model of human hepatocyte senescence and, for the first time, characterised the hepatocyte SASP using a transcriptomic approach. Senescent hepatocytes upregulate characteristic SASP factors such as IL-8, and selectively promote recruitment of inflammatory (GM-CSF-derived) macrophages. SASP-mediated recruitment of inflammatory cells may be a key mechanism by which senescence contributes to injury resolution or pathogenesis in chronic liver disease. Future studies on novel SASP factors that we have identified are likely to provide mechanistic insights into such processes.
COMMENTS
Background
Cellular senescence, a form of permanent replicative arrest, is a phenomenon associated with aging, inflammation and cancer. Although widely studied in cancer biology, the role of cellular senescence in the pathogenesis of inflammatory diseases, such as chronic liver disease, is not known. Hepatocyte senescence is frequently observed in all chronic liver diseases of hepatocellular origin, even at early stages of disease progression. It is important to understand the impact of hepatocyte senescence on liver disease, since it may have both protective and pathological roles.
Research frontiers
The global burden of liver disease is steadily increasing, particularly liver cancer, which develops in the setting of chronic disease. Hepatocyte senescence is associated with progression of chronic liver disease and the development of liver cancer, but how senescence contributes to disease outcomes is not well understood.
Innovations and breakthroughs
Authors developed a novel model of stress-induced hepatocyte senescence and used it to demonstrate that senescent human hepatocytes adopt a hyper-secretory phenotype, which is likely to condition their microenvironment and contribute to disease pathogenesis. They used microarray and proteomic analysis to characterise senescent hepatocytes and identify candidate mediators; and confirmed the functional relevance of senescence-associated secretory phenotype by demonstrating that conditioned media from senescent hepatocytes elicits inflammatory macrophage migration.
Applications
The results of this study suggest senescent hepatocytes recruit inflammatory macrophages, and identify candidate mediators of this process, which represent targets for validation in vivo. Their novel model will facilitate investigations into the mechanistic basis of inflammatory cell recruitment, and the impact of senescent hepatocyte secretory products on diverse cell types implicated in liver disease progression.
Peer review
The authors developed a great model of human hepatocyte senescence and they characterised the hepatocyte senescence associated secretory phenotype (SASP) using a transcriptomic approach. Senescent hepatocytes upregulate characteristic SASP factors such as IL-8, and selectively promote recruitment of macrophages. SASP-mediated recruitment of inflammatory cells may be a key mechanism by which senescence contributes to injury resolution or pathogenesis in chronic liver disease.
Footnotes
Supported by the National Health and Medical Research Council of Australia (NHMRC), APP1044650 and APP1003108; the Queensland Government’s Smart State Health and Medical Research Fund; the Princess Alexandra Hospital Research and Development Foundation and The Australian Liver Foundation; Irvine KM is the recipient of the Australian Liver Foundation Pauline Hall Fellowship; Powell EE is the recipient of an NHMRC Practitioner Fellowship, APP1004242; Sweet MJ is the recipient of an Australian Research Council (ARC) Future Fellowship, FT100100657; and an honorary NHMRC Senior Research Fellowship, APP1003470; Hill MM is the recipient of an ARC Future Fellowship, FT120100251
P- Reviewer: Freire-De-Lima CG, Kuan YH, Vetvicka V S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, Coleman N, Alexander G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33–42. doi: 10.1053/j.gastro.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima T, Moriguchi M, Katagishi T, Sekoguchi S, Nishikawa T, Takashima H, Kimura H, Minami M, Itoh Y, Kagawa K, et al. Premature telomere shortening and impaired regenerative response in hepatocytes of individuals with NAFLD. Liver Int. 2006;26:23–31. doi: 10.1111/j.1478-3231.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 3.Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda H, Sasaki M, Sato Y, Harada K, Zen Y, Mitsui T, Nakanuma Y. Large cell change of hepatocytes in chronic viral hepatitis represents a senescent-related lesion. Hum Pathol. 2009;40:1774–1782. doi: 10.1016/j.humpath.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM, Walsh SN, Hayashi PH, Labundy J, Di Bisceglie AM. Hepatocyte senescence in end-stage chronic liver disease: a study of cyclin-dependent kinase inhibitor p21 in liver biopsies as a marker for progression to hepatocellular carcinoma. Liver Int. 2007;27:662–671. doi: 10.1111/j.1478-3231.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 7.Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 8.Wagayama H, Shiraki K, Yamanaka T, Sugimoto K, Ito T, Fujikawa K, Takase K, Nakano T. p21WAF1/CTP1 expression and hepatitis virus type. Dig Dis Sci. 2001;46:2074–2079. doi: 10.1023/a:1011977923941. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda H, Sasaki M, Sato Y, Harada K, Zen Y, Mitsui T, Nakanuma Y. Bile ductular cell reaction with senescent hepatocytes in chronic viral hepatitis is lost during hepatocarcinogenesis. Pathol Int. 2009;59:471–478. doi: 10.1111/j.1440-1827.2009.02395.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aravinthan A, Pietrosi G, Hoare M, Jupp J, Marshall A, Verrill C, Davies S, Bateman A, Sheron N, Allison M, et al. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One. 2013;8:e72904. doi: 10.1371/journal.pone.0072904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aravinthan A, Scarpini C, Tachtatzis P, Verma S, Penrhyn-Lowe S, Harvey R, Davies SE, Allison M, Coleman N, Alexander G. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol. 2013;58:549–556. doi: 10.1016/j.jhep.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies JM, Voskamp A, Dang TD, Pettit B, Loo D, Petersen A, Hill MM, Upham JW, Rolland JM, O’Hehir RE. The dominant 55 kDa allergen of the subtropical Bahia grass (Paspalum notatum) pollen is a group 13 pollen allergen, Pas n 13. Mol Immunol. 2011;48:931–940. doi: 10.1016/j.molimm.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki M, Ikeda H, Yamaguchi J, Miyakoshi M, Sato Y, Nakanuma Y. Bile ductular cells undergoing cellular senescence increase in chronic liver diseases along with fibrous progression. Am J Clin Pathol. 2010;133:212–223. doi: 10.1309/AJCPWMX47TREYWZG. [DOI] [PubMed] [Google Scholar]
- 20.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in senescence induction by BRAF. Cell. 2010;141:746–747. doi: 10.1016/j.cell.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 22.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26 Suppl 1:173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Yan J, Wang Z, Hua F, Yu J, Sun W, Li K, Liu H, Yang H, Lv Q, et al. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology. 2013;57:171–182. doi: 10.1002/hep.25991. [DOI] [PubMed] [Google Scholar]
- 25.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim JS, Park SH, Jang KL. Hepatitis C virus Core protein overcomes stress-induced premature senescence by down-regulating p16 expression via DNA methylation. Cancer Lett. 2012;321:154–161. doi: 10.1016/j.canlet.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 27.Tachibana Y, Nakamoto Y, Mukaida N, Kaneko S. Intrahepatic interleukin-8 production during disease progression of chronic hepatitis C. Cancer Lett. 2007;251:36–42. doi: 10.1016/j.canlet.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann HW, Seidler S, Gassler N, Nattermann J, Luedde T, Trautwein C, Tacke F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One. 2011;6:e21381. doi: 10.1371/journal.pone.0021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Y, Poon RT, Tsui HT, Chen WH, Li Z, Lau C, Yu WC, Fan ST. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res. 2003;9:5996–6001. [PubMed] [Google Scholar]
- 30.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 32.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 34.Hohenhaus DM, Schaale K, Le Cao KA, Seow V, Iyer A, Fairlie DP, Sweet MJ. An mRNA atlas of G protein-coupled receptor expression during primary human monocyte/macrophage differentiation and lipopolysaccharide-mediated activation identifies targetable candidate regulators of inflammation. Immunobiology. 2013;218:1345–1353. doi: 10.1016/j.imbio.2013.07.001. [DOI] [PubMed] [Google Scholar]


