Abstract
AIM: To investigate the risk factors for 6-wk rebleeding and mortality in acute variceal hemorrhage (AVH) patients treated by percutaneous transhepatic variceal embolization (PTVE).
METHODS: A retrospective cohort study of AVH patients who had undergone PTVE treatment was conducted between January 2010 and December 2012. Demographic information, medical histories, physical examination findings, and laboratory test results were collected. The PTVE procedure was performed as a rescue therapy for patients who failed endoscopic and pharmacologic treatment. Survival analysis was estimated using the Kaplan-Meier method and compared using the log-rank test. The multivariate analysis was performed using the Cox regression test to identify independent risk factors for rebleeding and mortality.
RESULTS: One hundred and one patients were included; 71 were males and the average age was 51 years. Twenty-one patients rebled within 6 wk. Patients with high-risk stigmata, PTVE with trunk obliteration, and a hepatic vein pressure gradient (HVPG) ≥ 20 mmHg were at increased risk for rebleeding (OR = 5.279, 95%CI: 2.782-38.454, P = 0.003; OR = 4.309, 95%CI: = 2.144-11.793, P < 0.001; and OR = 1.534, 95%CI: 1.062-2.216, P = 0.022, respectively). Thirteen patients died within 6 wk. A model for end-stage liver disease (MELD) score ≥ 18 and an HVPG ≥ 20 mmHg were associated with 6-wk mortality (OR = 2.162, 95%CI: 1.145-4.084, P = 0.017 and OR = 1.423, 95%CI: 1.222-1.657, P < 0.001, respectively).
CONCLUSION: MELD score and HVPG in combination allow for early identification of patients with AVH who are at substantially increased risk of death over the short term.
Keywords: Acute variceal hemorrhage, Percutaneous transhepatic variceal embolization, Hepatic vein pressure gradient
Core tip: Acute variceal hemorrhage (AVH) is a medical emergency with a 20% mortality rate at 6 wk. Percutaneous transhepatic variceal embolization (PTVE) is a rescue therapy for endoscopic variceal ligation failure. Here we present a retrospective study to determine the risk factors for 6-wk rebleeding and mortality in AVH patients who have undergone PTVE. Patients with a model for end-stage liver disease score ≥ 18 and an HVPG ≥ 20 mmHg are at increased risk of death within 6 wk of an acute variceal bleeding episode. A transjugular intrahepatic portosystemic shunt or liver transplantation should be considered for this high-risk group.
INTRODUCTION
Gastroesophageal varices are present at diagnosis in nearly one-half of patients with cirrhosis. Variceal hemorrhage carries high rebleeding and mortality rates. In patients with esophageal varices (EV), the combination of endoscopic variceal ligation (EVL) and pharmacologic treatment is recommended as the standard of care for prevention of rebleeding[1]. Mortality with each episode of acute variceal hemorrhage (AVH) has decreased to the current level of approximately 20%[2]. A previous report showed that early rebleeding rate ranges from 30% to 40% within the first 6 wk, and is significantly associated with the risk of death within 6 wk[3]. Thus, research assessing the value of various risk factors in patients with AVH is important in an effort to identify the group of patients at high risk for rebleeding and mortality. A transjugular intrahepatic portosystemic shunt (TIPS) or liver transplantation should be considered for this high-risk group.
Percutaneous transhepatic variceal embolization (PTVE) was introduced in 1974 by Lunderquist et al[4] for the management of portal hypertension and EV. PTVE is a safe, easy to perform, and effective treatment for the control of AVH[5]. In the modified PTVE procedure, we used 2-octyl cyanoacrylate as an embolic material to obliterate the EV and peri-esophageal collaterals and feeding vessels[6]. Using multi-detector row computed tomography, a study showed that 2-octyl cyanoacrylate permanently retained in the para- and peri-varices in the vessels without a time-dependent decrease[7]. It is important to continue the obliteration of the feeding vessels and prevent the relapse of esophageal-gastric varices. The modified PTVE technique has been confirmed as an effective and safe method for the management of recurrent gastroesophageal varices and rebleeding compared with endoscopy therapy[8,9]. The combination of PTVE and EVL is more effective than EVL alone in the prevention and treatment of recurrent EV and rebleeding[10]. Thus, PTVE may be used as a rescue therapy for patients who fail endoscopic and pharmacologic treatment.
Some studies have identified the predictors of early rebleeding and mortality after initial EV bleeding in cirrhotic patients with endoscopic treatment[11-16]; however, no study has addressed the association between the predictors of outcome and mortality in cirrhotic patients with EV bleeding treated by PTVE. Studies assessing the value of various risk factors in patients with AVH are important as risk factors may offer a useful means of selection for entry into liver transplantation or they may identify a group of patients with a very high mortality. The early use of TIPS in patients with cirrhosis and at high risk for variceal bleeding was associated with marked and significant reductions in treatment failure and mortality[13]. The aim of this study was to identify the risk factors for rebleeding and mortality in cirrhotic patients admitted to a hospital with a first variceal bleed and treated by PTVE as a rescue therapy after failed endoscopic variceal ligation within 6 wk.
MATERIALS AND METHODS
Patients
Consecutive patients with liver cirrhosis and AVH admitted to our hospital, a tertiary center, were retrospectively analyzed between January 2010 and December 2012. The inclusion criteria were as follows: (1) cirrhosis confirmed by full clinical history, physical examination, laboratory testing, and imaging examinations; (2) patients admitted to hospital within 24 h after the symptom onset, with AVH diagnosed by upper gastrointestinal endoscopy when varices were bleeding actively or showed stigmata of recent bleeding and/or if fresh blood was observed in the stomach and varices were the only potential source of bleeding; and (3) PTVE was performed as a rescue therapy in patients who had uncontrolled severe bleeding or recurrent bleeding from EV during and after band ligation.
The exclusion criteria were as follows: (1) concomitant hepatic cell cancer (HCC) or other cancers; (2) severe hypertension, coronary heart disease, cardiopulmonary insufficiency, or chronic renal insufficiency; (3) previous TIPS placement or endoscopic treatment of varices by sclerotherapy or band ligation; and (4) insufficient data on survival or incomplete medical records.
Therapeutic interventions for variceal bleeding
When admitted to our hospital, all patients were managed by fluid resuscitation and an infusion of vasoactive drugs (octreotide or somatostatin), and the infusion was continued for a total of 3-5 d. Prophylactic oral norfloxacin (400 mg, twice a day) or intravenous ciprofloxacin (1 g, once a day) was administered for 7 d. Sengstaken-Blakemore balloon tamponade was used if necessary. Patients with hemodynamic instability or a significant drop in the hemoglobin level (< 8 g/dL) were given packed red blood cell (PRBC) transfusions to a hemoglobin of 8 g/dL. Upper gastrointestinal endoscopy to identify the source of bleeding was performed on all patients within 24 h of presentation; endoscopic variceal band ligation was performed if the source of gastrointestinal bleeding was believed to be from EV. If band ligation was not feasible because blood obscured the visual field, or if band ligation failed to control bleeding, the PTVE procedure was performed under radiologic guidance, as described previously[6]. The wedged hepatic venous pressure (WHVP) and the free hepatic venous pressure (FHVP) were measured before PTVE, and the hepatic venous pressure gradient (HVPG) was calculated (HVPG = WHVP - FHVP). Propranolol with or without isosorbide mononitrate was used for prevention of recurrent bleeding if patients had no contraindications.
Variable definitions and data collection
Patient outcome was obtained from hospital records and telephone contacts. We included only the first episode for each patient. The primary end point of the study was the first episode of recurrent variceal bleeding 6 wk after the procedure. The secondary end point was death due to primary liver disease in 6 wk. Rebleeding was defined according to the Baveno criteria as recurrence of bleeding evidenced by new melena or hematemesis, requirement for > 2 units of PRBCs in a 24 h time period, and hemodynamic instability[17]. Time zero was defined as the day of the PTVE procedure.
Demographic information, medical history, physical examination with vital signs, documentation of etiology of liver disease and presenting clinical symptoms, ascites, encephalopathy, and Child-Turcotte-Pugh (CTP) classification were collected. The number of blood units transfused within 72 h of admission was recorded. The grade of ascites was based on the definitions of the International Ascites Club[18].
Blood for laboratory testings, including complete blood count, prothrombin time, serum creatinine, total bilirubin, serum albumin, serum sodium, aspartate aminotransferase, and alanine aminotransferase levels was also drawn on the first day of variceal bleeding.
Findings at endoscopy were documented, including sites of varices, stage of EV, presence of active bleeding, and stigmata of high-risk varices. The stage of variceal size was based on the general rules established by the Japanese Research Society for Portal Hypertension[19]. High-risk varices were defined as the presence of an adherent clot or white nipple or red signs on varices (cherry red spot, red wale sign, or hematocystic spots).
The filling range of cyanoacrylate in EVs and the feeding vessels by PTVE was based on the following definitions: (1) complete obliteration, with at least 3 cm of the lower EVs and peri- and para-EVs, as well as the adventitial plexus of the gastric cardia and fundus filled with cyanoacrylate; (2) partial obliteration, with the varices surrounding the gastric cardia, fundus, and the feeding vessels being obliterated with cyanoacrylate, but without reaching the lower EVs; and (3) trunk obliteration, with the main branch of the left gastric vein being filled with cyanoacrylate, but without reaching the varices surrounding the gastric cardia or fundus[7].
MELD scores were calculated according to the following formula: MELD score = 0.957 × ln (creatinine mg/dL) + 0.378 × ln (bilirubin mg/dL) + 1.120 × ln (INR) + 6.43.
Statistical analysis
Continuous data are expressed as mean ± SD, unless specified otherwise. Descriptive statistics (number and percentages) were used to describe discrete data. Survival analysis was estimated using the Kaplan-Meier method and compared using the log-rank test. The multivariate analysis was performed using the Cox regression test to identify independent risk factors for rebleeding and mortality. The Statistical Package for Social Sciences (version 17.0; SPSS, Inc., Chicago, IL, United States) was used, and P < 0.05 was regarded as significant.
RESULTS
Between January 2010 and December 2012, 137 cirrhotic patients with AVH underwent PTVE as rescue treatment; 36 patients were excluded from the analysis because of HCC (n = 5), technical failures (n = 4), previous placement of TIPS or endoscopic treatment (n = 23), and incomplete medical records (n = 4). Therefore, the number of patients who met the inclusion criteria and were analyzed in the current study was 101. The gastric coronary vein was the main blood vessel for EV in 89 patients. Forty-six patients had varying degrees of contribution from the short gastric and posterior gastric veins. All of the feeding vessels were obliterated with cyanoacrylate. Sengstaken-Blakemore balloon tamponade was used in five patients. Propranolol with or without isosorbide mononitrate was used in 94 patients; the other seven patients did not use propranolol or isosorbide mononitrate because of contraindications (glaucoma, n = 2; sinus bradycardia < 50 bpm, n = 2; arterial hypotension with systolic pressure < 85 mmHg, n = 2; and asthma, n = 1). The clinical characteristics of the patients are shown in Table 1.
Table 1.
Clinical and biological characteristics of the study population n (%)
| Characteristic | n = 101 |
| Gender (male/female) | 71 (70.3%)/30 (29.7%) |
| Age (yr) | 51 ± 12 |
| Etiology of liver disease n (%) Viral (HBV and/or HCV) Alcohol Others | 76 (75.2) 19 (18.8) 6 (5.9) |
| Clinical presentation of bleeding n (%) Melena Hematemesis Both melena and hematemesis | 22 (21.8) 32 (31.7) 47 (46.5) |
| Systolic blood pressure at presentation (mmHg) | 114 ± 21 |
| Bleeding source n (%) Esophageal varices Gastric varices | 85 (84.2) 16 (15.8) |
| High risk stigmata of variceal bleeding (yes/no) | 89 (88.1)/12 (11.9) |
| Active variceal bleeding at endoscopy (yes/no) | 27 (26.7)/74 (73.3) |
| Hemoglobin (g/dL) | 9.9 ± 2.4 |
| White blood cells (109/L) | 4.78 ± 3.55 |
| Platelets (109/L) | 111 ± 102 |
| Aspartate aminotransferase level (U/L) | 39 ± 15 |
| Alanine aminotransferase level (U/L) | 29 ± 15 |
| Serum sodium level (mmol/L) | 132 ± 11 |
| Serum creatinine (μmol/L) | 57 ± 18 |
| Albumin (g/L) | 34 ± 6 |
| Total bilirubin (μmol/L) | 24.5 ± 12.3 |
| Prothrombin time (s) | 16.7 ± 2.3 |
| Presence of ascites n (%) 0 I II III | 47 (46.5) 31 (30.7) 18 (17.8) 5 (5.0) |
| Requiring blood transfusion within 72 h (yes/no) | 38 (37.6)/63 (62.4) |
| Units of PRBCs transfused within 72 h | 6 ± 2 |
| HVPG (mmHg) | 18 ± 4 |
| Obliteration range of PTVE n (%) Complete Partial Trunk | 63 (62.4) 21 (20.8) 17 (16.8) |
| Child-Turcotte-Pugh classification n (%) A B C | 15 (14.9) 61 (60.4) 25 (24.8) |
| MELD score | 15 ± 5 |
PRBCs: Packed red blood cells; HVPG: Hepatic vein pressure gradient; PTVE: Percutaneous transhepatic variceal embolization.
Risk factors for rebleeding within 6 wk following PTVE treatment
Twenty-one (20.8%) patients rebled within 6 wk of the PTVE procedure. Recurrent bleeding occurred in a range of 3-32 d following PTVE. Among 21 patients with rebleeding, 5 had bleeding from EVL-induced ulcers, 12 from EV, 3 from gastric varices, and 1 from an unknown site. High-risk stigmata of variceal bleeding, PTVE with trunk obliteration, and an HVPG ≥ 20 mmHg were independent risk factors for rebleeding as revealed by the Kaplan-Meier method. Figure 1 shows survival curves according to independent predictor variables. In multivariable analyses using Cox regression, high-risk stigmata of variceal bleeding, the obliteration range of PTVE, and an HVPG ≥ 20 mmHg were significantly associated with the risk of rebleeding; high-risk stigmata of variceal bleeding was the variable with the highest odds ratio (OR = 5.279; 95%CI: 2.782-38.454; Table 2).
Figure 1.
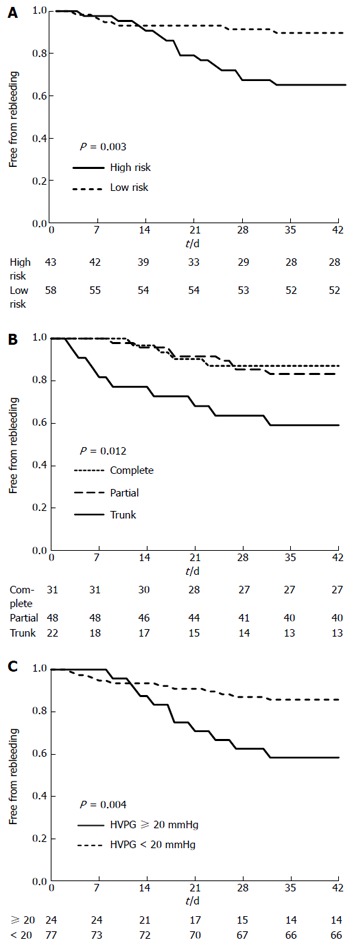
Kaplan-Meier plots showing the cumulative incidence of rebleeding in 6 wk stratified according to (A) risk stigmata of variceal bleeding, (B) obliteration range of percutaneous transhepatic variceal embolization, and (C) hepatic vein pressure gradient. The curves are compared using a log-rank test. PTVE: Percutaneous transhepatic variceal embolization; HVPG: Hepatic vein pressure gradient.
Table 2.
Independent risk factors associated with rebleeding as revealed by Cox regression analysis
| Variable | B | SE | Wals | P value | OR |
95%CI |
|
| Lower | Upper | ||||||
| High risk stigmata of variceal bleeding | 4.267 | 1.435 | 8.843 | 0.003 | 5.279 | 2.782 | 38.454 |
| Obliteration range of PTVE | 2.068 | 0.540 | 14.663 | 0.000 | 4.309 | 2.144 | 11.793 |
| HVPG | 0.428 | 0.188 | 5.209 | 0.022 | 1.534 | 1.062 | 2.216 |
PTVE: Percutaneous transhepatic variceal embolization; HVPG: Hepatic vein pressure gradient.
Risk factors for 6-wk mortality after PTVE
Thirteen (12.9%) patients died within the 6-wk follow-up period. Among these patients, six died of uncontrolled EV bleeding, five of hepatic failure, one of hepatorenal syndrome, and one of hepatic encephalopathy. Cox regression analysis revealed that the MELD score and HVPG were significantly associated with 6-wk mortality after PTVE (Table 3). Figure 2 shows the survival curves according to independent predictor variables. Stratification of patients according to MELD score (MELD ≥ 18 or MELD < 18) revealed a significant increase in 6-wk mortality after PTVE between patients with MELD scores ≥ 18 or < 18 (P = 0.008; Figure 2A). The HVPG was also significantly associated with 6-wk mortality after PTVE (P < 0.001; Figure 2B). Interestingly, CTP class (A vs B/C) was not predictive of mortality.
Table 3.
Independent prognostic factors associated with mortality as revealed by Cox regression analysis
| Variable | B | SE | Wals | P value | OR |
95%CI |
|
| Lower | Upper | ||||||
| MELD | 0.771 | 0.324 | 5.652 | 0.017 | 2.162 | 1.145 | 4.084 |
| HVPG | 0.353 | 0.078 | 20.691 | 0.000 | 1.423 | 1.222 | 1.657 |
MELD: Model for end-stage liver disease; HVPG: Hepatic vein pressure gradient.
Figure 2.
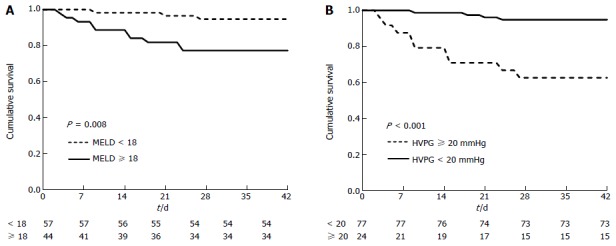
Kaplan-Meier plots showing the cumulative incidence of death in 6 wk stratified according to model for end-stage liver disease (A) and hepatic vein pressure gradient (B). The curves are compared using a log-rank test. MELD: Model for end-stage liver disease; HVPG: Hepatic vein pressure gradient.
Adverse effects
Adverse effects were observed in 21 (20.8%) patients following PTVE. Transient upper abdominal pain (n = 16), fever (n = 14), and bleeding at the liver puncture site (n = 3) developed in patients following PTVE. All of the adverse effects were minor and alleviated by pharmacologic therapy.
DISCUSSION
Specific risk factors that predict early rebleeding and mortality after variceal bleeding with PTVE treatment have not been studied. In the present study, we found that high-risk stigmata of variceal bleeding, the obliteration range of PTVE, and an HVPG ≥ 20 mmHg are significantly predictive of 6-wk rebleeding in patients with cirrhosis who are hospitalized with an acute variceal bleed and treated by PTVE as rescue treatment. By complete and permanent obliteration of the lower EVs, peri- and para-EVs, and the adventitial plexus of the gastric cardia and fundus, complete obliteration of PTVE can reduce the risk of variceal recurrence, and prevent bleeding from EV, while varices tend to reoccur over time following partial and incomplete trunk obliteration of PTVE.
Both HVPG measurement and endoscopic features as prognostic indicators of early rebleeding in patients with EVL treatment have been reported in some studies. A retrospective study conducted by Lee et al[20] showed that early recurrent hemorrhage in cirrhotic patients is significantly associated with more EV ligations due to the extensive surface area of the mucosal injury and post-banding ulcers. Xu et al[21] showed that the severity of varices is one of the main factors affecting early rebleeding after EVL.
The rate of mortality within 6 wk after cessation of initial EV bleeding in our study was 12.9%, which was similar with the lower rates reported in previous studies (range, 8%-46%)[22-25]. Our study also demonstrated that MELD and HVPG are related to early mortality for EV patients following PTVE.
Several studies have investigated the accuracy of the MELD score in predicting mortality after AVH. In a retrospective study of 172 cirrhotic patients admitted for esophageal variceal hemorrhage, Amitrano et al[26] showed that patients with an MELD score > 15 had significantly higher mortality at 6 wk than patients with an MELD score ≤ 15. By regression analysis of 256 patients with AVH in a randomized, prospective trial, Bambha et al[27] demonstrated that patients with a MELD score ≥ 18, those transfused with ≥ 4 units of packed erythrocytes within the first 24 h or those being actively bleeding at the time of endoscopy had increased mortality within 6 wk. Based on a study by Suk et al[28], the efficacy of HVPG and MELD is excellent for predicting the survival of patients with decompensated liver cirrhosis. Ripoll et al[29] showed that MELD was the only predictor of death in decompensated patients based on multivariate analysis. Reverter et al[11] developed an MELD-based model that accurately predicts mortality among patients with AVH; MELD values ≥ 19 predicted ≥ 20% mortality, whereas MELD scores < 11 predicted < 5% mortality. MELD also was a significant predictor of mortality for patients with variceal bleeding admitted to intensive care units[30]. Wang et al[31] reported that HVPG measurement may help identify a subset of patients with low MELD scores who have a higher mortality. Our study showed that MELD score and HVPG measurement can be used as stratified factors to discern high-risk patients and make a decision to proceed to TIPS or liver transplantation earlier.
Additionally, we found that the etiology of liver disease (alcohol vs viral vs others), active bleeding at the index endoscopy, volume of blood transfusion in 72 h, and CTP class were not correlated with the risk of rebleeding and mortality based on univariate analyses. These results are somewhat discordant with several other studies focusing on the prognosis of variceal bleeding. In unselected cirrhotic patients, Amitrano et al[32] concluded that CTP class C was an independent predictor of 5-d failure; mortality was mainly related to the severity of liver failure. Bambha et al[27] demonstrated that patients who received ≥ 4 units of packed erythrocytes within the first 24 h or were actively bleeding at the time of endoscopy had an increased mortality rate within 6 wk.
Differences in patient sampling (percentage of alcoholics and percentage of CTP class C cirrhotic patients), variables recorded, techniques of endoscopic intervention, or dissimilar study end points could explain the discrepancies. In the current study, the source of bleeding (esophagus or stomach) and the activity of bleeding (active or recent) were defined according to the Baveno V consensus[17]. EVL was defined as a primary therapy; patients underwent endoscopy therapy as soon as they were hemodynamically stable and PTVE was a rescue therapy when EVL failed. Furthermore, we only analyzed the first bleeding episode. It is very important to distinguish the first from the subsequent bleeding episodes because associated mortality is different and this may lead to biases in studies when pooling the two types of episodes[33].
Interestingly, we found that the risk factors for rebleeding and mortality were different. Our study therefore suggests that although rebleeding is the major cause of cirrhosis-associated deaths, death is influenced not only by the severity of the bleeding episode itself, but also by the severity of the underlying liver disease and concomitant diseases.
There were two limitations in the current study. First, PTVE is not a standard treatment for AVH. EVL is the recommended form of endoscopic therapy for AVH; rebleeding after EVL may be managed by a second attempt at endoscopic therapy[1]. If rebleeding is severe, PTFE-covered TIPS is likely the best option[34]; however, TIPS is a complex procedure, and in some patients, such as those with variant anatomy, portal vein thrombosis, hepatic vein thrombosis, or pre-existing TIPS, TIPS creation may be extremely difficult[35]. Contraindications and complications of TIPS also restrict its use in cirrhosis patients[36]. Modified PTVE with 2-octyl cyanoacrylate has been confirmed as an effective and safe method for preventing rebleeding of EV and gastric varices[9,10,37]. Variceal embolotherapy during TIPS procedures is a rational approach to reducing recurrent bleeding rates after TIPS placement. According to a recent study, the TIPS plus embolization regimen may reduce the risk of recurrent variceal bleeding during the first 6 months after the TIPS procedure by preventing shunt dysfunction, which may improve liver function and quality of life[38], Therefore, we used PTVE as rescue therapy for EVL failure, and the current study demonstrated the risk factors for rebleeding and mortality after AVH with PTVE treatment.
Second, an absence of standardization at the time of entry also affected the study. Difference in the time of entry could lead to different results. As Burroughs et al[39] reported, the starting point for analysis following variceal hemorrhage is an important confounding variable when calculating survival and rebleeding. Changing the starting point for analysis after variceal hemorrhage leads to completely different conclusions. Usually, the entry time is the day patients are admitted to the hospital in studies involving risk factors related to rebleeding and mortality in AVH or EVL-treated patients. However, in the current study, our aim was to determine the risk factors related to rebleeding and mortality after PTVE treatment, so we chose the day of the PTVE procedure as time zero, which avoided rebleeding and mortality before PTVE.
In conclusion, the current study demonstrated that stigmata of variceal bleeding, the obliteration range of PTVE, and an HVPG ≥ 20 mmHg are significant and strong predictors of short-term rebleeding 6 wk after AVH treated by PTVE. We also demonstrated that patients with an MELD score ≥ 18 and an HVPG ≥ 20 mmHg are at increased risk of death within 6 wk of an acute variceal bleeding episode. Together these factors allow for early identification of patients with AVH who are at substantially increased risk of death over the short term. Such patients would also probably benefit from early TIPS or liver transplantation. Furthermore, the PTVE procedure combined with TIPS may improve survival in AVH patients and is worthy of further study.
COMMENTS
Background
Acute variceal hemorrhage (AVH) is a medical emergency with a 20% mortality rate at 6 wk. Recurrent variceal bleeding is very frequent and risk factors for early rebleeding and mortality in AVH patients are ill-defined.
Research frontiers
Research assessing the value of various risk factors for AVH patients is important in an effort to identify the group of patients at high risk for rebleeding and mortality. Percutaneous transhepatic variceal embolization (PTVE) is a rescue therapy for endoscopic variceal ligation failure. Specific risk factors that predict early rebleeding and mortality after variceal bleeding with PTVE treatment have not been studied.
Innovations and breakthroughs
In previous studies involving risk factors for early rebleeding in patients who had undergone EVL treatment, it was reported that severity of varices, MELD score, transfusion, and Child class were related to AVH rebleeding and mortality. However, specific risk factors that predict early rebleeding and mortality after variceal bleeding with PTVE treatment have not been studied. In the present retrospective cohort study, we found that high-risk stigmata, PTVE with trunk obliteration, and an HVPG ≥ 20 mmHg are predictors of variceal rebleeding within 6 wk, and patients with an MELD score ≥ 18 and an HVPG ≥ 20 mmHg are at increased risk of death within 6 wk. In combination, these factors allow for early identification of patients with AVH who are at substantially increased risk of rebleeding or death over the short term. Such patients would probably benefit from early TIPS or liver transplantation.
Applications
The current study results suggest that patients with an MELD score ≥ 18 and an HVPG ≥ 20 mmHg are at increased risk of death within 6 wk after an acute variceal bleeding episode. Research assessing the value of various risk factors in patients with AVH is important in an effort to identify the group of patients at high risk for rebleeding and mortality. A transjugular intrahepatic portosystemic shunt (TIPS) or liver transplantation should be considered for this high-risk group.
Terminology
Percutaneous transhepatic variceal embolization (PTVE) is the earliest intervention performed for the treatment of intractable variceal bleeding. During PTVE, the portal vein is catheterized by a percutaneous transhepatic approach and the gastric vein feeding the varix is embolized with ethanol, steel coils, or cyanoacrylate glue. The hepatic venous pressure gradient (HVPG) is currently the most commonly used parameter for portal pressure measurement, i.e., the difference between the wedged and free hepatic venous pressures. HVPG represents the gradient between pressures in the portal vein and the intra-abdominal portion of the inferior vena cava.
Peer review
This is an interesting study debating a well-chosen topic. The study adequately addresses several points of an on-going debate regarding AVH, rebleeding risk, and treatment.
Footnotes
P- Reviewer: Cao GW, Narciso-Schiavon JL, Pan WS, Streba CT S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 2.del Olmo JA, Peña A, Serra MA, Wassel AH, Benages A, Rodrigo JM. Predictors of morbidity and mortality after the first episode of upper gastrointestinal bleeding in liver cirrhosis. J Hepatol. 2000;32:19–24. doi: 10.1016/s0168-8278(01)68827-5. [DOI] [PubMed] [Google Scholar]
- 3.Sempere L, Palazón JM, Sánchez-Payá J, Pascual S, de Madaria E, Poveda MJ, Carnicer F, Zapater P, Pérez-Mateo M. Assessing the short- and long-term prognosis of patients with cirrhosis and acute variceal bleeding. Rev Esp Enferm Dig. 2009;101:236–248. [PubMed] [Google Scholar]
- 4.Lunderquist A, Vang J. Transhepatic catheterization and obliteration of the coronary vein in patients with portal hypertension and esophageal varices. N Engl J Med. 1974;291:646–649. doi: 10.1056/NEJM197409262911303. [DOI] [PubMed] [Google Scholar]
- 5.L’Herminé C, Chastanet P, Delemazure O, Bonnière PL, Durieu JP, Paris JC. Percutaneous transhepatic embolization of gastroesophageal varices: results in 400 patients. AJR Am J Roentgenol. 1989;152:755–760. doi: 10.2214/ajr.152.4.755. [DOI] [PubMed] [Google Scholar]
- 6.Zhang CQ, Liu FL, Liang B, Xu HW, Xu L, Feng K, Liu ZC. A modified percutaneous transhepatic varices embolization with 2-octyl cyanoacrylate in the treatment of bleeding esophageal varices. J Clin Gastroenterol. 2009;43:463–469. doi: 10.1097/MCG.0b013e31817ff90f. [DOI] [PubMed] [Google Scholar]
- 7.Sun A, Shi YJ, Xu ZD, Tian XG, Hu JH, Wang GC, Zhang CQ. MDCT angiography to evaluate the therapeutic effect of PTVE for esophageal varices. World J Gastroenterol. 2013;19:1563–1571. doi: 10.3748/wjg.v19.i10.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang CQ, Liu FL, Liang B, Sun ZQ, Xu HW, Xu L, Feng K, Liu ZC. A modified percutaneous transhepatic variceal embolization with 2-octyl cyanoacrylate versus endoscopic ligation in esophageal variceal bleeding management: randomized controlled trial. Dig Dis Sci. 2008;53:2258–2267. doi: 10.1007/s10620-007-0106-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Tian XG, Li Y, Zhang CQ, Liu FL, Cui Y, Liu JY. Comparison of modified percutaneous transhepatic variceal embolization and endoscopic cyanoacrylate injection for gastric variceal rebleeding. World J Gastroenterol. 2013;19:706–714. doi: 10.3748/wjg.v19.i5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian S, Tian XG, Hu JH, Wang GC, Zhang CQ. Percutaneous transhepatic variceal embolization combined with endoscopic ligation for the prevention of variceal rebleeding. J Dig Dis. 2013;14:388–395. doi: 10.1111/1751-2980.12049. [DOI] [PubMed] [Google Scholar]
- 11.Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412–419.e3. doi: 10.1053/j.gastro.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Hunter SS, Hamdy S. Predictors of early re-bleeding and mortality after acute variceal haemorrhage. Arab J Gastroenterol. 2013;14:63–67. doi: 10.1016/j.ajg.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Pagán JC, Di Pascoli M, Caca K, Laleman W, Bureau C, Appenrodt B, Luca A, Zipprich A, Abraldes JG, Nevens F, Vinel JP, Sauerbruch T, Bosch J. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58:45–50. doi: 10.1016/j.jhep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Wang MT, Liu T, Ma XQ, He J. Prognostic factors associated with rebleeding in cirrhotic inpatients complicated with esophageal variceal bleeding. Chin Med J (Engl) 2011;124:1493–1497. [PubMed] [Google Scholar]
- 15.Chen WT, Lin CY, Sheen IS, Huang CW, Lin TN, Lin CJ, Jeng WJ, Huang CH, Ho YP, Chiu CT. MELD score can predict early mortality in patients with rebleeding after band ligation for variceal bleeding. World J Gastroenterol. 2011;17:2120–2125. doi: 10.3748/wjg.v17.i16.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanbiervliet G, Giudicelli-Bornard S, Piche T, Berthier F, Gelsi E, Filippi J, Anty R, Arab K, Huet PM, Hebuterne X, et al. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case-control study. Aliment Pharmacol Ther. 2010;32:225–232. doi: 10.1111/j.1365-2036.2010.04331.x. [DOI] [PubMed] [Google Scholar]
- 17.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 19.Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213–218. doi: 10.1016/s0016-5107(81)73224-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee SW, Lee TY, Chang CS. Independent factors associated with recurrent bleeding in cirrhotic patients with esophageal variceal hemorrhage. Dig Dis Sci. 2009;54:1128–1134. doi: 10.1007/s10620-008-0454-0. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Ji F, Xu QW, Zhang MQ. Risk factors for predicting early variceal rebleeding after endoscopic variceal ligation. World J Gastroenterol. 2011;17:3347–3352. doi: 10.3748/wjg.v17.i28.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952–954. doi: 10.1016/S0140-6736(03)12778-X. [DOI] [PubMed] [Google Scholar]
- 23.González A, Augustin S, Pérez M, Dot J, Saperas E, Tomasello A, Segarra A, Armengol JR, Malagelada JR, Esteban R, et al. Hemodynamic response-guided therapy for prevention of variceal rebleeding: an uncontrolled pilot study. Hepatology. 2006;44:806–812. doi: 10.1002/hep.21343. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva C, Aracil C, Colomo A, Lopez-Balaguer JM, Piqueras M, Gonzalez B, Torras X, Guarner C, Balanzo J. Clinical trial: a randomized controlled study on prevention of variceal rebleeding comparing nadolol + ligation vs. hepatic venous pressure gradient-guided pharmacological therapy. Aliment Pharmacol Ther. 2009;29:397–408. doi: 10.1111/j.1365-2036.2008.03880.x. [DOI] [PubMed] [Google Scholar]
- 25.González A, Augustin S, Dot J, Pérez M, Abu-Suboh M, Romero A, Segarra A, Armengol JR, Esteban R, Guardia J, et al. Adding banding ligation is effective as rescue therapy to prevent variceal rebleeding in haemodynamic non-responders to pharmacological therapy. Dig Liver Dis. 2012;44:55–60. doi: 10.1016/j.dld.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Amitrano L, Guardascione MA, Bennato R, Manguso F, Balzano A. MELD score and hepatocellular carcinoma identify patients at different risk of short-term mortality among cirrhotics bleeding from esophageal varices. J Hepatol. 2005;42:820–825. doi: 10.1016/j.jhep.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Bambha K, Kim WR, Pedersen R, Bida JP, Kremers WK, Kamath PS. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut. 2008;57:814–820. doi: 10.1136/gut.2007.137489. [DOI] [PubMed] [Google Scholar]
- 28.Suk KT, Kim CH, Park SH, Sung HT, Choi JY, Han KH, Hong SH, Kim DY, Yoon JH, Kim YS, et al. Comparison of hepatic venous pressure gradient and two models of end-stage liver disease for predicting the survival in patients with decompensated liver cirrhosis. J Clin Gastroenterol. 2012;46:880–886. doi: 10.1097/MCG.0b013e31825f2622. [DOI] [PubMed] [Google Scholar]
- 29.Ripoll C, Lastra P, Rincón D, Catalina V, Bañares R. Comparison of MELD, HVPG, and their changes to predict clinically relevant endpoints in cirrhosis. Scand J Gastroenterol. 2012;47:204–211. doi: 10.3109/00365521.2011.645500. [DOI] [PubMed] [Google Scholar]
- 30.Corbett C, Murphy N, Olliff S, Mangat KS, Tripathi D. A case-control study of transjugular intrahepatic portosystemic stent shunts for patients admitted to intensive care following variceal bleeding. Eur J Gastroenterol Hepatol. 2013;25:344–351. doi: 10.1097/MEG.0b013e32835aa414. [DOI] [PubMed] [Google Scholar]
- 31.Wang YW, Huo TI, Yang YY, Hou MC, Lee PC, Lin HC, Lee FY, Chi CW, Lee SD. Correlation and comparison of the model for end-stage liver disease, portal pressure, and serum sodium for outcome prediction in patients with liver cirrhosis. J Clin Gastroenterol. 2007;41:706–712. doi: 10.1097/MCG.0b013e31802dabb3. [DOI] [PubMed] [Google Scholar]
- 32.Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, DeNucci C, Lombardi G, Martino R, Menchise A, Orsini L, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107:1872–1878. doi: 10.1038/ajg.2012.313. [DOI] [PubMed] [Google Scholar]
- 33.Le Moine O, Adler M, Bourgeois N, Delhaye M, Devière J, Gelin M, Vandermeeren A, Van Gossum A, Vereerstraeten A, Vereerstraeten P. Factors related to early mortality in cirrhotic patients bleeding from varices and treated by urgent sclerotherapy. Gut. 1992;33:1381–1385. doi: 10.1136/gut.33.10.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afdhal NH, Curry MP. Early TIPS to improve survival in acute variceal bleeding. N Engl J Med. 2010;362:2421–2422. doi: 10.1056/NEJMe1003400. [DOI] [PubMed] [Google Scholar]
- 35.Ferral H, Bilbao JI. The difficult transjugular intrahepatic portosystemic shunt: alternative techniques and “tips” to successful shunt creation. Semin Intervent Radiol. 2005;22:300–308. doi: 10.1055/s-2005-925556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 37.Tian X, Wang Q, Zhang C, Liu F, Cui Y, Liu F, Liu J. Modified percutaneous transhepatic variceal embolization with 2-octylcyanoacrylate for bleeding gastric varices: long-term follow-up outcomes. AJR Am J Roentgenol. 2011;197:502–509. doi: 10.2214/AJR.10.6005. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Li X, Wei B, Tong H, Zhang MG, Huang ZY, Cao JW, Tang CW. Recurrent variceal bleeding and shunt patency: prospective randomized controlled trial of transjugular intrahepatic portosystemic shunt alone or combined with coronary vein embolization. Radiology. 2013;268:900–906. doi: 10.1148/radiol.13120800. [DOI] [PubMed] [Google Scholar]
- 39.Burroughs AK, Mezzanotte G, Phillips A, McCormick PA, McIntyre N. Cirrhotics with variceal hemorrhage: the importance of the time interval between admission and the start of analysis for survival and rebleeding rates. Hepatology. 1989;9:801–807. doi: 10.1002/hep.1840090602. [DOI] [PubMed] [Google Scholar]


